Photocatalytic Nanocomposites for the Protection of European Architectural Heritage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Set-Up of Nanomaterials
- -
- the first nanocomposite, labeled WNC, is a formulation based on alkyl alkoxy silane oligomers (15% w/w) with TiO2 NPs (0.96% w/w) in water; and,
- -
- the second nanocomposite, labeled ANC, is based on alkyl alkoxy silane monomers (40% w/w) with TiO2 NPs (0.12% w/w) in 2-propanol.
2.2. Set-Up of Treated Specimens for Testing
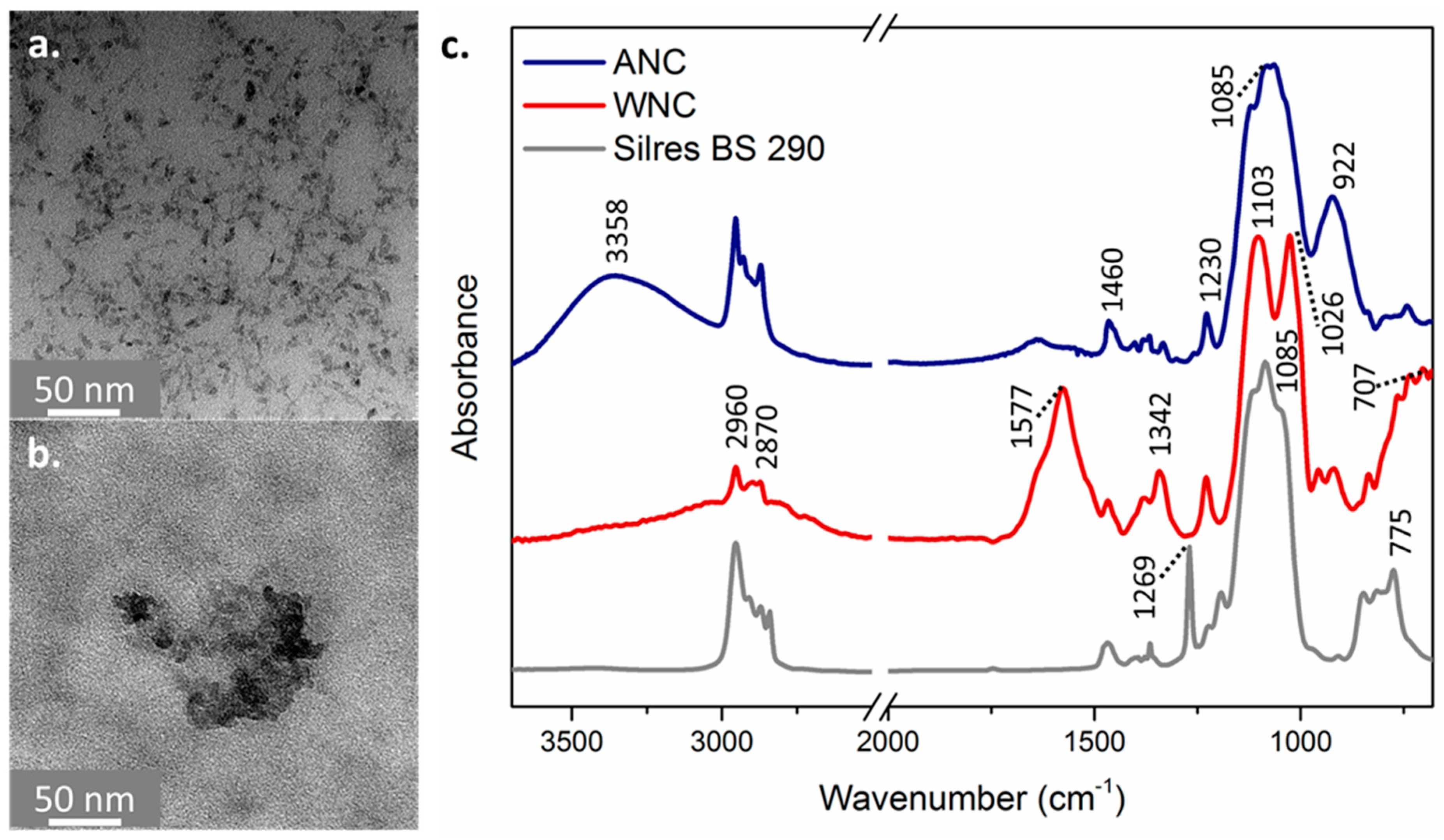
2.3. Characterization of the Nanocomposites
2.4. Evaluation of the Performances of Nanocomposites
3. Results and Discussion
3.1. Characterization of the Nanocomposites
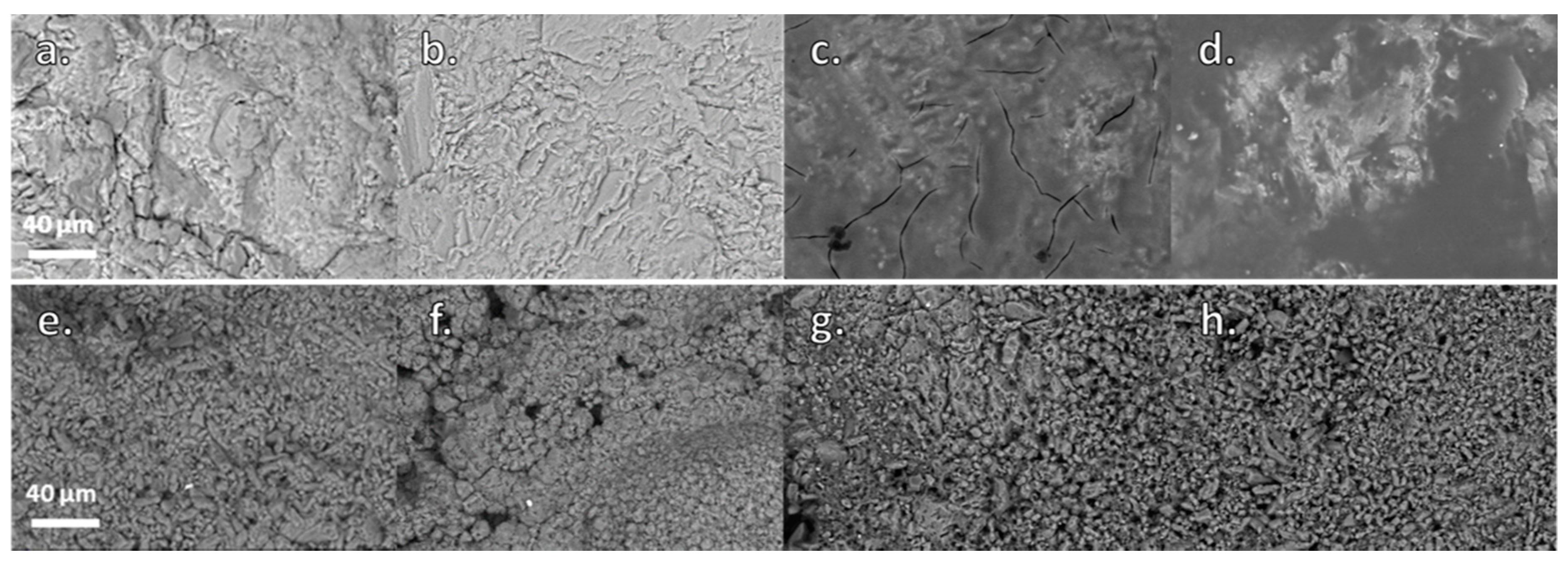
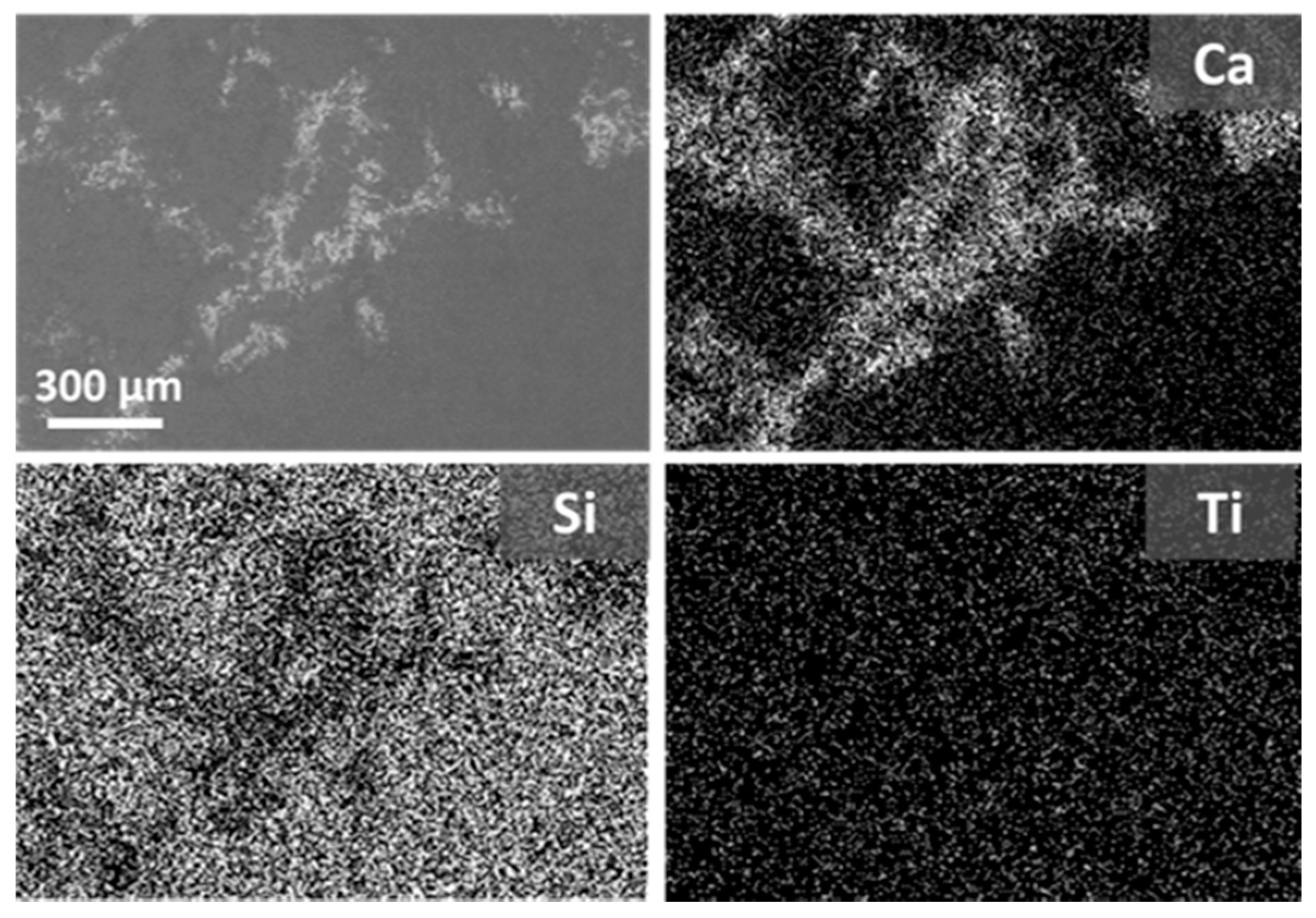
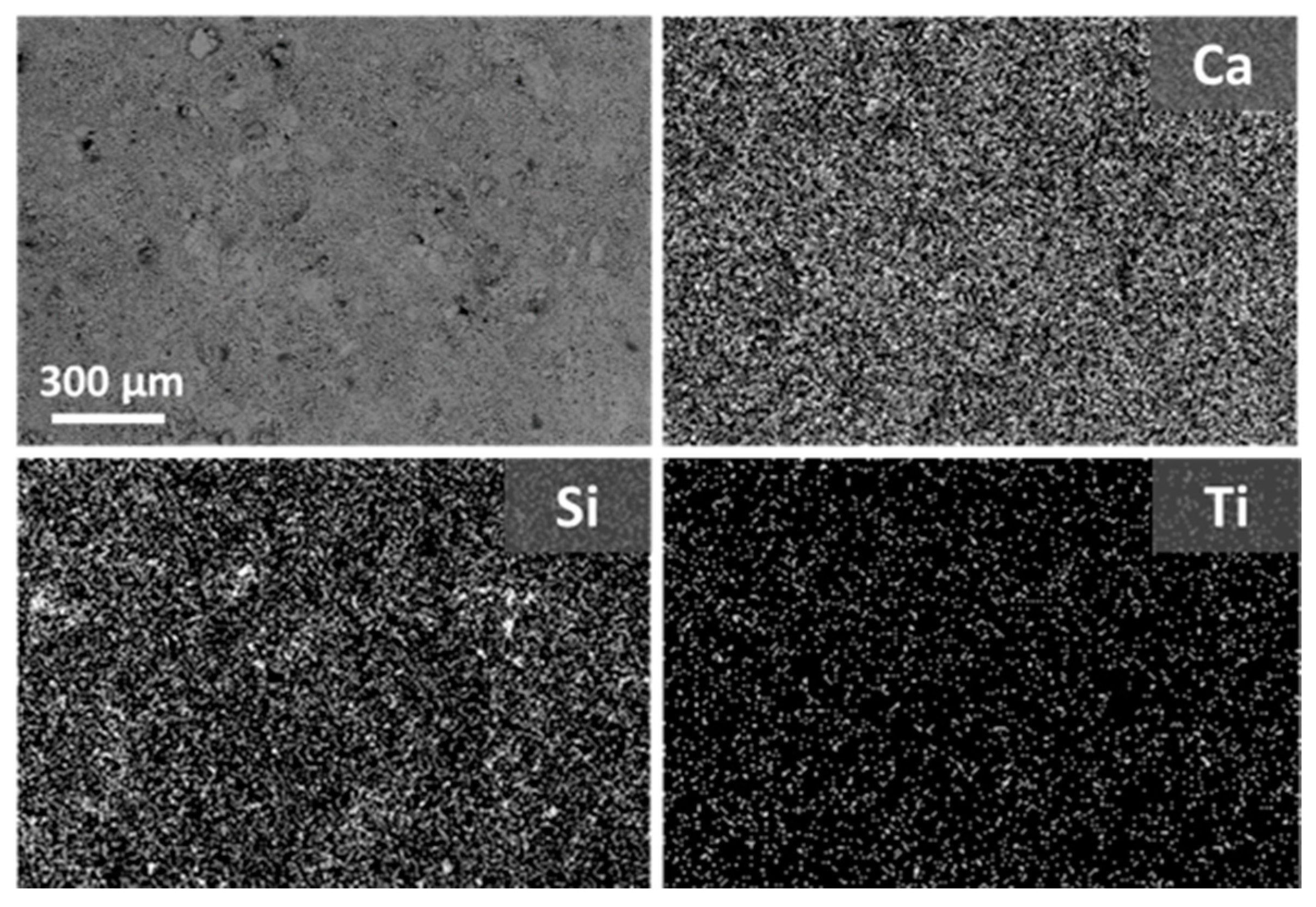
3.2. Evaluation of the Surface Morphology of the Treated Specimens
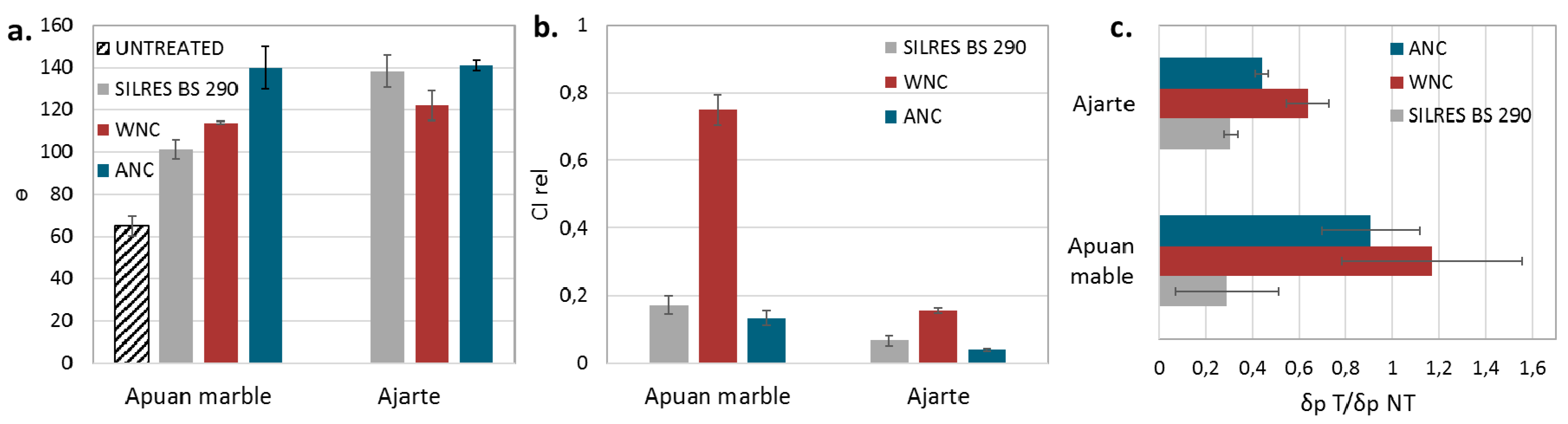
3.3. Evaluation of the Surface Colour Compatibility
3.4. Evaluation of the Surface Wettability, Water Absorption and Water Vapour Permeability
3.5. Evaluation of the Photocatalytic Activity of the Nanocomposites
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Doehne, E.F.; Price, C.A. Stone Conservation: An Overview of Current Research; Getty Conservation Institute: Los Angeles, CA, USA, 2010. [Google Scholar]
- Watt, J.; Tidblad, J.; Kucera, V.; Hamilton, R. The Effects of Air Pollution on Cultural Heritage; Springer: Berlin, Germany, 2009. [Google Scholar]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. C. R. Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Smart surfaces for architectural heritage: Preliminary results about the application of TiO2-based coatings on travertine. J. Cult. Herit. 2012, 13, 204–209. [Google Scholar] [CrossRef]
- Bergamonti, L.; Alfieri, I.; Lorenzi, A.; Montenero, A.; Predieri, G.; Barone, G.; Mazzoleni, P.; Pasquale, S.; Lottici, P.P. Nanocrystalline TiO2 by sol–gel: Characterisation and photocatalytic activity on modica and comiso stones. Appl. Surf. Sci. 2013, 282, 165–173. [Google Scholar] [CrossRef]
- Gherardi, F.; Colombo, A.; D’Arienzo, M.; Di Credico, B.; Goidanich, S.; Morazzoni, F.; Simonutti, R.; Toniolo, L. Efficient self-cleaning treatments for built heritage based on highly photo-active and well-dispersible TiO2 nanocrystals. Microchem. J. 2016, 126, 54–62. [Google Scholar] [CrossRef]
- Quagliarini, E.; Graziani, L.; Diso, D.; Licciulli, A.; D’Orazio, M. Is nano-TiO2 alone an effective strategy for the maintenance of stones in cultural heritage? J. Cult. Herit. 2017, in press. [Google Scholar] [CrossRef]
- Toniolo, L.; Gherardi, F. The protection of marble surfaces: The challenge to develop suitable nanostructured treatments. In Advanced Materials for the Conservation of Stone; Hosseini, M., Karapanagiotis, I., Eds.; Springer International Publishing: New York, NY, USA, 2017; Volume 1, in press. [Google Scholar]
- Cappelletti, G.; Fermo, P.; Camiloni, M. Smart hybrid coatings for natural stones conservation. Prog. Org. Coat. 2015, 78, 511–516. [Google Scholar] [CrossRef]
- Colangiuli, D.; Calia, A.; Bianco, N. Novel multifunctional coatings with photocatalytic and hydrophobic properties for the preservation of the stone building heritage. Constr. Build. Mater. 2015, 93, 189–196. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; Alvarez de Buergo, M.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Pinho, L.; Mosquera, M.J. Titania-silica nanocomposite photocatalysts with application in stone self-cleaning. J. Phys. Chem. C 2011, 115, 22851–22862. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2–SiO2–pdms nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156, 416–427. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Bergamonti, L.; Predieri, G.; Paz, Y.; Fornasini, L.; Lottici, P.P.; Bondioli, F. Enhanced self-cleaning properties of n-doped TiO2 coating for cultural heritage. Microchem. J. 2017, 133, 1–12. [Google Scholar] [CrossRef]
- EN16581:2014. Conservation of Cultural Heritage—Surface Protection for Porous Inorganic Materials—Laboratory Test Methods for the Evaluation of the Performance of Water Repellent Products; European Committee for Standardization: Brussels, Belgium, 2014. [Google Scholar]
- Gherardi, F.; Gulotta, D.; Goidanich, S.; Colombo, A.; Toniolo, L. On-site monitoring of the performance of innovative treatments for marble conservation in architectural heritage. Herit. Sci. 2017, 5, 4. [Google Scholar] [CrossRef]
- EN15802:2009. European Committee for Standardization Conservation of Cultural Property—Test Methods—Determination of Static Contact Angle; European Committee for Standardization: Brussels, Belgium, 2009. [Google Scholar]
- EN15801:2009. European Committee for Standardization: Conservation of Cultural Property—Test Methods—Determination of Water Absorption by Capillarity; European Committee for Standardization: Brussels, Belgium, 2009. [Google Scholar]
- Peruzzi, R.; Poli, T.; Toniolo, L. The experimental test for the evaluation of protective treatments: A critical survey of the “capillary absorption index”. J. Cult. Herit. 2003, 4, 251–254. [Google Scholar] [CrossRef]
- En 15803:2010. Conservation of Cultural Property—Test Methods—Determination of Water Vapour Permeability (δp); European Committee for Standardization: Brussels, Belgium, 2010. [Google Scholar]
- Tesser, E.; Antonelli, F.; Sperni, L.; Ganzerla, R.; Maravelaki, N.-P. Study of the stability of siloxane stone strengthening agents. Polym. Degrad. Stab. 2014, 110, 232–240. [Google Scholar] [CrossRef]
- Hasan, A.; Pandey, L.M. Kinetic studies of attachment and re-orientation of octyltriethoxysilane for formation of self-assembled monolayer on a silica substrate. Mater. Sci. Eng. C 2016, 68, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Téllez, L.; Rubio, J.; Rubio, F.; Morales, E.; Oteo, J.L. Ft-ir study of the hydrolysis and polymerization of tetraethyl orthosilicate and polydimethyl siloxane in the presence of tetrabutyl orthotitanate. Spectrosc. Lett. 2004, 37, 11–31. [Google Scholar] [CrossRef]
- García, O.; Malaga, K. Definition of the procedure to determine the suitability and durability of an anti-graffiti product for application on cultural heritage porous materials. J. Cult. Herit. 2012, 13, 77–82. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Superhydrophobic composite films produced on various substrates. Langmuir 2008, 24, 11225–11232. [Google Scholar] [CrossRef] [PubMed]
- Poli, T.; Toniolo, L. The challenge of protecting outdoor exposed monuments from atmospheric attack: Experience and strategy. In Fracture and Failure of Natural Building Stones; Kourkoulis, S.K., Ed.; Springer: Berlin, Germany, 2006. [Google Scholar]
- Charola, A.E. Water repellents and other “protective” treatments: A critical review, Hydrophobe III. In Proceedings of the 3rd International Conference on Surface Technology with Water Repellent Agents, Hannover, Germany, 25–25 September 2001; Aedificatio Publishers: Hannover, Germany, 2001. [Google Scholar]
- Hansen, C.M. Water transport and condensation in fluoropolymer films. Prog. Org. Coat. 2001, 42, 167–178. [Google Scholar] [CrossRef]
- Kronlund, D.; Bergbreiter, A.; Meierjohann, A.; Kronberg, L.; Lindén, M.; Grosso, D.; Smått, J.-H. Hydrophobization of marble pore surfaces using a total immersion treatment method—Product selection and optimization of concentration and treatment time. Prog. Org. Coat. 2015, 85, 159–167. [Google Scholar] [CrossRef]
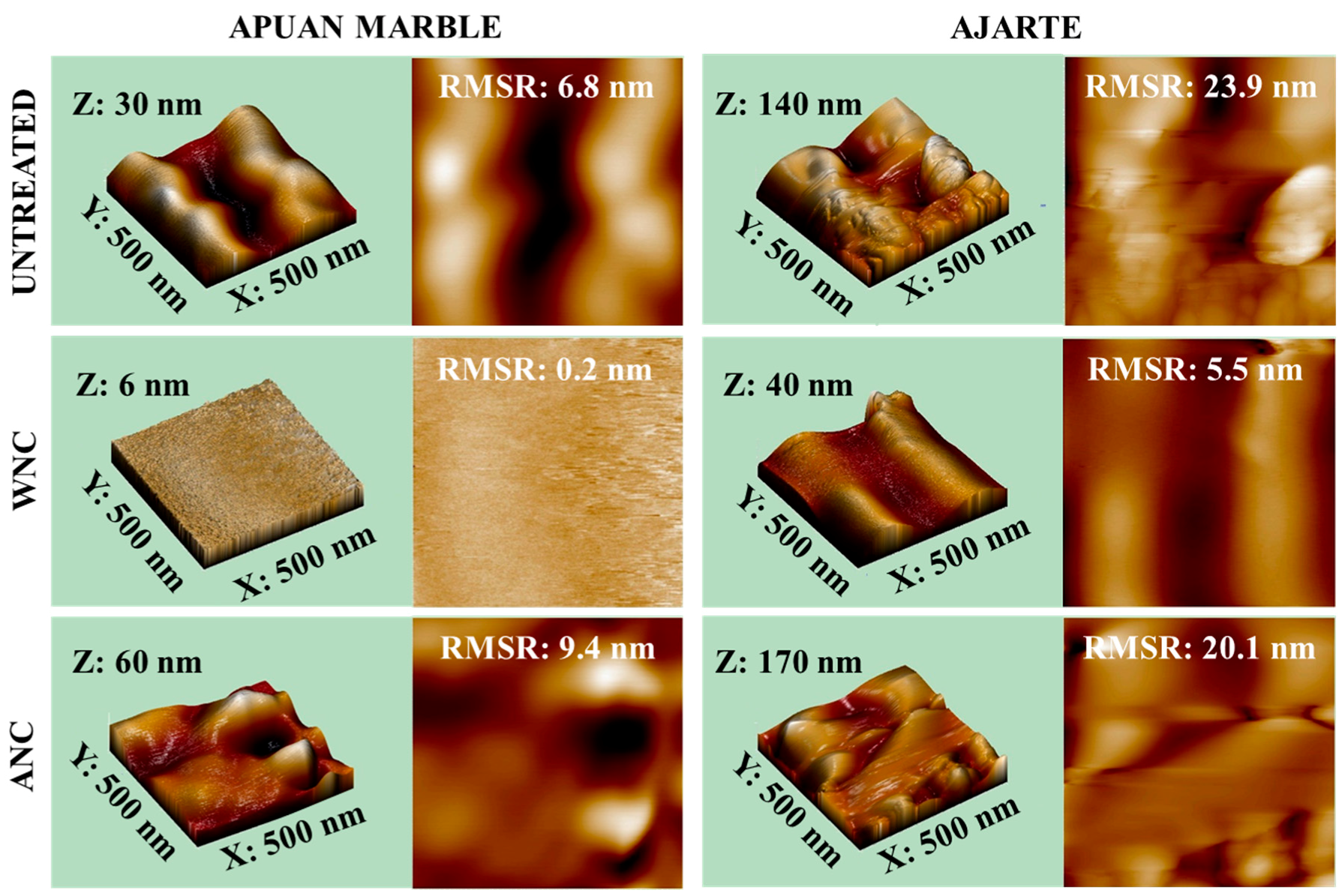
| Treatment | Apuan Marble | Ajarte |
|---|---|---|
| WNC | 0.3 ± 0.2 | 37 ± 5 |
| ANC | 1.3 ± 0.1 | 50 ± 6 |
| Lithotype | Total Open Porosity | Average Pore Diameter |
|---|---|---|
| Apuan marble | 0.7 | 0.08 |
| Ajarte | 23.5 | 0.17 |
| Lithotype | Treatment | ΔL* | Δa* | Δb* | ΔE* |
|---|---|---|---|---|---|
| Apuan marble | SILRES BS 290 | −1.69 ± 0.91 | 0.01 ± 0.01 | 0.50 ± 0.24 | 1.77 ± 0.92 |
| WNC | −1.23 ± 0.45 | −0.14 ± 0.17 | 0.73 ± 0.60 | 1.52 ± 0.54 | |
| ANC | −2.69 ± 0.76 | −0.05 ± 0.02 | 0.56 ± 0.11 | 2.75 ± 0.76 | |
| Ajarte | SILRES BS 290 | −5.40 ± 0.85 | 0.52 ± 0.08 | 2.06 ± 0.34 | 5.80 ± 0.91 |
| WNC | −0.94 ± 0.29 | −0.36 ± 0.06 | 1.08 ± 0.25 | 1.50 ± 0.28 | |
| ANC | −1.97 ± 0.77 | 0.21 ± 0.09 | −0.28 ± 0.87 | 2.19 ± 0.64 |
| Treatment | θ° nt | θ° t | CIrel | Qi nt | Qi t | CI nt | CI t | AC nt | AC t | δ t /δ nt |
|---|---|---|---|---|---|---|---|---|---|---|
| SILRES BS 290 | 58 ± 11 | 101 ± 5 | 0.17 ± 0.03 | 4.02 ± 1.24 | 0.80 ± 1.11 | 0.88 ± 0.11 | 0.75 ± 0.55 | 0.05 ± 0.04 | 0.003 ± 0.003 | 0.29 ± 0.22 |
| WNC | 66 ± 10 | 114 ± 1 | 0.75 ± 0.05 | 4.56 ± 2.64 | 3.61 ± 2.34 | 0.83 ± 0.08 | 0.81 ± 0.07 | 0.07 ± 0.07 | 0.026 ± 0.011 | 1.17 ± 0.38 |
| ANC | 72 ± 18 | 130 ± 10 | 0.13 ± 0.02 | 3.50 ± 0.17 | 0.57 ± 0.10 | 0.86 ± 0.04 | 0.70 ± 0.10 | 0.03 ± 0.01 | 0.006 ± 0.0004 | 0.91 ± 0.21 |
| Treatment | θ° nt | θ° t | CIrel | Qi nt | Qi t | CI nt | CI t | AC nt | AC t | δ t /δ nt |
|---|---|---|---|---|---|---|---|---|---|---|
| SILRES BS 290 | - | 138 ± 7 | 0.07 ± 0.01 | 420.70 ± 11.94 | 48.18 ± 13.42 | 0.86 ± 0.01 | 0.51 ± 0.03 | 4.80 ± 0.98 | 0.06 ± 0.01 | 0.31 ± 0.03 |
| WNC | - | 122 ± 7 | 0.16 ± 0.01 | 441.87 ± 22.94 | 105.56 ± 7.43 | 0.86 ± 0.01 | 0.56 ± 0.02 | 4.60 ± 1.33 | 0.21 ± 0.07 | 0.64 ± 0.09 |
| ANC | - | 141 ± 2 | 0.04 ± 0.01 | 415.71 ± 20.26 | 24.41 ± 3.56 | 0.85 ± 0.01 | 0.59 ± 0.02 | 4.48 ± 0.87 | 0.04 ± 0.01 | 0.44 ± 0.03 |
| Apuan Marble | Ajarte | |||
|---|---|---|---|---|
| Time (min.) | D*WNC/D*Silres | D*ANC/D*Silres | D*WNC/D*Silres | D*ANC/D*Silres |
| 30 | 2.16 | 0.71 | 5.64 | 0.15 |
| 40 | 1.86 | 0.75 | 6.40 | 1.14 |
| 90 | 1.58 | 0.76 | 3.90 | 1.95 |
| 150 | 1.34 | 1.30 | 3.31 | 2.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherardi, F.; Roveri, M.; Goidanich, S.; Toniolo, L. Photocatalytic Nanocomposites for the Protection of European Architectural Heritage. Materials 2018, 11, 65. https://doi.org/10.3390/ma11010065
Gherardi F, Roveri M, Goidanich S, Toniolo L. Photocatalytic Nanocomposites for the Protection of European Architectural Heritage. Materials. 2018; 11(1):65. https://doi.org/10.3390/ma11010065
Chicago/Turabian StyleGherardi, Francesca, Marco Roveri, Sara Goidanich, and Lucia Toniolo. 2018. "Photocatalytic Nanocomposites for the Protection of European Architectural Heritage" Materials 11, no. 1: 65. https://doi.org/10.3390/ma11010065





