Hydrophilicity, Viscoelastic, and Physicochemical Properties Variations in Dental Bone Grafting Substitutes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blocks
2.2. Granules
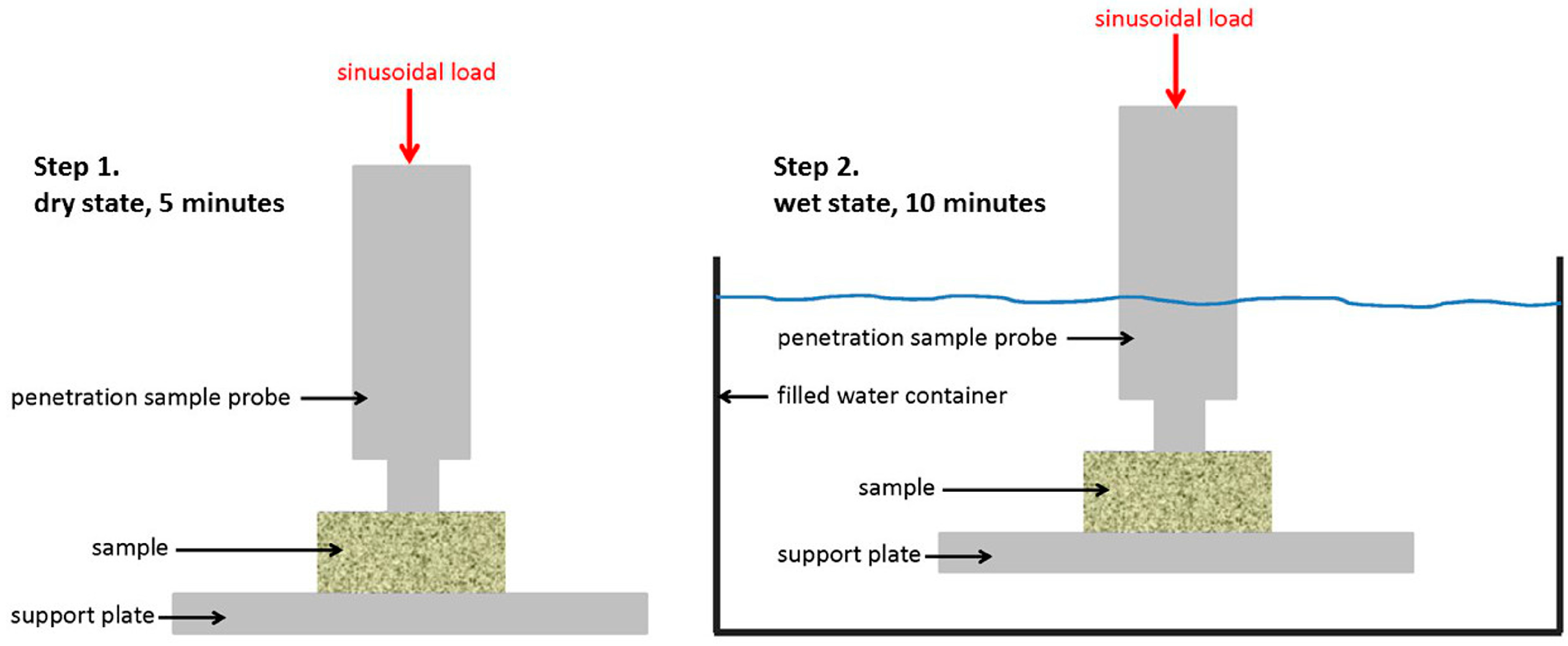
2.3. Dynamic Mechanical Analysis
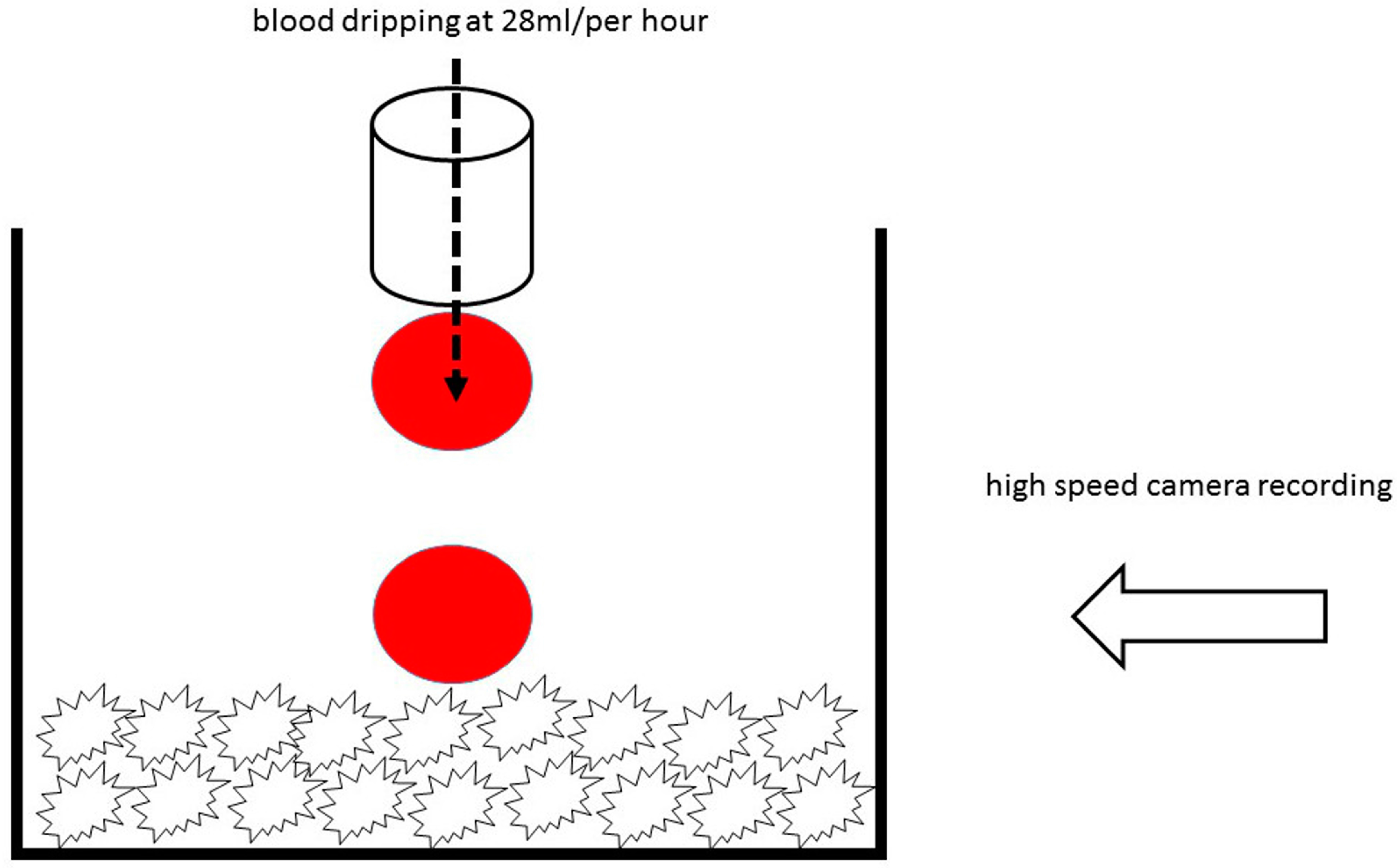
2.4. Hydrophilicity Analysis by High Speed Microscopy Imaging
2.5. Physico-Chemical Analysis
3. Results
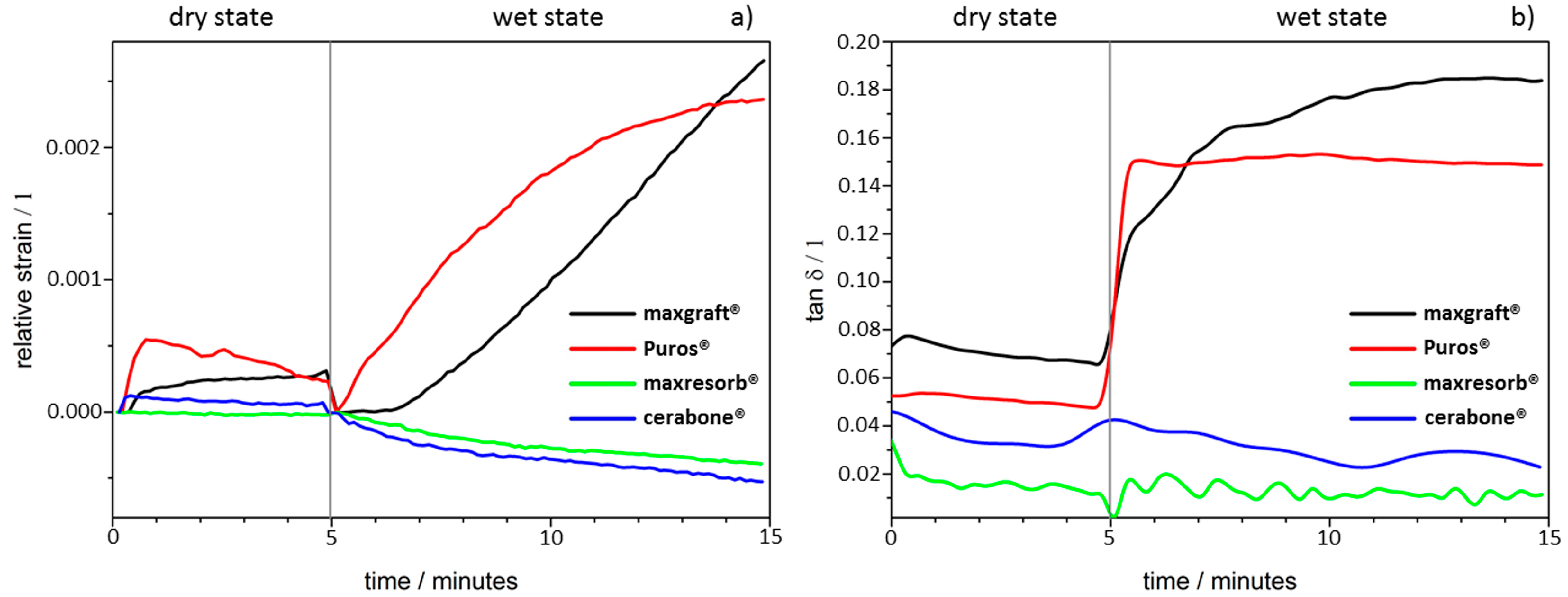
3.1. Dynamic Mechanical Analysis
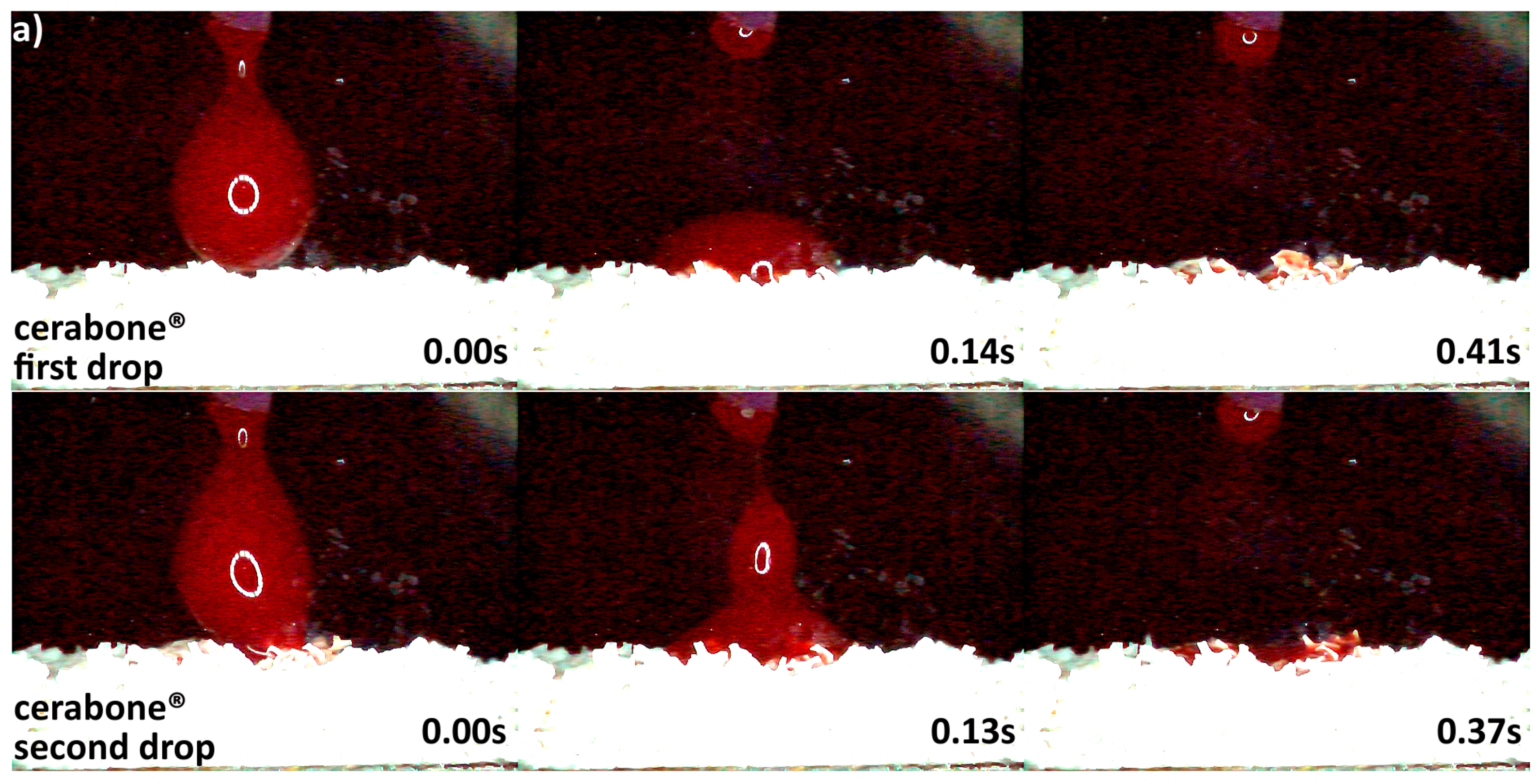
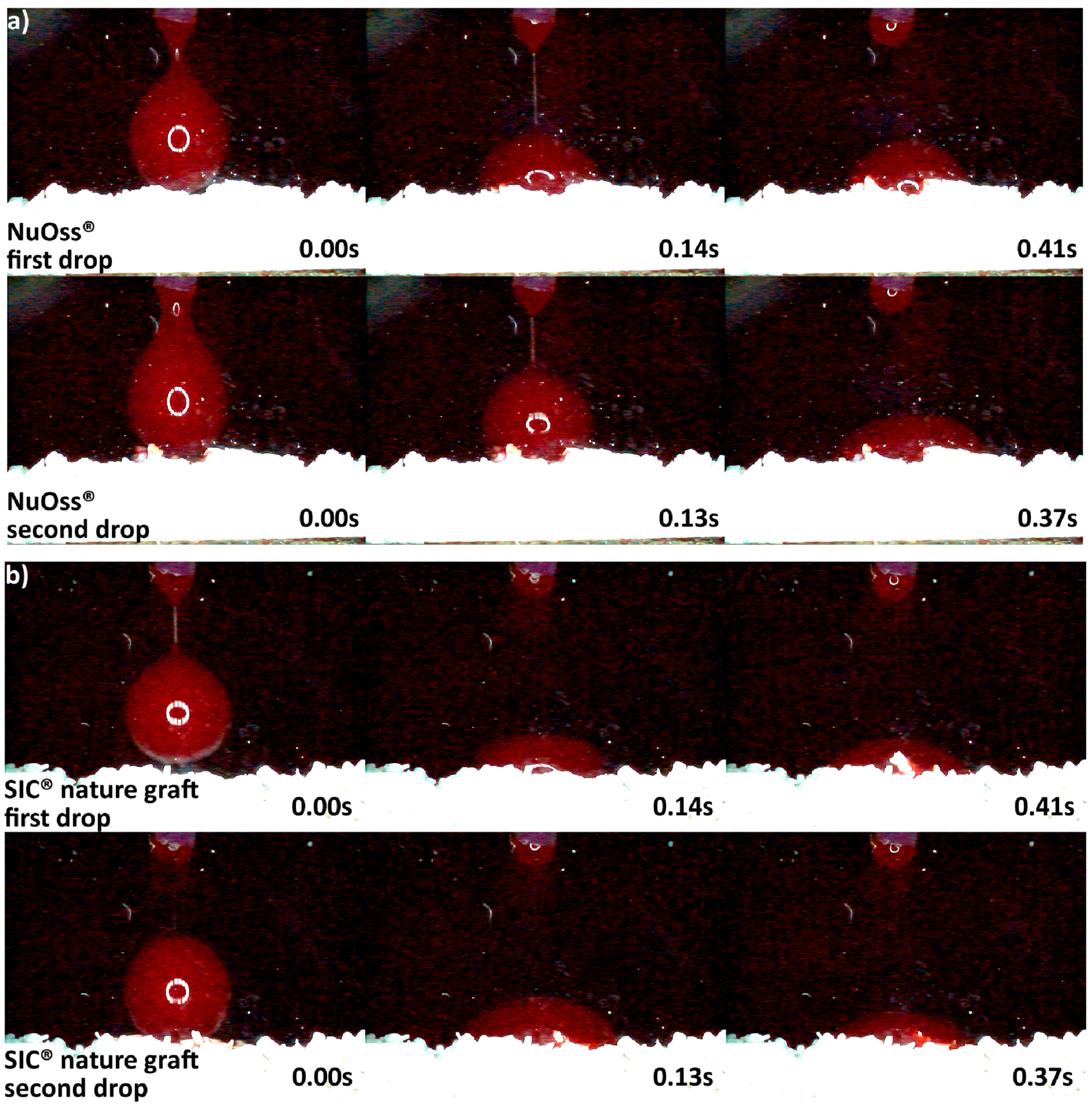
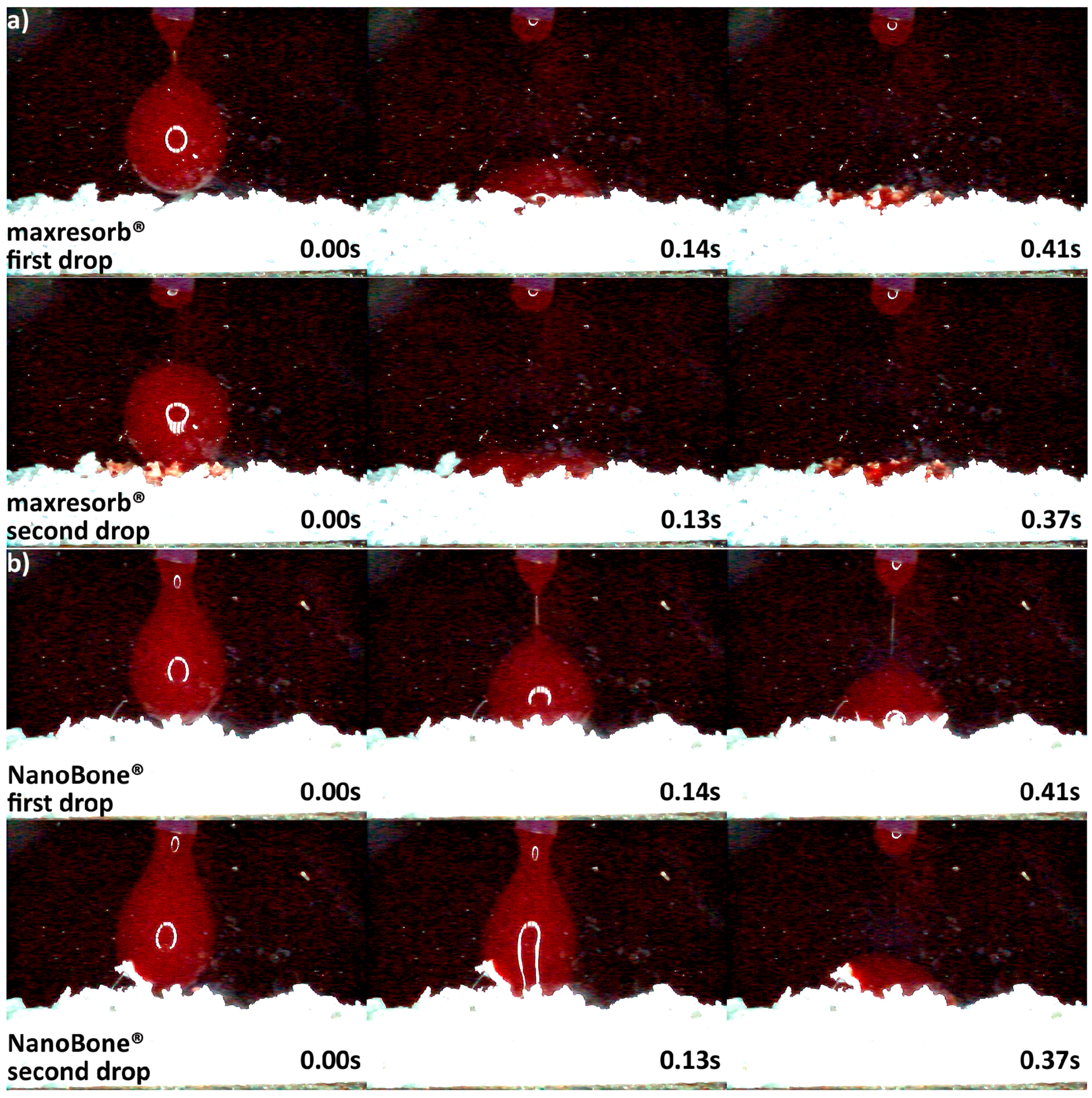
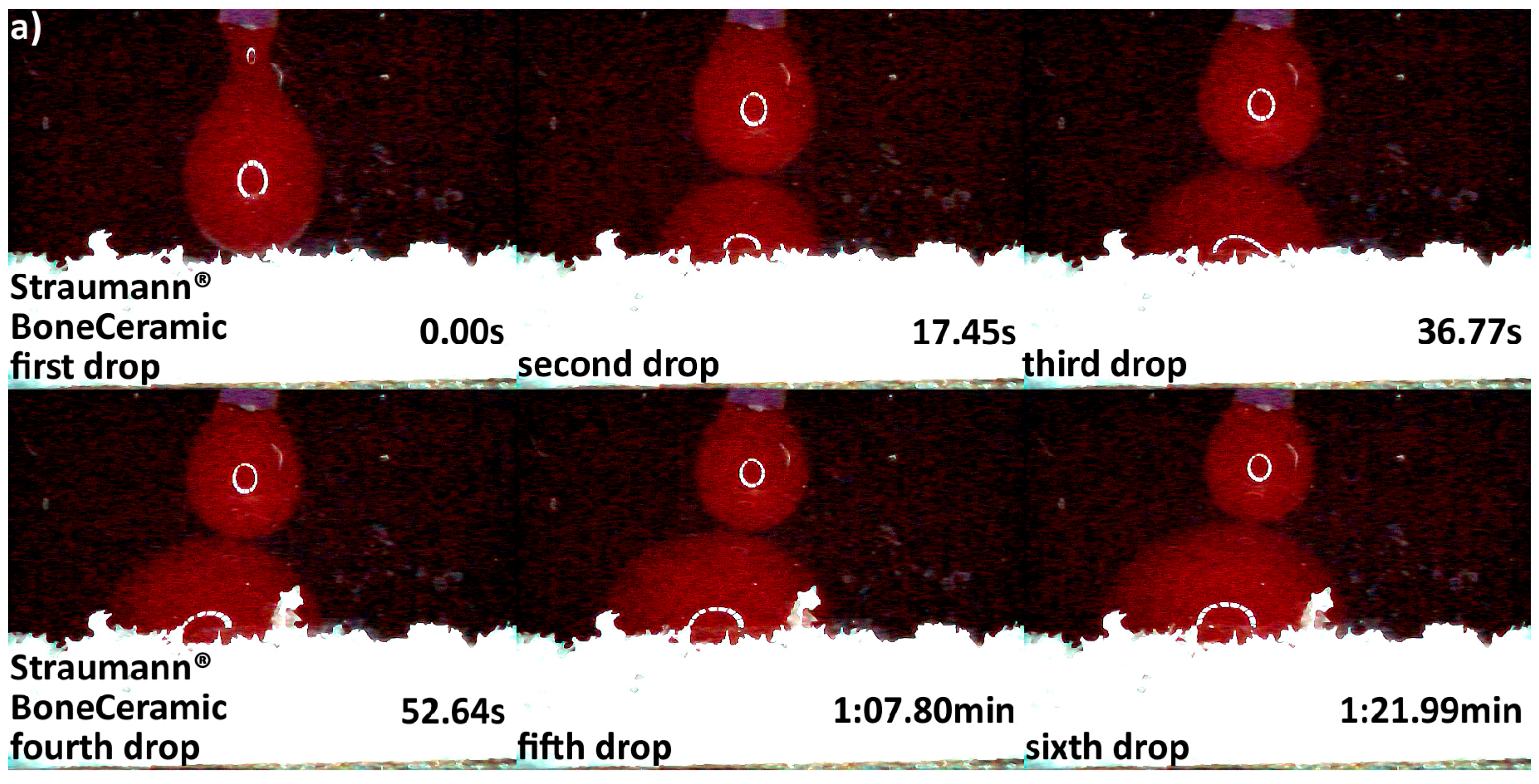
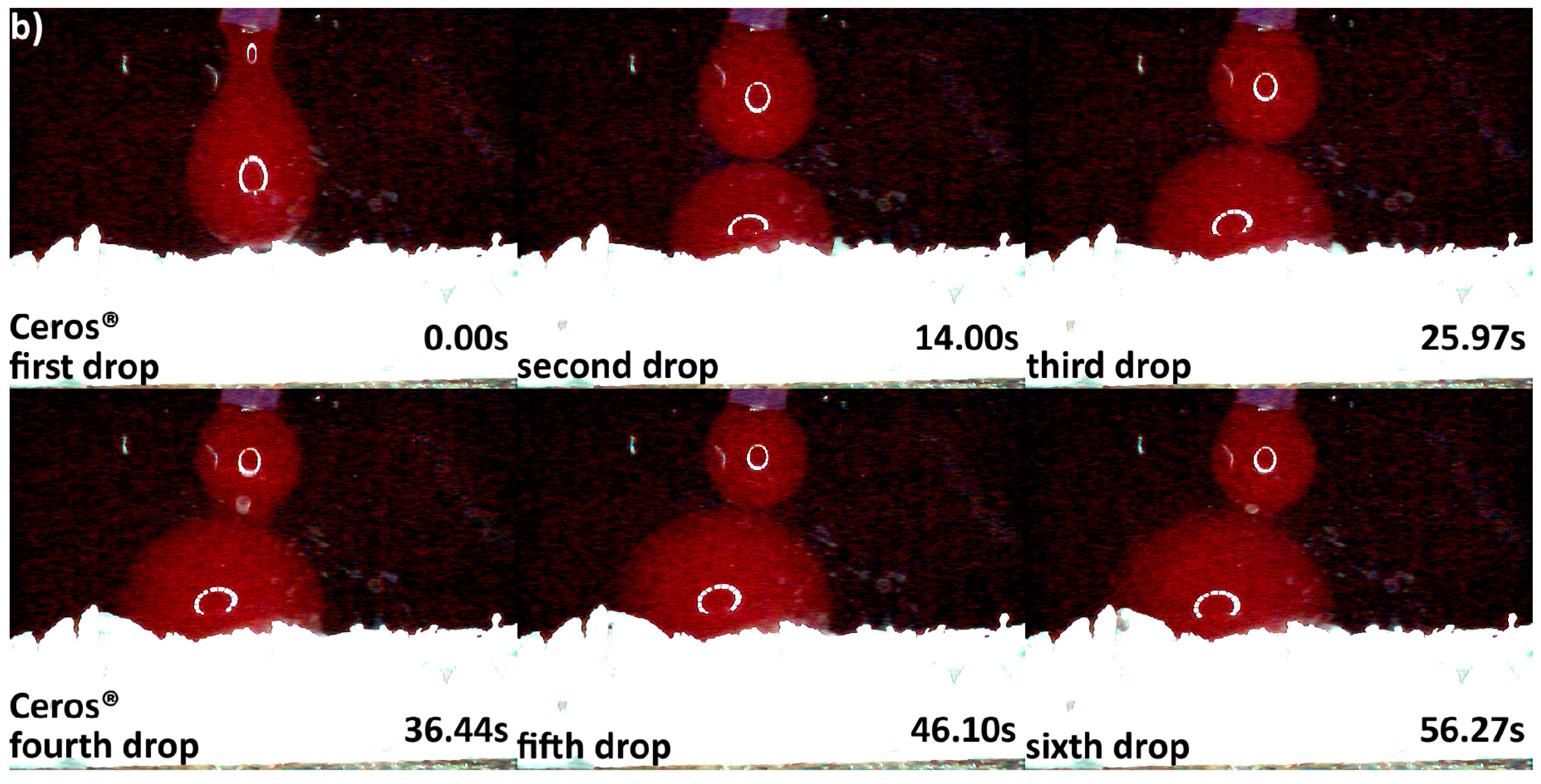
3.2. Hydrophilicity Analysis
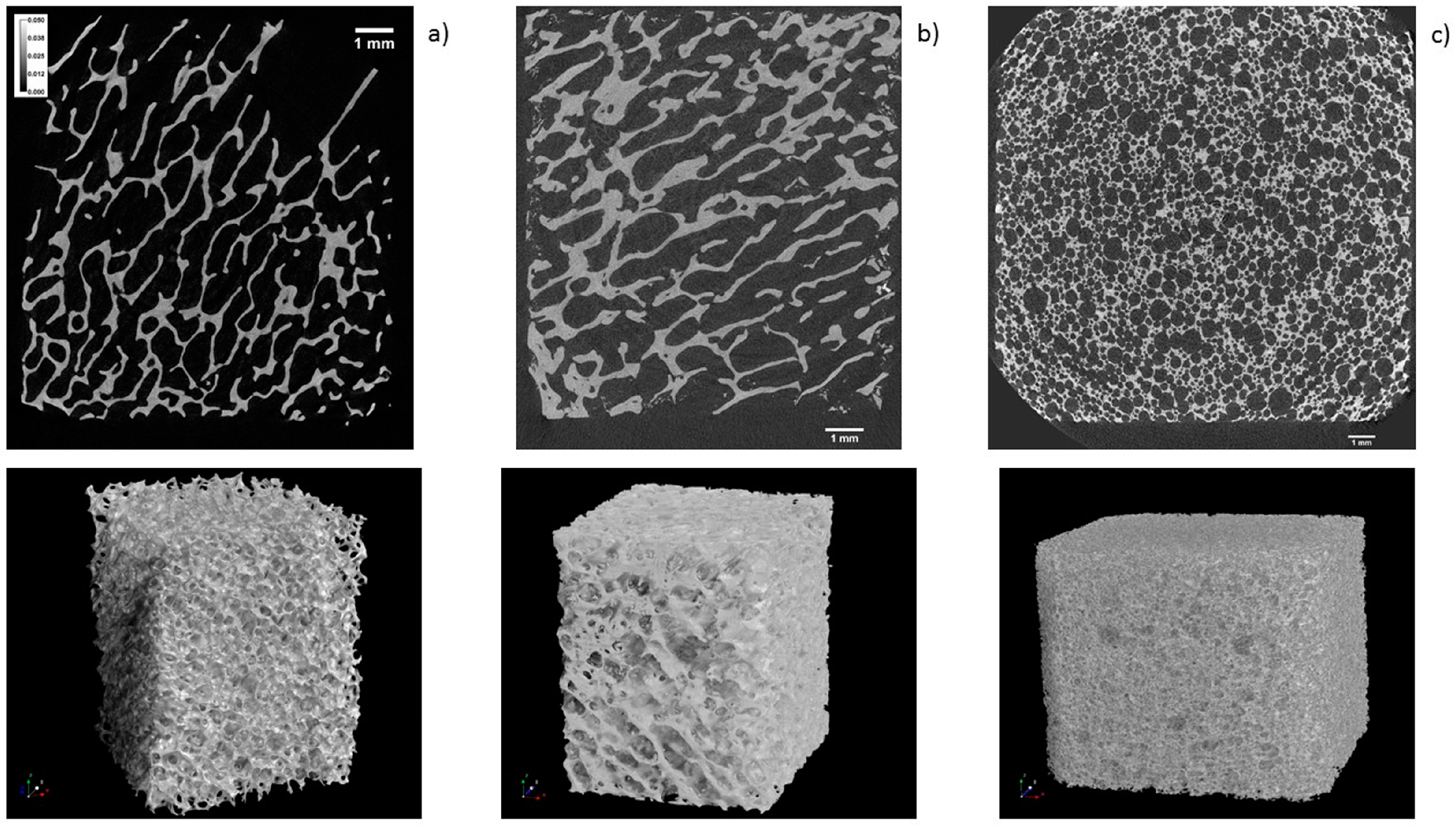
3.3. Micro Computed Tomography Analysis
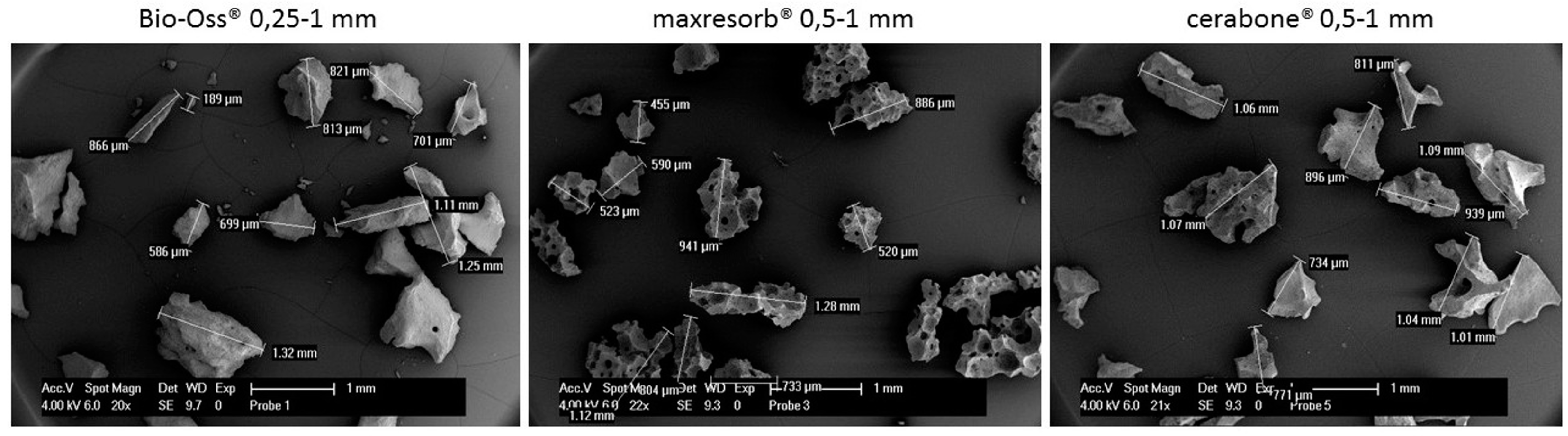
3.4. Scanning Electron Microscopy Analysis
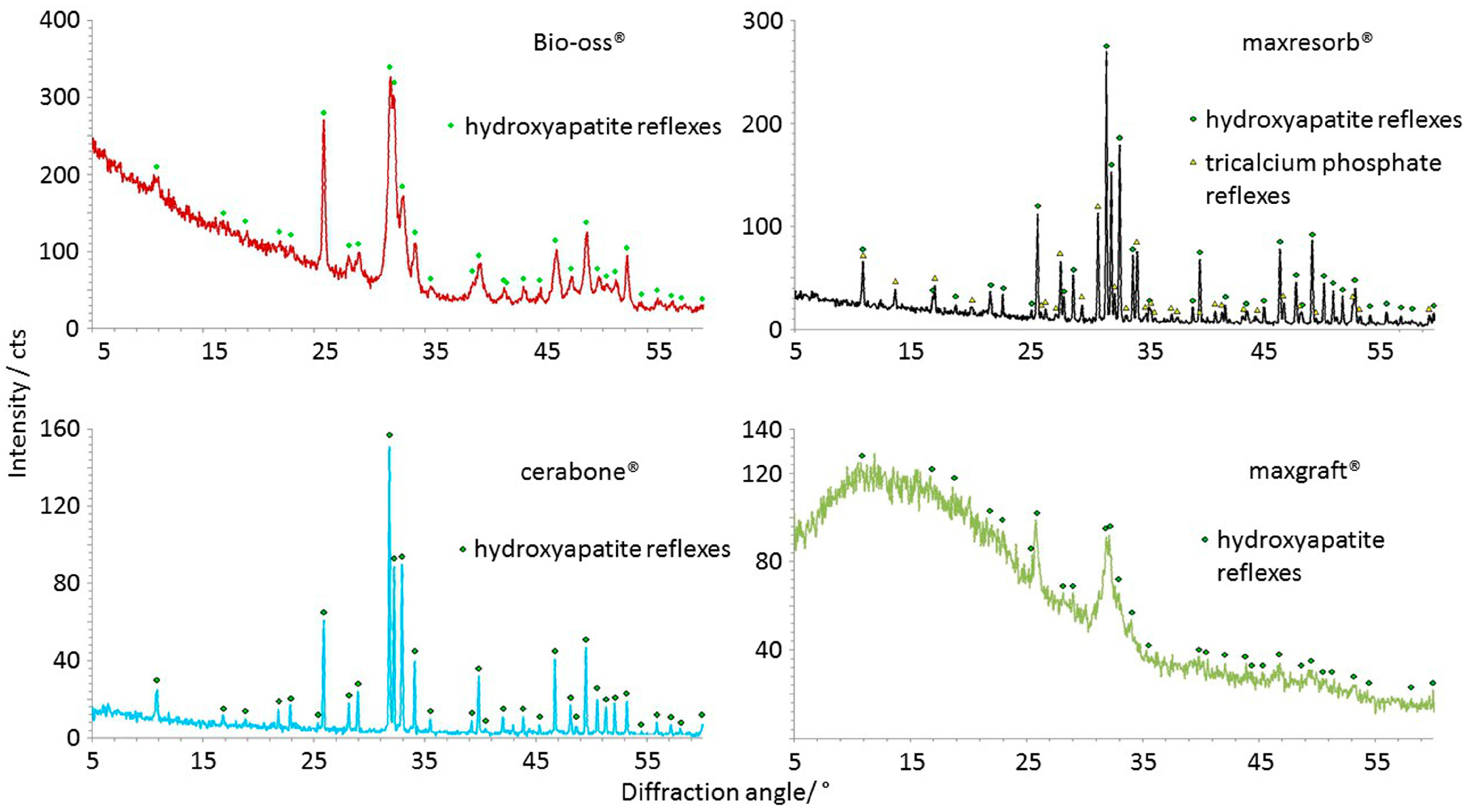
3.5. Chemical Structure, Mineral Phases, and Crystallinity Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Griffin, K.S.; Davis, K.M.; McKinley, T.O.; Anglen, J.O.; Chu, T.M.G.; Boerckel, J.D.; Kacena, M.A. Evolution of Bone Grafting: Bone Grafts and Tissue Engineering Strategies for Vascularized Bone Regeneration. Clin. Rev. Bone Min. Metab. 2015, 13, 232–244. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Javaid, M.A.; Hamdan, N.; Hashmi, R. Bone Regeneration Using Bone Morphogenetic Proteins and Various Biomaterial Carriers. Materials 2015, 8, 1778–1816. [Google Scholar] [CrossRef] [PubMed]
- Group, M.R. The European Market for Dental Bone Graft Substitutes. Implant Dent. 2003, 12, 3–5. [Google Scholar] [CrossRef]
- iData Research. European Market for Dental Bone Graft Substitutes and Other Biomaterials; iData Research: Burnaby, BC, Canada, 2013. [Google Scholar]
- Transparency Market Research. Dental Membrane and Bone Graft Substitutes Market; Transparency Market Research: Albany, NY, USA, 2016. [Google Scholar]
- Dumitrescu, A.L. Bone Grafts and Bone Graft Substitutes in Periodontal Therapy. In Chemicals in Surgical Periodontal Therapy; Springer: Berlin, Germany, 2011; p. 307. ISBN 3642182259. [Google Scholar]
- BDIZ EDI. Cologne Classification of Alveolar Ridge Defects (CCARD). In Proceedings of the 8th European Consensus Conference of BDIZ EDI, Cologne, Germany, 9 Februery 2013. [Google Scholar]
- Sheikh, Z.; Drager, J.; Zhang, Y.L.; Abdallah, M.N.; Tamimi, F.; Barralet, J. Controlling Bone Graft Substitute Microstructure to Improve Bone Augmentation. Adv. Healthc. Mater. 2016, 5, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Chee, W.; Jivraj, S. Failures in implant dentistry. Br. Dent. J. 2007, 202, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Oates, T.W.; Valderrama, P.; Bischof, M.; Nedir, R.; Jones, A.; Simpson, J.; Toutenburg, H.; Cochran, D.L. Enhanced implant stability with a chemically modified SLA surface: A randomized pilot study. Int. J. Oral Maxillofac. Implants 2007, 22, 755–760. [Google Scholar] [PubMed]
- Gorna, K.; Gogolewski, S. Preparation, degradation, and calcification of biodegradable polyurethane foams for bone graft substitutes. J. Biomed. Mater. Res. A 2003, 67, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.; Joly, P.; Bergmann, C.; Korus, G.; Duda, G.N. The Impact of Substrate Stiffness and Mechanical Loading on Fibroblast-Induced Scaffold Remodeling. Tissue Eng. Part A 2012, 18, 1804–1817. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Goldstein, M.; Raviv, E.; Hirsch, A.; Ranly, D.M.; Boyan, B.D. Clinical evaluation of demineralized bone allograft in a hyaluronic acid carrier for sinus lift augmentation in humans: A computed tomography and histomorphometric study. Clin. Oral Implants Res. 2007, 18, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, P.; Niculescu-Morzsa, E.; Zwickl, H.; Halbwirth, F.; Pichler, M.; Matzner, M.; Gottsauner-Wolf, F.; Nehrer, S. Investigation of bone allografts representing different steps of the bone bank procedure using the CAM-model. ALTEX 2010, 27, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.; Schädel-Höpfner, M.; Berns, T.; Sitter, H.; Gotzen, L. Influence of processing and sterilization on the mechanical properties of pins made from bovine cortical bone. Unfallchirurg 2003, 106, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.F.; Reis, R.L.; Cunha, A.M. Dynamic Mechanical Analysis in Polymers for Medical Applications. In Polymer Based Systems on Tissue Engineering, Replacement and Regeneration SE—10; Reis, R., Cohn, D., Eds.; NATO Science Series; Springer: Amsterdam, The Netherlands, 2002; Volume 86, pp. 139–164. ISBN 978-1-4020-1001-9. [Google Scholar]
- Bidan, C.M.; Kommareddy, K.P.; Rumpler, M.; Kollmannsberger, P.; Fratzl, P.; Dunlop, J.W.C. Geometry as a Factor for Tissue Growth: Towards Shape Optimization of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2013, 2, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Seidel, P.; Dingeldein, E. Cerabone®—Eine Spongiosa-Keramik bovinen Ursprungs. Materwiss. Werksttech. 2004, 35, 208–212. [Google Scholar] [CrossRef]
- Schnettler, R.; Franke, J.; Rimashevskiy, D.; Zagorodniy, N.; Batpenov, N.; Unger, R.E.; Wenisch, S.; Barbeck, M. Allogeneic Bone Grafting Materials—Update of the Current Scientific Status. New Tech. Traumatol. Orthop. 2017, 23. [Google Scholar] [CrossRef]
- Doktor, T.; Valach, J.; Kytyr, D.; Jiroušek, O. Pore Size Distribution of Human Trabecular Bone—Comparison of Intrusion Measurements with Image Analysis. In Proceedings of the 17th International Conference Engineering Mechanics 2011, Svratka, Czech Republic, 9–12 May 2011. [Google Scholar]
- Gosau, M.; Viale-Bouroncle, S.; Eickhoff, H.; Prateeptongkum, E.; Reck, A.; Götz, W.; Klingelhöffer, C.; Müller, S.; Morsczeck, C. Evaluation of implant-materials as cell carriers for dental stem cells under in vitro conditions. Int. J. Implant Dent. 2015, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, B.; Petersen, A.; Strube, P.; Mehta, M.; Duda, G.N. Intra-operatively customized implant coating strategies for local and controlled drug delivery to bone. Adv. Drug Deliv. Rev. 2012, 64, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone replacement materials and techniques used for achieving vertical alveolar bone augmentation. Materials 2015, 8, 2953–2993. [Google Scholar] [CrossRef]
- Xavier, S.P.; Santos, T.S.; Sehn, F.P.; Silva, E.R.; Garcez-Filho, J.A.; Martins-Filho, P.R.S. Maxillary sinus grafting with fresh frozen allograft versus bovine bone mineral: A tomographic and histological study. J. Craniomaxillofac. Surg. 2016, 44, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Favero, G.; Labanca, M. Biomaterials in maxillofacial surgery: Membranes and grafts. Int. J. Biomed. Sci. 2011, 7, 81–88. [Google Scholar] [PubMed]
- Artzi, Z.; Wasersprung, N.; Weinreb, M.; Steigmann, M.; Prasad, H.S.; Tsesis, I. Effect of suided tissue regeneration on newly formed Bone and cementum in periapical tissue healing after endodontic surgery: An in vivo study in the cat. J. Endod. 2012, 38, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Riachi, F.; Naaman, N.; Tabarani, C.; Aboelsaad, N.; Aboushelib, M.N.; Berberi, A.; Salameh, Z. Influence of material properties on rate of resorption of two bone graft materials after sinus lift using radiographic assessment. Int. J. Dent. 2012, 2012, 737262. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Perrotti, V.; Piattelli, A.; Iezzi, G. Eight-year results of site retention of anorganic bovine bone and anorganic bovine matrix. J. Oral Implantol. 2013, 39, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Danilchenko, S.N.; Koropov, A.V.; Protsenko, I.Y.; Sulkio-Cleff, B.; Sukhodub, L.F. Thermal behavior of biogenic apatite crystals in bone: An X-ray diffraction study. Cryst. Res. Technol. 2006, 41, 268–275. [Google Scholar] [CrossRef]
- Scarano, A.; Carinci, F.; Assenza, B.; Piattelli, M.; Murmura, G.; Piattelli, A. Vertical ridge augmentation of atrophic posterior mandible using an inlay technique with a xenograft without miniscrews and miniplates: Case series. Clin. Oral Implants Res. 2011, 22, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Konermann, A.; Staubwasser, M.; Dirk, C.; Keilig, L.; Bourauel, C.; Götz, W.; Jäger, A.; Reichert, C. Bone substitute material composition and morphology differentially modulate calcium and phosphate release through osteoclast-like cells. Int. J. Oral Maxillofac. Surg. 2016, 43, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Delgado-Ruíz, R.A.; Ramírez-Fernández, M.P.; Maté-Sánchez, J.E.; Ortiz-Ruiz, A.; Marcus, A. Histomorphometric and mineral degradation study of Ossceram®: A novel biphasic B-tricalcium phosphate, in critical size defects in rabbits. Clin. Oral Implants Res. 2012, 23, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Handschel, J.; Simonowska, M.; Naujoks, C.; Depprich, R.A.; Ommerborn, M.A.; Meyer, U.; Kübler, N.R. A histomorphometric meta-analysis of sinus elevation with various grafting materials. Head Face Med. 2009, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kattz, J. The effects of various cleaning and sterilization processes on allograft bone incorporation. J. Long-Term Eff. Med. Implants 2010, 20, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Froum, S.J.; Wallace, S.S.; Elian, N.; Cho, S.C.; Tarnow, D.P. Comparison of mineralized cancellous bone allograft (Puros) and anorganic bovine bone matrix (Bio-Oss) for sinus augmentation: Histomorphometry at 26 to 32 weeks after grafting. Int. J. Periodontics Restor. Dent. 2006, 26, 543–551. [Google Scholar]
- Gultekin, B.A.; Cansiz, E.; Borahan, O.; Mangano, C.; Kolerman, R.; Mijiritsky, E.; Yalcin, S. Evaluation of Volumetric Changes of Augmented Maxillary Sinus With Different Bone Grafting Biomaterials. J. Craniofac. Surg. 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Minichetti, J.C.; D’Amore, J.C.; Hong, A.Y.J.; Cleveland, D.B. Human Histologic Analysis of Mineralized Bone Allograft (Puros) Placement Before Implant Surgery. J. Oral Implantol. 2004, 30, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.M.; Nataraj, C.; Jaw, R.; Deigl, E.; Bursac, P. Demineralized bone matrix as an osteoinductive biomaterial and in vitro predictors of its biological potential. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Tadic, D.; Epple, M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Booms, P.; Lorenz, J.; Kirkpatrick, C.J.; Sader, R.A. Potential lack of “standardized” processing techniques for production of allogeneic and xenogeneic bone blocks for application in humans. Acta Biomater. 2014, 10, 3557–3562. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Herten, M.; Berndsen, K.; Fienitz, T.; Ritter, L.; Dreiseidler, T.; Zöller, J. Impact of Citric Acid Etching on Biocompatibility and Osseous Organisation of a Natural Bovine Bone Mineral: Preliminary Results of an In Vitro/In-Vivo Study. In World Congress on Medical Physics and Biomedical Engineering, September 7–12, 2009, Munich, Germany; Vol. 25/11 Biomedical Engineering for Audiology, Ophthalmology, Emergency Dental Medicine; Dössel, O., Schlegel, W.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 259–262. ISBN 978-3-642-03891-4. [Google Scholar]



| Specimen | Residual Mass in % |
|---|---|
| Bio-Oss® | 92.64 |
| maxresorb® | 98.61 |
| cerabone® | 99.52 |
| maxgraft® | 61.48 |
| Properties | Xenograft | Synthetic | Allograft |
|---|---|---|---|
| Dimensional changes and molecular mobility (Figure 3) | High rigidity and stiff, brittleness due purely ceramic nature | High rigidity and stiff, brittleness due purely ceramic nature | Swelling due presence of organic material |
| Resorption rate | Low due highly crystalline natural HA structure | Medium due synthetic HA and ß-TCP structure | Fast due low crystallinity, amorphous structure and organic material presence |
| Volume stability at the grafting site | High due low resorption rate | Medium due to bi-phasic resorption rate | Low due fast resorption rate |
| Regenerative mechanism | Slow due penetration of newly formed bone and integration in the porosity | Medium due parallel new bone formation and remodelling | Fast new bone formation and remodelling due fast resorption rate and organic content |
| Hydrophilicity (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7) | Variations due mineral purity, crystallinity, particle distribution size | Variations due chemical structure, particle distribution size | Variations due acetone use during manufacturing |
| Macroscopic structure (Figure 8) | Labyrinth-like | Foam-like | Labyrinth-like |
| Particles structure and surface (Figure 9 and Figure 10) | Irregular structure and rough surface | Foam-like structure and grain-like surface | Irregular structure and fiber-like surface |
| Chemical structure (Figure 11) | P–O, O–H due water, additional hydroxyapatite O–H in cerabone®, additional CO32− in Bio-Oss® | P–O, O–H due water, additional hydroxyapatite O–H | P–O, O–H due water, CO32−, C–H, N–H |
| Crystalline structure (Figure 12) | Hydroxyapatite, narrow peaks and a low baseline in cerabone® due high crystallinity; broader peaks due lower crystallinity in Bio-Oss® | Hydroxyapatite, β-tricalcium phosphate, narrow peaks and a low baseline due high crystallinity | Hydroxyapatite, very broad peaks and a high baseline due low crystallinity and amorphous structure |
| Impurities (Table 1) | Low water content and only a traces of carbon dioxide in cerabone®; chemically bound water and carbon dioxide in Bio-Oss® | Low water content and only traces of carbon dioxide | Organic material |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trajkovski, B.; Jaunich, M.; Müller, W.-D.; Beuer, F.; Zafiropoulos, G.-G.; Houshmand, A. Hydrophilicity, Viscoelastic, and Physicochemical Properties Variations in Dental Bone Grafting Substitutes. Materials 2018, 11, 215. https://doi.org/10.3390/ma11020215
Trajkovski B, Jaunich M, Müller W-D, Beuer F, Zafiropoulos G-G, Houshmand A. Hydrophilicity, Viscoelastic, and Physicochemical Properties Variations in Dental Bone Grafting Substitutes. Materials. 2018; 11(2):215. https://doi.org/10.3390/ma11020215
Chicago/Turabian StyleTrajkovski, Branko, Matthias Jaunich, Wolf-Dieter Müller, Florian Beuer, Gregory-George Zafiropoulos, and Alireza Houshmand. 2018. "Hydrophilicity, Viscoelastic, and Physicochemical Properties Variations in Dental Bone Grafting Substitutes" Materials 11, no. 2: 215. https://doi.org/10.3390/ma11020215





