On the Potential of Bulk Metallic Glasses for Dental Implantology: Case Study on Ti40Zr10Cu36Pd14
Abstract
:1. Introduction
2. Results
2.1. Materials General Characterization
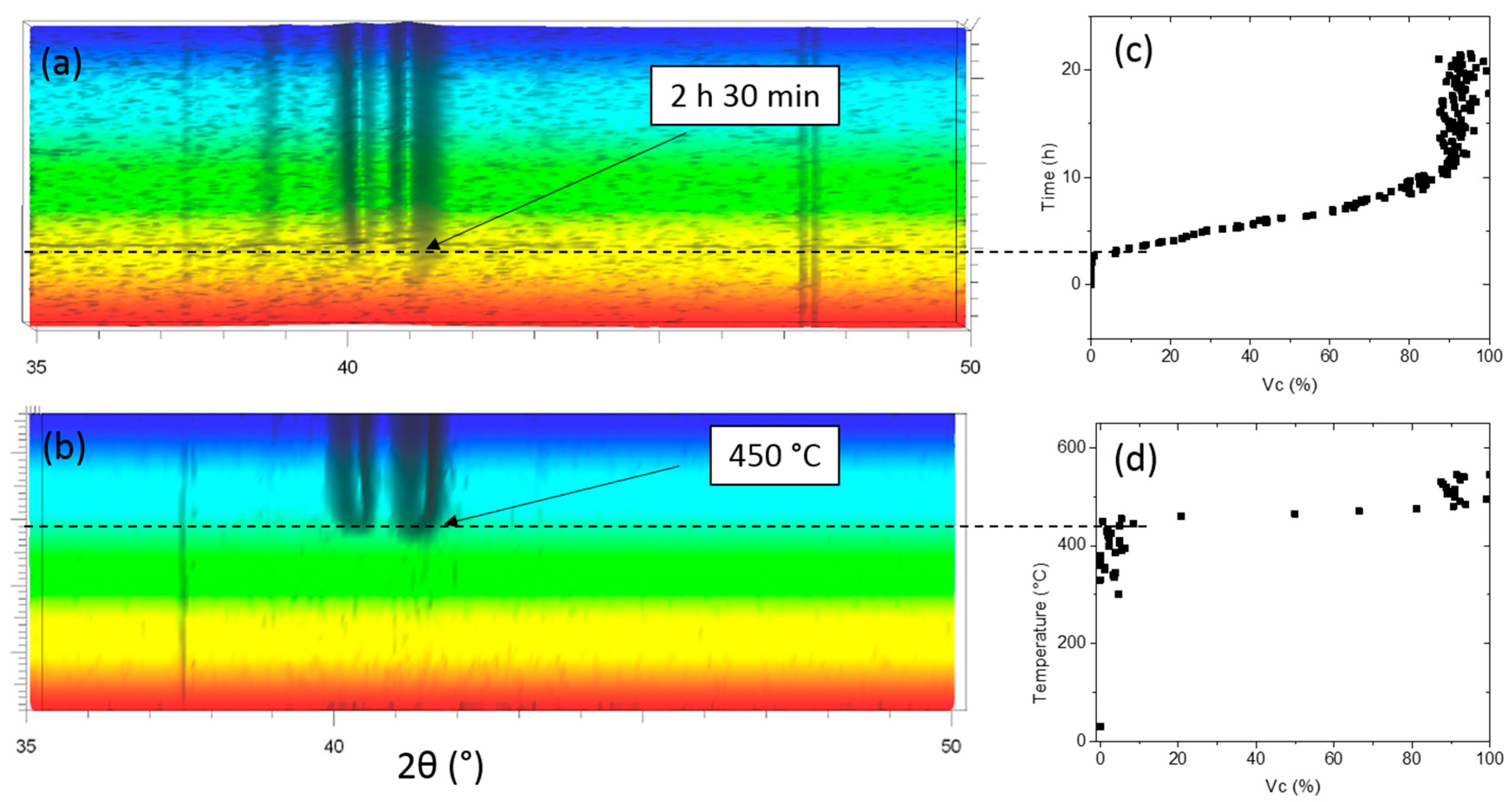
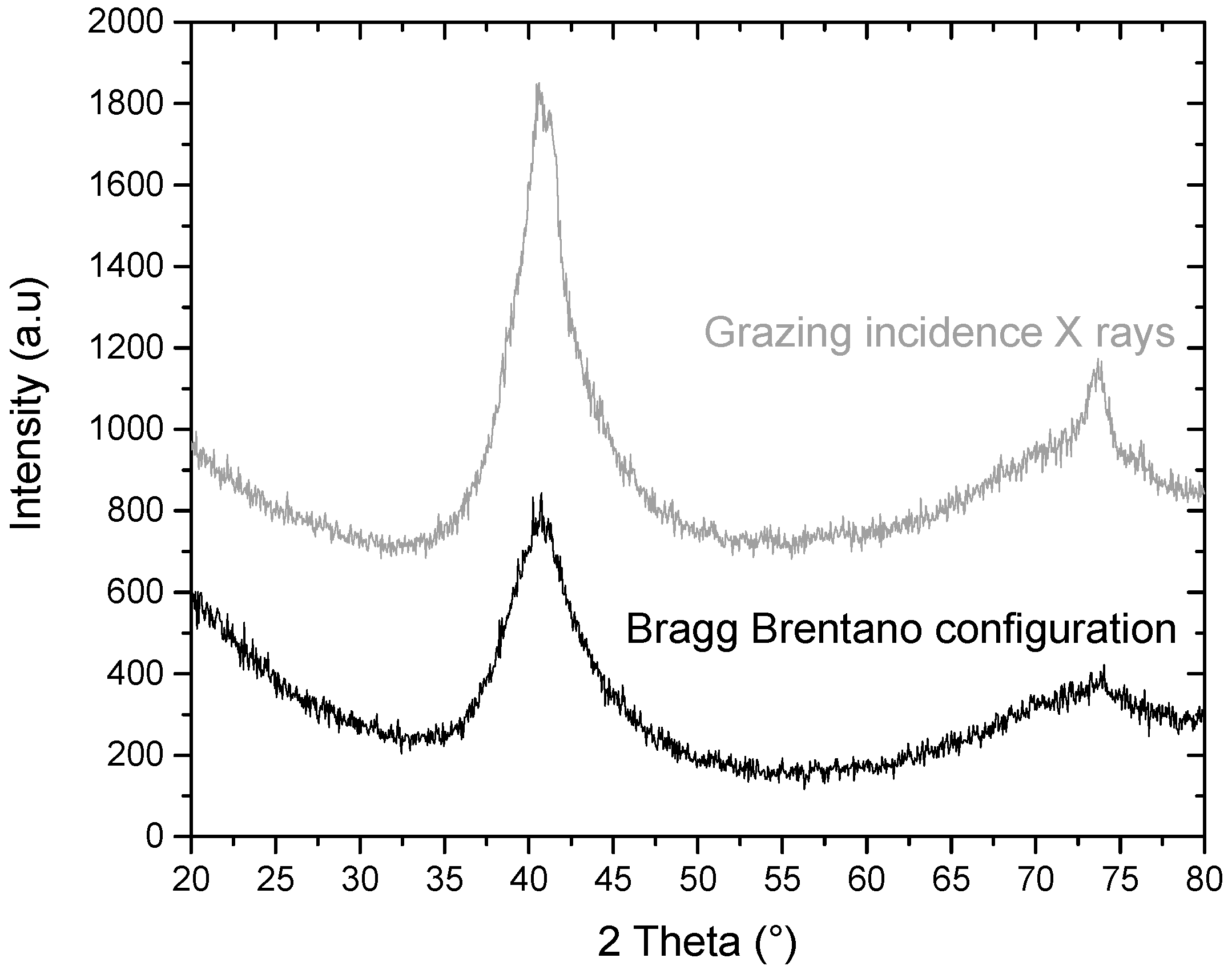
2.1.1. Structural Analysis
2.1.2. Thermal Stability
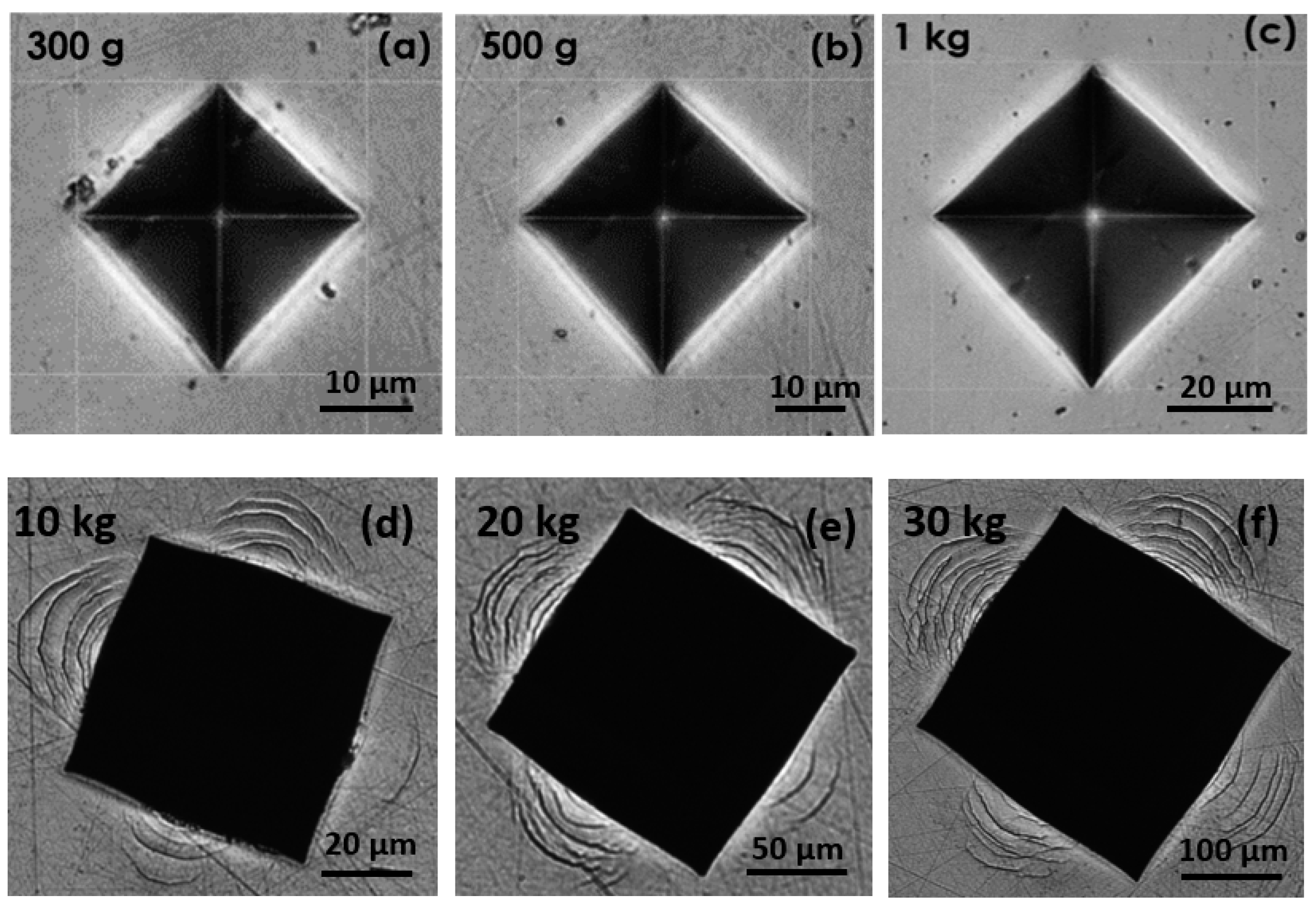
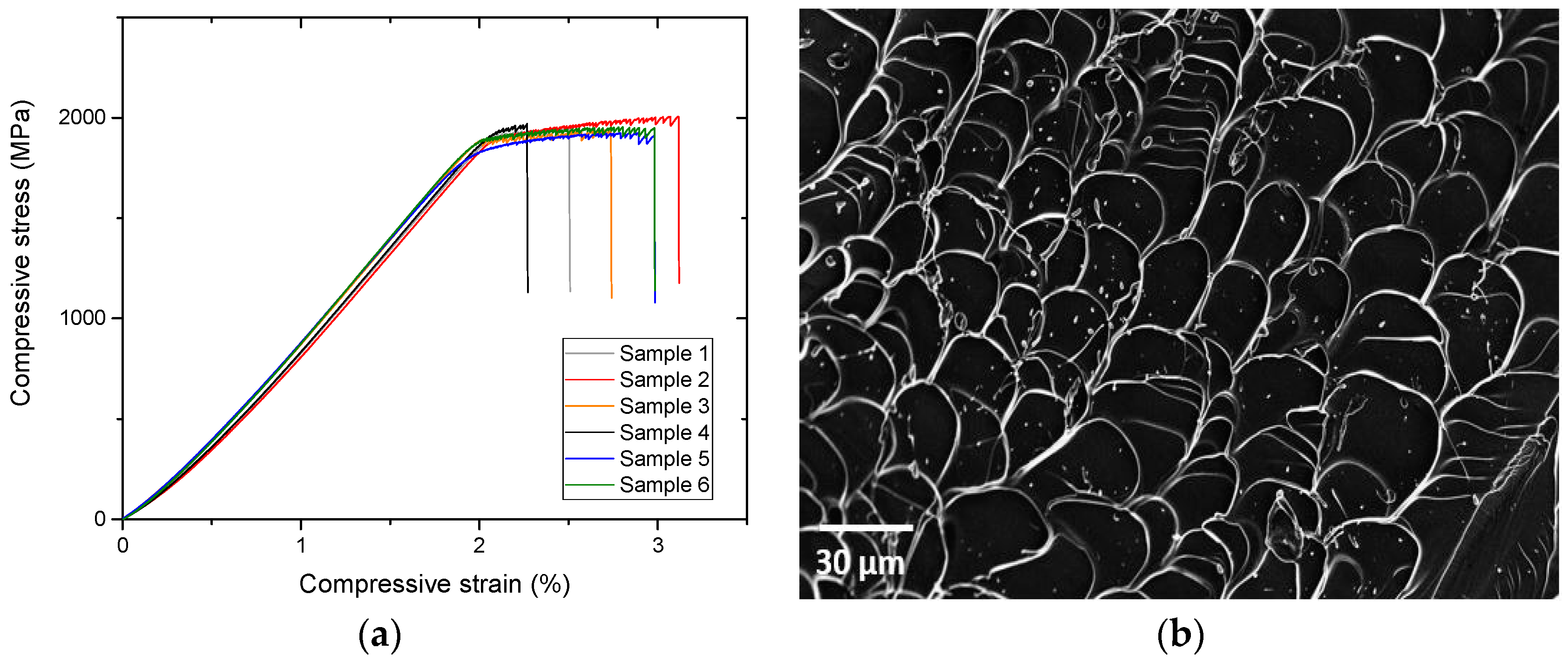
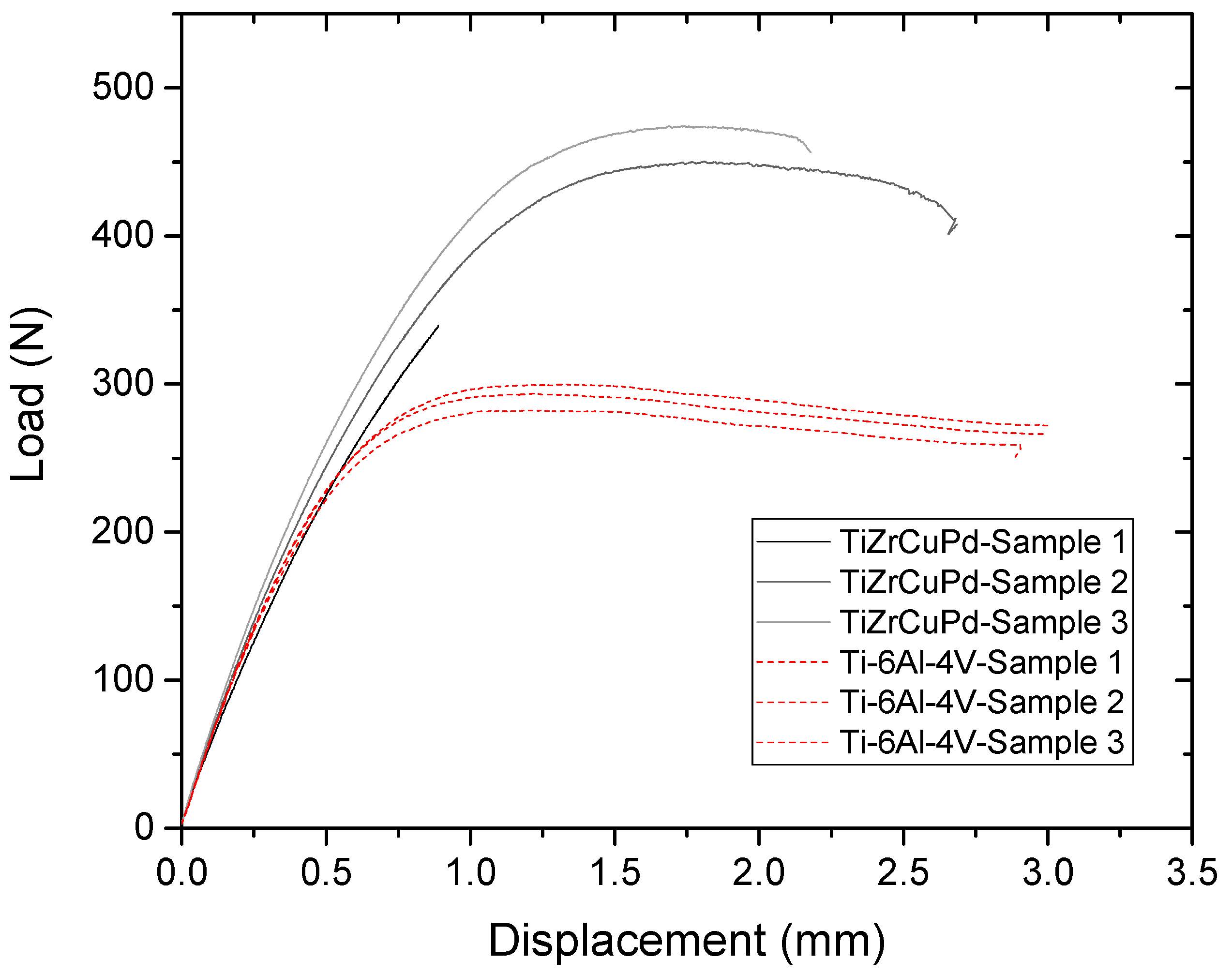
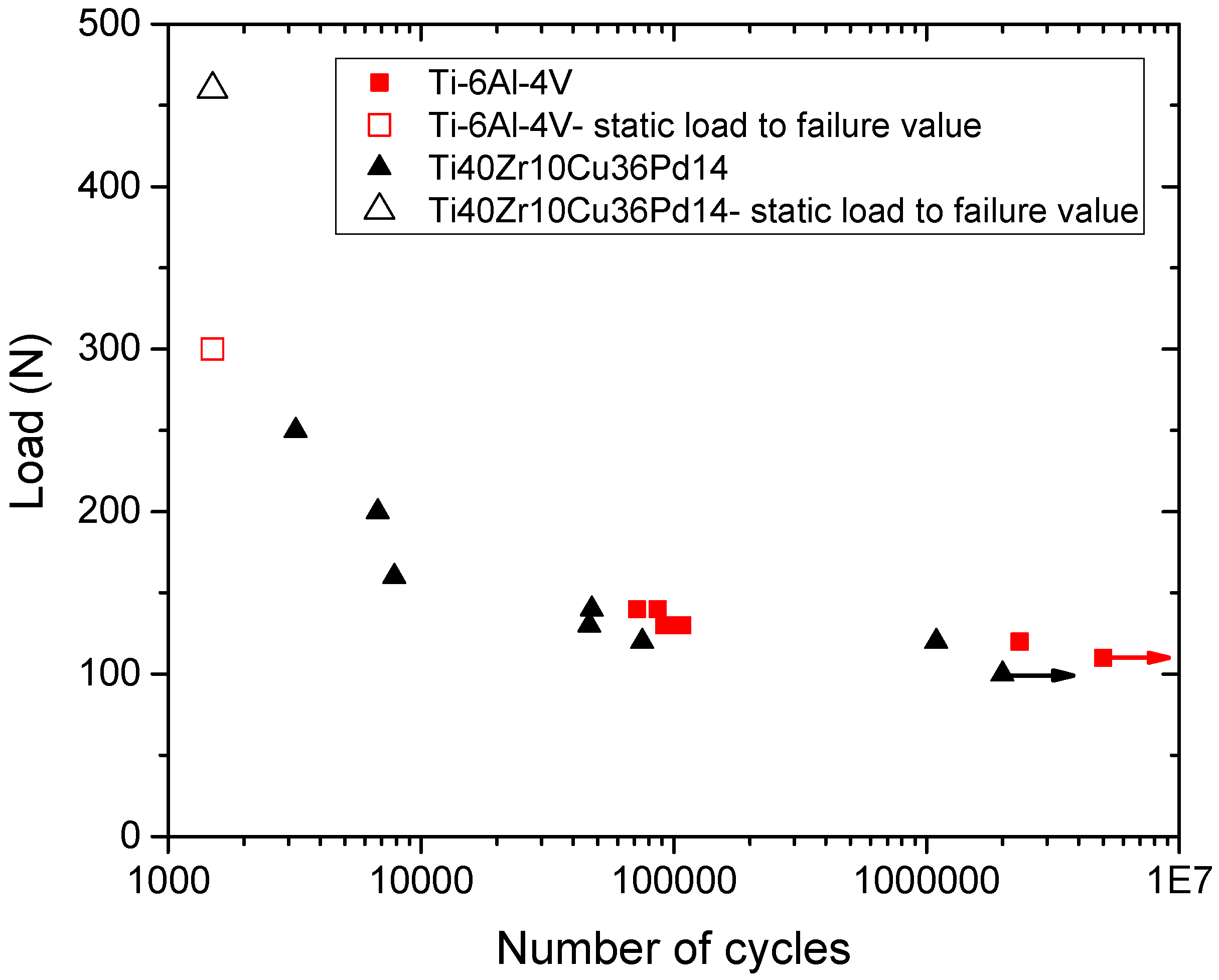
2.1.3. Mechanical Characterization
2.2. Characterization for a Future Biomedical Application
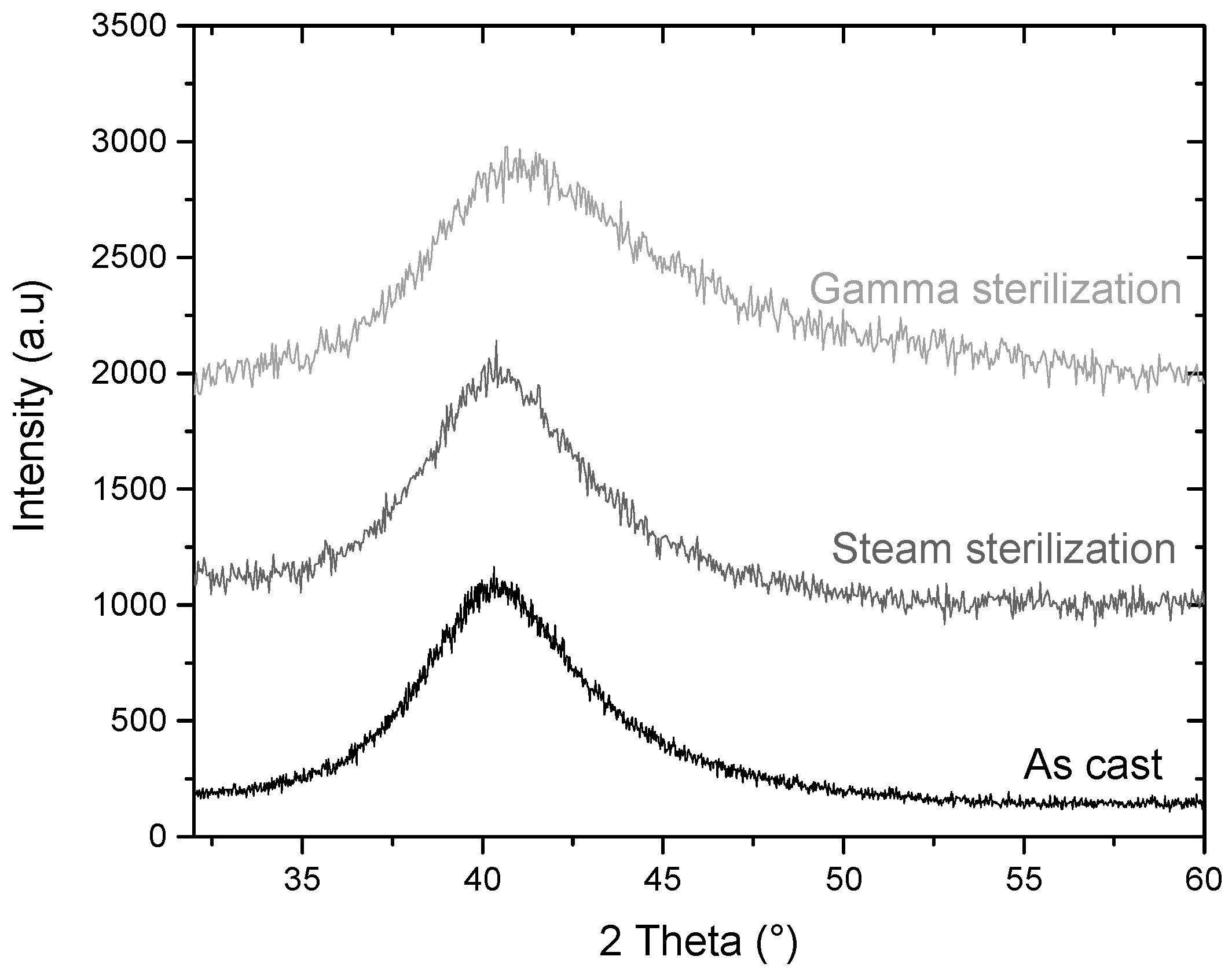
2.2.1. Resistance to Sterilization
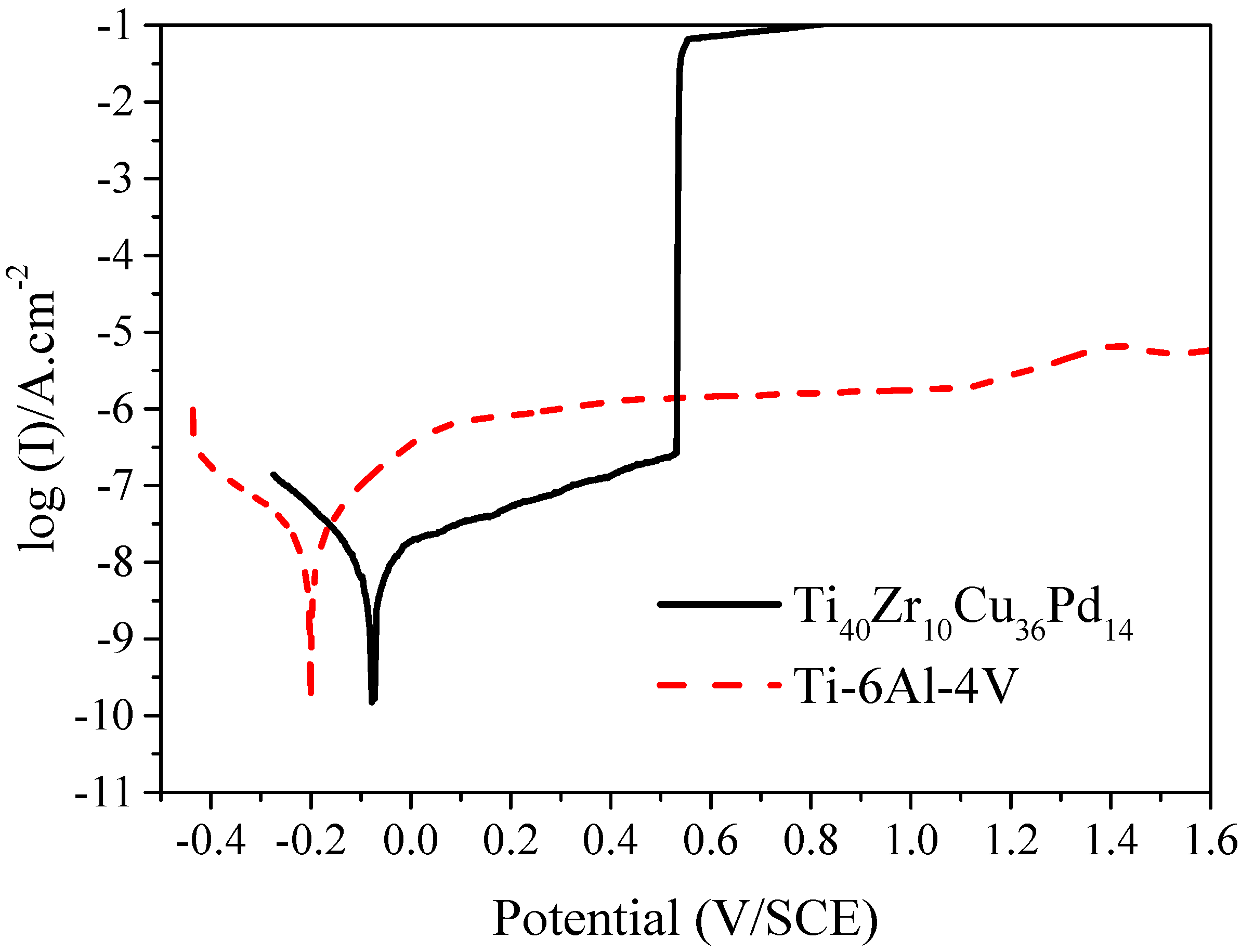
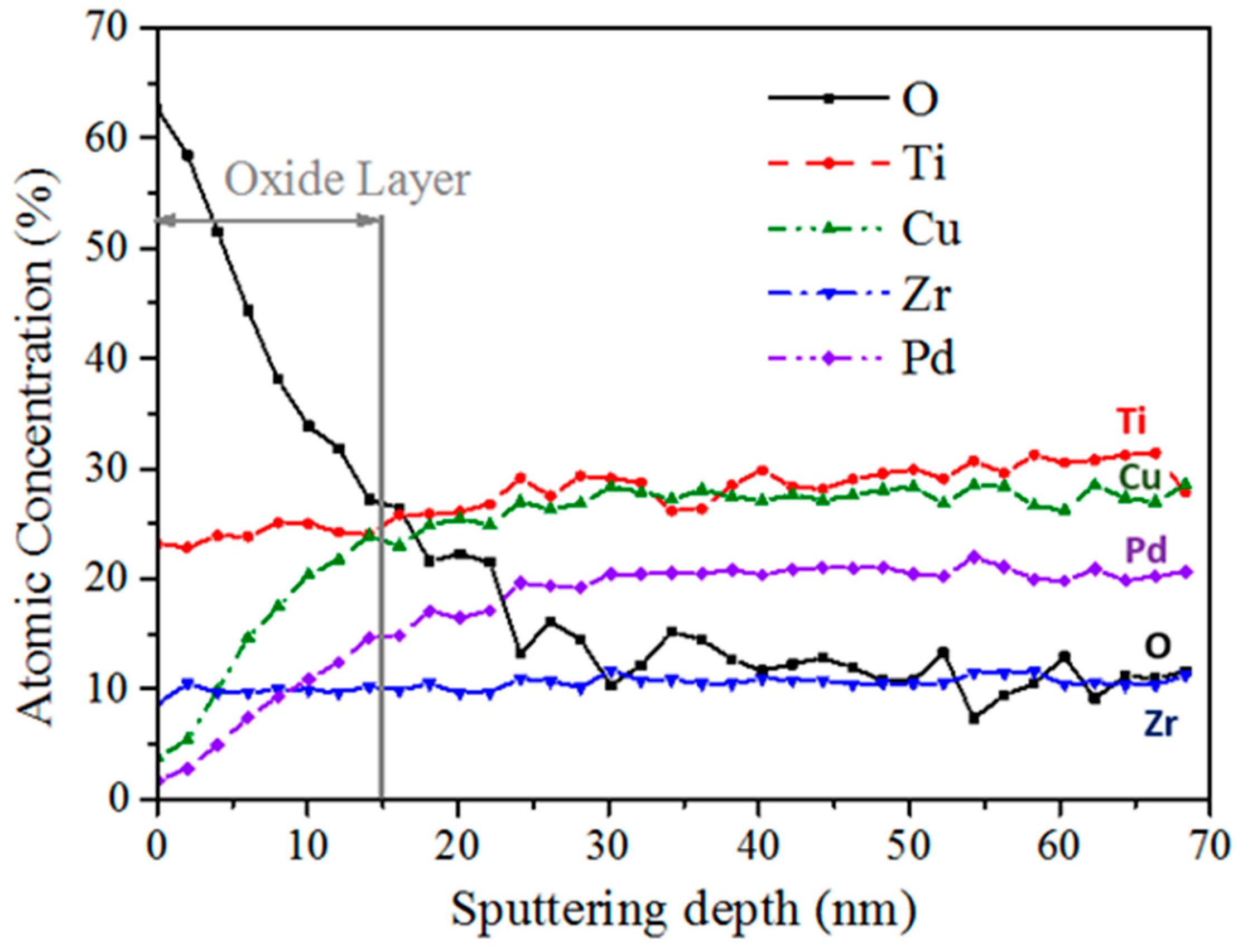
2.2.2. Corrosion Resistance
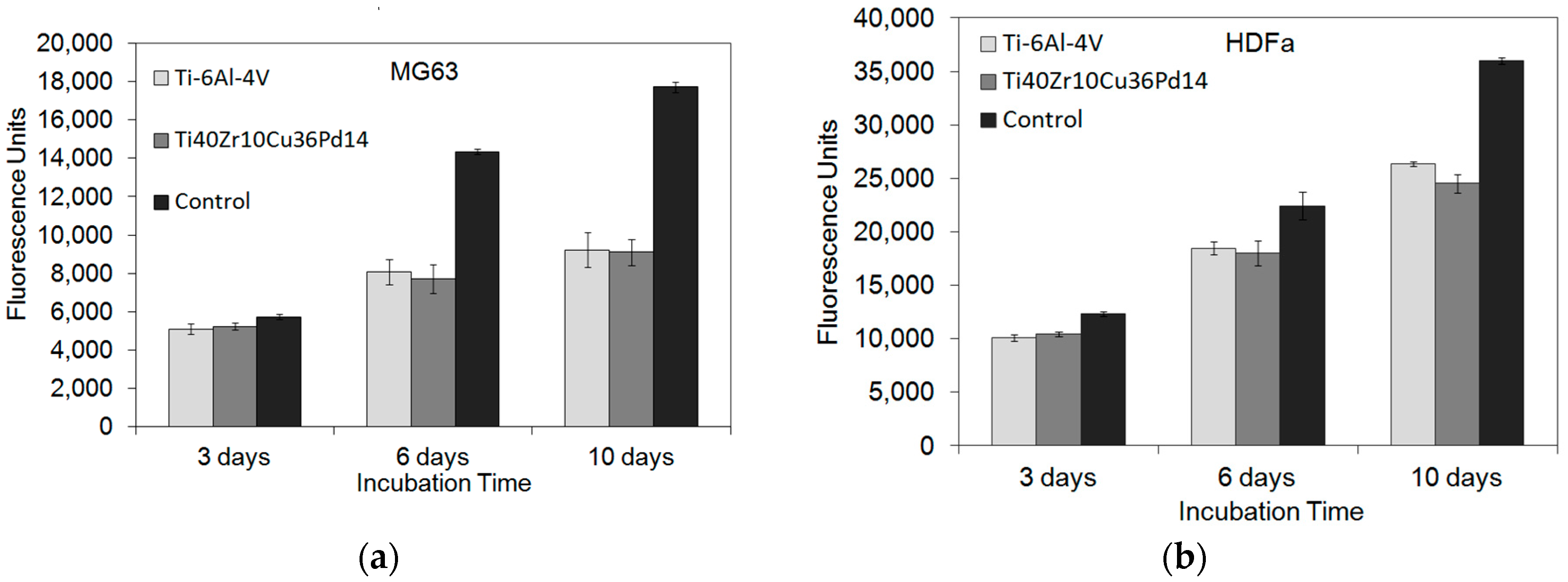
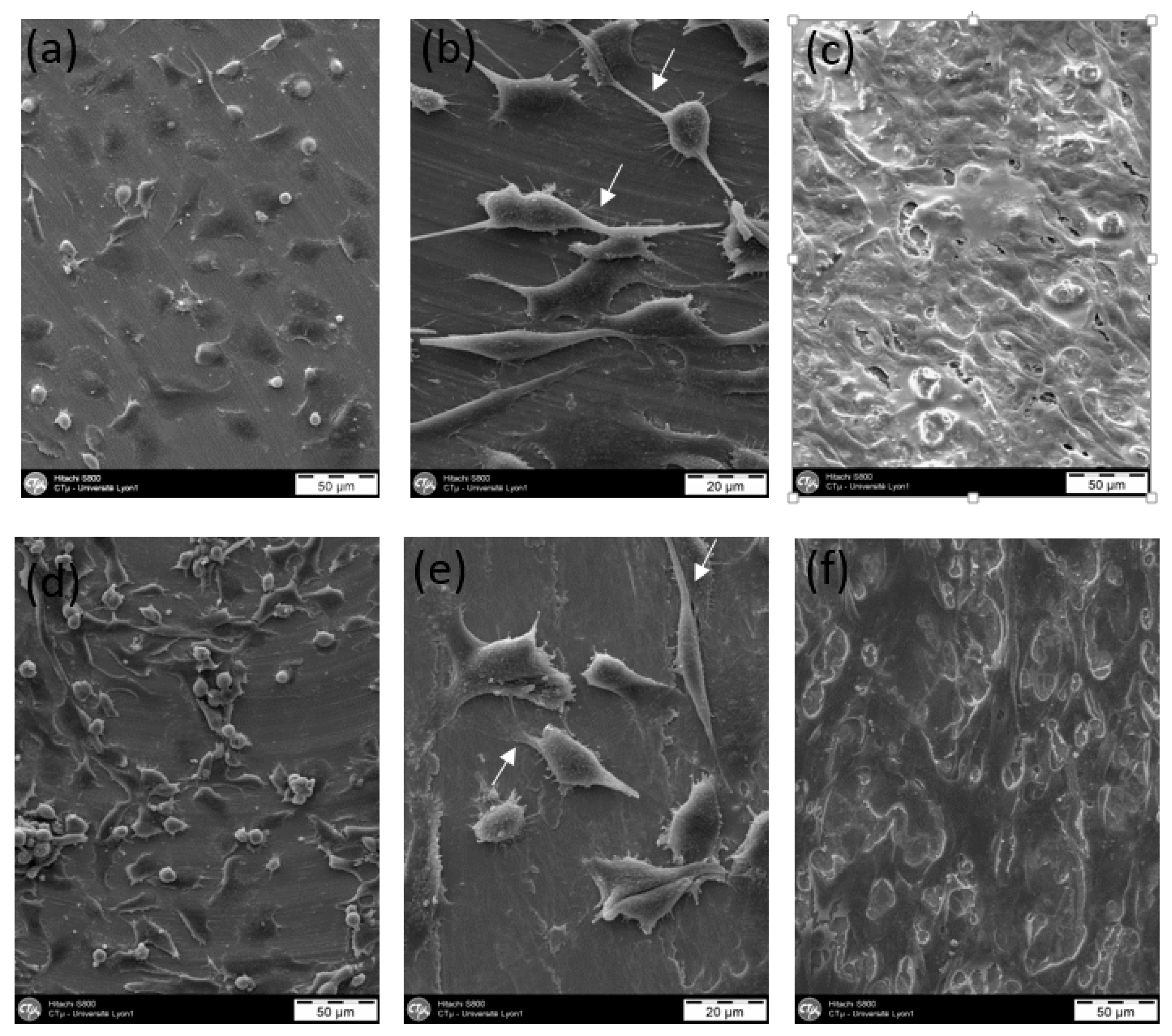
2.2.3. Cytocompatibility
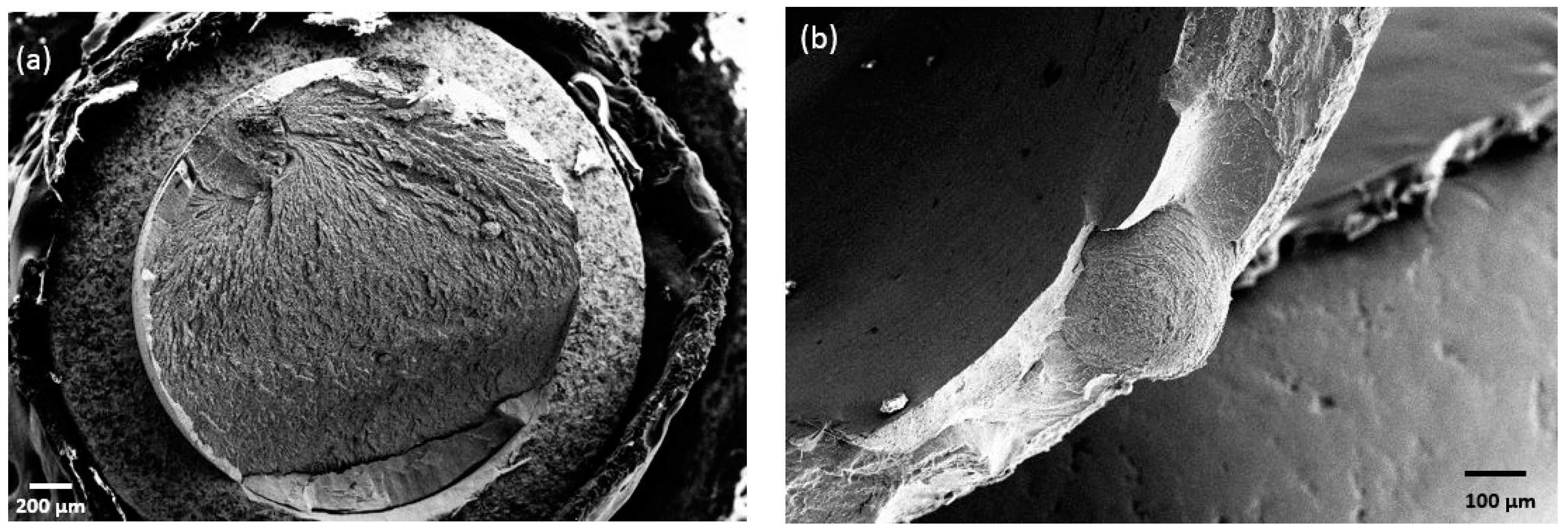
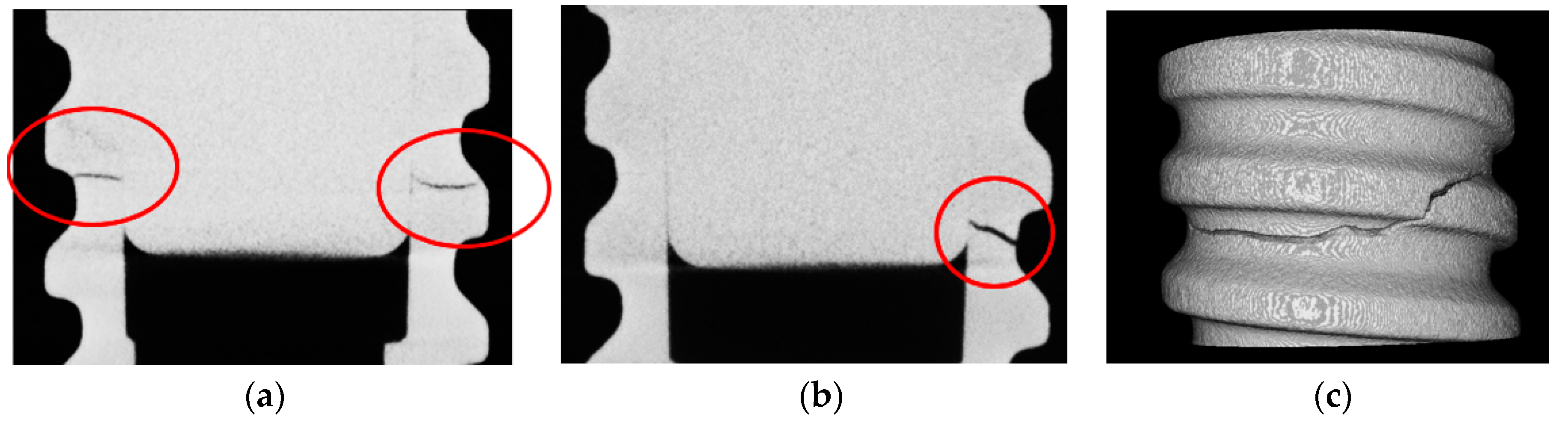
2.2.4. Tests on Dental Implants
3. Discussion
3.1. Glass Forming Ability, Processing Chain and Overall Stability
3.2. Mechanical Performances
3.3. Corrosion Resistance and Cytocompatibility
3.4. Limits of the Study and Further Work
4. Materials and Methods
4.1. Materials Characterization
4.1.1. Materials
4.1.2. Structural Analysis
4.1.3. Thermal Stability
4.1.4. Mechanical Characterization
4.2. Characterization for a Potential Biomedical Application
4.2.1. Resistance to Sterilization
- -
- Heat treatment in autoclave performed in water steam at 134 °C, under a 2-bar pressure for 20 h (mimicking 20 sterilization cycles),
- -
- Gamma rays (a total dose of 100 kGy was applied by subsequent conventional 25–40 kGy medical devices irradiations).
4.2.2. Corrosion Resistance
4.2.3. Cytocompatibility
4.3. Characterization of Implant-Abutment Assemblies
Tests on Dental Implants
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ASM International. Handbook of Materials for Medical Devices; Davis, J.R., Ed.; Materials Park: Novelty, OH, USA, 2003; ISBN 0-87170-790-X. [Google Scholar]
- Li, H.F.; Zheng, Y.F. Recent advances in bulk metallic glasses for biomedical applications. Acta Biomater. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Amiya, K.; Nishiyama, N.; Inoue, A.; Masumoto, T. Mechanical strength and thermal stability of Ti-based amorphous alloys with large glass forming ability. Mater. Sci. Eng. C 1994, 692–696. [Google Scholar] [CrossRef]
- Matsumoto, T.; Zhang, T.; Inoue, A. Amorphous (Ti, Zr, Hf)-Ni-Cu ternary alloys with a wide supercooled liquid region. Mater. Sci. Eng. 1994, 181–182. [Google Scholar] [CrossRef]
- Johnson, W.J.; Lin, X.H. Formation of Ti-Zr-Cu-Ni bulk metallic glasses. J. Appl. Phys. Lett. 1995, 78, 6514–6519. [Google Scholar] [CrossRef]
- Glade, S.C.; Hays, C.C. Thermophysical properties and glass forming ability in the alloy series (Ti100-xZrx)45(Ni14.5Cu85.5)55. Appl. Phys. Lett. 2002, 8, 3096–3098. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, Y.C.; Kim, W.T.; Kim, D.H. Ti based bulk metallic glasses with high specific strength. Mater. Trans. 2004, 45, 595–598. [Google Scholar] [CrossRef]
- Inoue, A. High strength bulk amorphous alloys with low critical cooling rates. Mater. Trans. JIM 1995, 36, 866–875. [Google Scholar] [CrossRef]
- Kim, Y.C.; Bae, D.H.; Kim, W.T. Glass forming ability and crystallization behavior of Ti-based amorphous alloys with high specific strength. J. Non-Cryst. Solids 2003, 325, 242–250. [Google Scholar] [CrossRef]
- Park, J.M.; Chang, H.J.; Han, K.H.; Kim, W.T.; Kim, D.H. Enhancement of plasticity in Ti-rich Ti–Zr–Be–Cu–Ni bulk metallic glasses. Scr. Mater. 2005, 53, 1–6. [Google Scholar] [CrossRef]
- Guo, F.Q.; Wang, H.J.; Poon, S.J.; Shiflet, G.J. Ductile titanium-based glassy alloy ingots. Appl. Phys. Lett. 2005, 86, 091907. [Google Scholar] [CrossRef]
- Tang, M.Q.; Zhang, H.F.; Zhu, Z.W.; Fu, H.M.; Wang, A.M.; Li, H.; Hu, Z.Q. TiZr-base Bulk Metallic Glass with over 50 mm in Diameter. J. Mater. Sci. Technol. 2010, 26, 481–486. [Google Scholar] [CrossRef]
- Gong, P.; Yao, K.F.; Shao, Y. Effects of Fe addition on glass-forming ability and mechanical properties of Ti–Zr–Be bulk metallic glass. J. Alloys Compd. 2012, 536, 26–29. [Google Scholar] [CrossRef]
- Jiang, B.J.; Hofmann, D.; Jarvis, D.J.; Fecht, H. Low-Density High-Strength Bulk Metallic Glasses and Their Composites: A Review. Adv. Eng. Mater. 2015, 17, 761–780. [Google Scholar] [CrossRef]
- Zhu, S.L.; Wang, X.M.; Qin, F.X.; Inoue, A. A new Ti-based bulk glassy alloy with potential for biomedical application. Mater. Sci. Eng. A 2007, 459, 233–237. [Google Scholar] [CrossRef]
- Qin, F.; Yoshimura, M.; Wang, X.; Zhu, S.; Kawashima, A.; Asami, K.; Inoue, A. Corrosion Behavior of a Ti-Based Bulk Metallic Glass and Its Crystalline Alloys. Mater. Trans. 2007, 48, 1855–1858. [Google Scholar] [CrossRef]
- Lütjering, G. Property optimization through microstructural control in titanium and aluminium alloys. Mater. Sci. Eng. 1999, 117–126. [Google Scholar] [CrossRef]
- Gong, P.; Deng, L.; Jin, J.; Wang, S.; Wang, X.; Yao, K. Review on the Research and Development of Ti-Based Bulk Metallic Glasses. Metals 2016, 6, 264. [Google Scholar] [CrossRef]
- Wang, H.; Park, E.S.; Oak, J.J.; Setyawan, A.D.; Zhu, S.L.; Wada, T.; Wang, X.M.; Takeuchi, A.; Kato, H. Effect of cobalt microalloying on the glass forming ability of Ti-Cu-Pd-Zr metallic glass. J. Non-Cryst. Solids 2013, 379, 155–160. [Google Scholar] [CrossRef]
- Qin, F.X.; Wang, X.M.; Inoue, A. Effect of annealing on microstructure and mechanical property of a Ti-Zr-Cu-Pd bulk metallic glass. Intermetallics 2007, 15, 1337–1342. [Google Scholar] [CrossRef]
- Zhu, S.L.; Wang, X.M.; Qin, F.X.; Yoshimura, M.; Inoue, A. New TiZrCuPd Quaternary Bulk Glassy Alloys with Potential of Biomedical Applications. Mater. Trans. 2007, 48, 2445–2448. [Google Scholar] [CrossRef]
- Blanquer, A.; Hynowska, A.; Nogues, C.; Ibanez, E.; Sort, J.; Baro, M.D.; Özkale, B.; Pané, S.; Pellicer, E.; Barrios, L. Effect of Surface Modifications of Ti40Zr10Cu38Pd12 Bulk Metallic Glass and Ti-6Al-4V Alloy on Human Osteoblasts In Vitro Biocompatibility. PLoS ONE 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fornell, J.; van Steenberge, N.; Varea, A.; Rossinyol, E.; Pellicer, E.; Surinach, S.; Baro, M.D.; Sort, J. Enhanced mechanical properties and in vitro corrosion behavior of amorphous and devitrified Ti40Zr10Cu38Pd12 metallic glass. J. Mech. Behav. Biomed. Mater. 2011, 4, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Sypien, A.; Czeppe, T.; Garzel, G.; Litynska-Dobrzynska, L.; Latuch, J.; Chinh, N.Q. Thermal stability and mechanical properties of the TiCuZrPd glasses with 10, 14 and 20 at. % Pd. J. Alloys Compd. 2015, 615, S108–S112. [Google Scholar] [CrossRef]
- Cui, Z.D.; Zhu, S.L.; Yang, X.J. Effect of hydroxyapatite content on the microstructure, thermal and mechanical properties of Ti-based glassy alloy/hydroxyapatite composite prepared by spark plasma sintering. Intermetallics 2011, 572–576. [Google Scholar] [CrossRef]
- Qin, F.; Dan, Z.; Wang, X.; Xie, G.; Inoue, A. Ti-based Bulk Metallic Glasses for Biomedical Applications. Biomed. Eng. Trends Mater. Sci. 2011, 249–268. [Google Scholar] [CrossRef]
- Apreutesei, M.; Steyer, P.; Billard, A.; Joly-Pottuz, L.; Esnouf, C. Zr-Cu thin film metallic glasses: An assessment of the thermal stability and phases’ transformation mechanisms. J. Alloys Compd. 2015, 619, 284–292. [Google Scholar] [CrossRef]
- Zhu, S.; Xie, G.; Qin, F.; Wang, X.; Inoue, A. Effect of Minor Sn Additions on the Formation and Properties of TiCuZrPd Bulk Glassy Alloy. Mater. Trans. 2012, 53, 500–503. [Google Scholar] [CrossRef]
- Apreutesei, M.; Steyer, P.; Joly-Pottuz, L.; Billard, A.; Qiao, J.; Cardinal, S.; Sanchette, F.; Pelletier, J.M.; Esnouf, C. Microstructural, thermal and mechanical behavior of co-sputtered binary Zr-Cu thin film metallic glasses. Thin Solid Films 2014, 561, 53–59. [Google Scholar] [CrossRef]
- Guan, B.; Shi, X.; Dan, Z.; Xie, G.; Niinomi, M.; Qin, F. Corrosion behavior, mechanical properties and cell cytotoxity of Zr-based bulk metallic glasses. Intermetallics 2016, 72, 69–75. [Google Scholar] [CrossRef]
- Yamaura, S.I.; Zhu, S.; Abe, K.; Xie, G. Ultrasonic Fatigue of Ti40Zr10Cu34Pd14Sn2 Glassy Alloy. Open J. Met. 2014, 4, 56–64. [Google Scholar] [CrossRef]
- Liu, Y.; Pang, S.; Li, H.; Hu, Q.; Chen, B.; Zhang, T. Formation and properties of Ti-based Ti-Zr-Cu-Fe-Sn-Si bulk metallic glasses with different (Ti + Zr)/Cu ratios for biomedical application. Intermetallics 2016, 72, 36–43. [Google Scholar] [CrossRef]
- Pang, S.; Liu, Y.; Li, H.; Sun, L.; Li, Y.; Zhang, T. New Ti-based Ti-Cu-Zr-Fe-Sn-Si-Ag bulk metallic glass for biomedical applications. J. Alloys Compd. 2015, 625, 323–327. [Google Scholar] [CrossRef]
- Sarac, B.; Bera, S.; Balakin, S.; Stoica, M.; Calin, M.; Eckert, J. Hierarchical surface patterning of Ni- and Be-free Ti- and Zr-based bulk metallic glasses by thermoplastic net-shaping. Mater. Sci. Eng. C 2017, 73, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Sarac, B.; Balakin, S.; Ramasamy, P.; Stoica, M.; Calin, M.; Eckert, J. Micro-patterning by thermoplastic forming of Ni-free Ti-based bulk metallic glasses. Mater. Des. 2017, 120, 204–211. [Google Scholar] [CrossRef]
- Sarac, B.; Bera, S.; Spieckermann, F.; Balakin, S.; Stoica, M.; Calin, M.; Eckert, J. Micropatterning kinetics of different glass-forming systems investigated by thermoplastic net-shaping. Scr. Mater. 2017, 137, 127–131. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T.; Wennerberg, A.T. Suggested guidelines for the topographic evaluation of implant surfaces. Int. J. Oral Maxillofac. Implants 2000, 15, 331–344. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. On Implant Surfaces: A Review of Current Knowledge and Opinions. Int. J. Oral Maxillofac. Implants 2009, 24, 63–74. [Google Scholar]
- Shah, L.H.; Bun, T.; Nagata, S.; Shikama, T. The effects of gamma ray on the mechanical properties of Zr-based bulk metallic glass. IJAME 2012, 6, 713–721. [Google Scholar] [CrossRef]
- Shah, L.H.; Tsuchiya, B.; Nagata, S.; Shikama, T. The effect of gamma-rays on the electrical properties of Zr55Ni5Al10Cu30 bulk metallic glass. J. Nucl. Mater. 2011, 417, 822–825. [Google Scholar] [CrossRef]
- Park, D.G.; Song, H.; Cheong, Y.M. Effects of gamma irradiation and thermal annealing on the co-based amorphous ribbon. IEEE Trans. Magn. 2011, 47, 2835–2837. [Google Scholar] [CrossRef]
- Xi, X.K.; Zhao, D.Q.; Pan, M.X.; Wang, W.H.; Wu, Y.; Lewandowski, J.J. Fracture of brittle metallic glasses: Brittleness or plasticity. Phys. Rev. Lett. 2005, 94, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.F. Materials Selection in Mechanical Design, 3rd ed.; Elsevier Butterworth-Heinemann Edition: Waltham, MA, USA, 2005; ISBN 9780080468648. [Google Scholar]
- Inoue, A.; Zhang, T. Fabrication of bulky Zr-based glassy alloys by suction casting into copper mold. Mater. Trans. JIM 1995, 36, 1184–1187. [Google Scholar] [CrossRef]
- Wall, J.J.; Fan, C.; Liaw, P.K.; Liu, T.; Choo, H. A combined drop/suction-casting machine for the manufacture of bulk-metallic-glass materials. Rev. Sci. Instrum. 2006, 77. [Google Scholar] [CrossRef]
- Schroers, B.J. Processing of Bulk Metallic Glass. Adv. Mater. 2010, 22, 1566–1597. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Strunskus, T.; Ladebush, H.; Zaporojtchenko, V.; Faupel, F. XPS study of the initial oxidation of the bulk metallic glass Zr46.75Ti8.25Cu7.5Ni10Be27.5. J. Mater. Sci. 2008, 5495–5503. [Google Scholar] [CrossRef]
- Tang, J.L.; Zhu, Q.H.; Wang, Y.Y.; Apreutesei, M.; Wang, H.; Steyer, P.; Chamas, M.; Billard, A. Insights on the role of copper addition on corrosion and mechanical properties in binary Zr-Cu metallic glass coatings. Coatings 2007, 7, 223. [Google Scholar] [CrossRef]
- Hiromoto, S.; Asami, K.; Tsai, A.P.; Hanawa, T. Surface characterization of amorphous Zr-Al-(Ni,Cu) alloys immersed in cell culture medium. Mater. Trans. JIM 2002, 261–266. [Google Scholar] [CrossRef]
- Pang, S.; Zhang, T.; Kimura, H.; Asami, K.; Inoue, A. Corrosion behavior of Zr-(Nb)-Al-Ni-Cu glassy alloys. JIM 2000, 1490–1494. [Google Scholar] [CrossRef]
- Raju, V.R.; Kühn, U.; Wolff, U.; Scheider, F.; Eckert, J.; Reiche, R.; Gebert, A. Corrosion behavior of Zr-based bulk glass-forming alloys containing Nb or Ti. Mater. Lett. 2002, 173–177. [Google Scholar] [CrossRef]
- Oak, J.J.; Louzguine-Luzgin, D.V.; Inoue, A. Fabrication of Ni-free Ti-based bulk metallic glassy alloy having potential for application as biomaterial, and investigation of its mechanical properties, corrosion, and crystallization behavior. J. Mater. Res. 2007, 22, 1346–1353. [Google Scholar] [CrossRef]
- Ding, C.; Liu, X.; Chu, P.K. Surface modification of titanium, titanium alloys and related materials for biomedical applications. Mater. Sci. Eng. 2004, 49–121. [Google Scholar] [CrossRef]
- Darnell, J.; Lodish, H.; Baltimore, D. Molecular cell biology. Q. Rev. Biol. 1988, 63, 77–78. [Google Scholar] [CrossRef]
- Breme, J.; Veltn, D.; Biehl, V.; Aubertin, F.; Valeske, B.; Possart, W. Preparation of TiO2 layers on cp-Ti and Ti-6Al-4V by thermal and anodic oxidation and by sol-gel coating techniques and their characterization. J. Biomed. Mater. Res. 2002, 18–28. [Google Scholar] [CrossRef]
- Breslin, C.B.; Aziz-Kerrzo, M.; Conroy, K.G.; Fenelon, A.M.; Farrell, S.T. Electrochemical studies on the stability and corrosion resistance of titanium-based implant materials. Biomaterials 2001, 1531–1539. [Google Scholar] [CrossRef]
- Wever, D.J.; Veldhuizen, A.G.; de Vries, J.; Bussher, H.J.; Uges, D.R.A.; van Horn, J.R. Electrochemical and surface characterization of a nickel-titanium alloy. Biomaterials 1998, 761–769. [Google Scholar] [CrossRef]
- Li, J.; Shi, S.; Zhu, Z.; He, Q.; Ai, H.; Xu, J. Zr61Ti2Cu25Al12 metallic glass for potential use in dental implants: Biocompatibility assessment by in vitro cellular responses. Mater. Sci. Eng. C 2013, 33, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, S.H.; Kim, M.S.; Lee, E.J.; Oh, H.G.; Oh, W.M.; Park, S.W.; Kim, W.J.; Lee, G.J.; Choi, N.G.; et al. Varying Ti-6A1-4V surface roughness induces different early morphologic and molecular responses in MG63 osteoblast-like cells. J. Biomed. Mater. Res. Part A 2005, 74, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Zheng, Y.F.; Wei, S.C.; Li, M. In vitro study on Zr-based bulk metallic glasses as potential biomaterials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Li, H.F.; Cheng, Y.; Zheng, Y.F.; Ruan, L.Q. In vitro and in vivo studies on Ti-based bulk metallic glass as potential dental implant material. Mater. Sci. Eng. C 2013, 33, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Blanquer, A.; Pellicer, E.; Hynowska, A.; Barrios, L.; Ibanoz, E.; Baro, M.D.; Sort, J.; Noguès, C. In vitro biocompatibility assessment of Ti40Cu38Zr10Pd12 bulk metallic glass. J. Mater. Sci. Mater. Med. 2014, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Syverud, M.; Dahl, J.E.; Hero, H.; Morisbak, E. Corrosion and biocompatibility of palladium alloy castings. Dent. Mater. 2001, 17, 7–13. [Google Scholar] [CrossRef]
- Zhang, E.; Zheng, L.; Liu, J.; Bai, B.; Liu, C. Influence of Cu content on the cell biocompatibility of Ti–Cu sintered alloys. Mater. Sci. Eng. 2015, 46, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Addison, O.; Davenport, A.J. A synergistic effect of albumin and H2O2 accelerates corrosion of Ti6Al4V. Acta Biomater. 2015, 26, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Yu, F. Corrosion of Titanium for Biomedical Applications. Ph.D. Thesis, School of Metallurgy and Materials, University of Birmingham, Birmingham, UK, March 2015. [Google Scholar]
- Buffiere, J.Y.; Maire, E.; Adrien, J.; Masse, J.P.; Boller, E. In Situ Experiments with X ray Tomography: An Attractive Tool for Experimental Mechanics. Exp. Mech. 2010, 289–305. [Google Scholar] [CrossRef]
- Abramoff, M.; Magalhaes, P.; Ram, S.J. Image processing with ImageJ. J. Biophotonics Int. 2004, 36–42. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image. Nat. Methods 2009, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Vergnol, G.; Ginsac, N.; Rivory, P.; Meille, S.; Chenal, J.M.; Balvay, S.; Chevalier, J.; Hartmann, D.J. In vitro and in vivo evaluation of a polylactic acid-bioactive glass composite for bone fixation devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 180–191. [Google Scholar] [CrossRef] [PubMed]
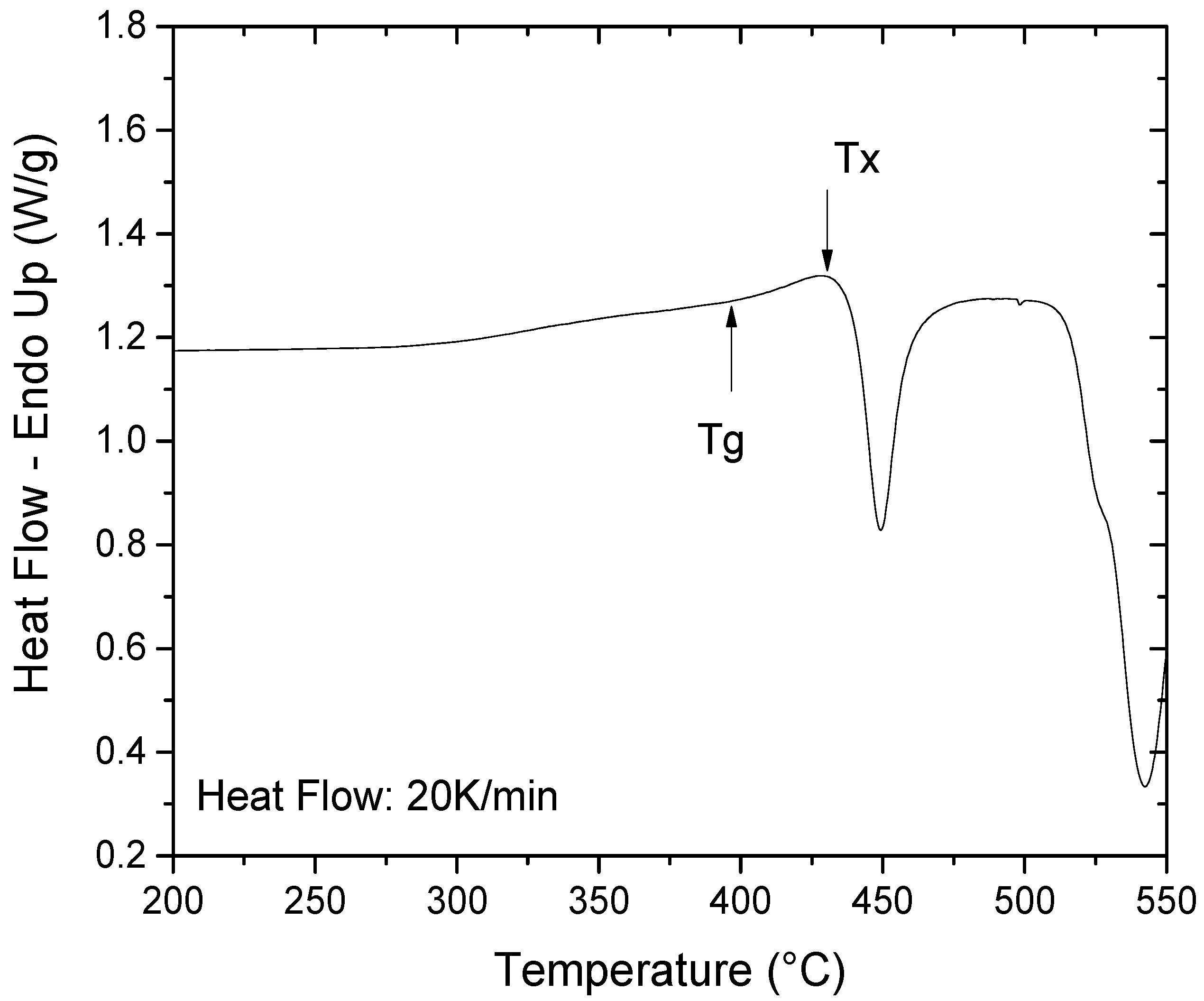

| Chemical Composition (At %) | Thermal Stability | Phases and Crystallization Temperature (°C) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ti | Cu | Pd | Zr | Tg (°C) | Tx (°C) | ΔTx (°C) | PdTi2 | Cu3Zr8 | CuTi |
| 40.2 | 36.4 | 14 | 9.4 | 394 | 432 | 38 | 450 | 450 | 450 |
| Alloy | Corrosion Properties | Mechanical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ecorr. (V/SCE) | Icorr. (A/cm2) | Rp (MΩ/cm2) | E (GPa) | Hardness (HV1) | σm 1 (GPa) | σy 2 (MPa) | εel (%) | εp (%) | σs 3 (N∙m/kg) | |
| BMG | −0.07 | ~6 × 10−9 | 2.0 | 96 | 556.4 | 2.01 | 1930 | 2 | 0.73 | 2.8 × 105 |
| Ti-6Al-4V | −0.2 | 1.5 × 10−8 | 0.5 | 115 | 341 | 0.97 | 1050 | 1 | 10 | 2.0 × 105 |
| Metallic Glasses | w (µm) | KQ (MPa.√m) | σy (MPa) | Reference |
|---|---|---|---|---|
| Ti40Zr10Cu36Pd14 | 19.5 () | 56 | 2010 () | Original |
| Ti40Zr10Cu36Pd14 | 20.3 | 55.6 | 1950 | [15] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liens, A.; Etiemble, A.; Rivory, P.; Balvay, S.; Pelletier, J.-M.; Cardinal, S.; Fabrègue, D.; Kato, H.; Steyer, P.; Munhoz, T.; et al. On the Potential of Bulk Metallic Glasses for Dental Implantology: Case Study on Ti40Zr10Cu36Pd14. Materials 2018, 11, 249. https://doi.org/10.3390/ma11020249
Liens A, Etiemble A, Rivory P, Balvay S, Pelletier J-M, Cardinal S, Fabrègue D, Kato H, Steyer P, Munhoz T, et al. On the Potential of Bulk Metallic Glasses for Dental Implantology: Case Study on Ti40Zr10Cu36Pd14. Materials. 2018; 11(2):249. https://doi.org/10.3390/ma11020249
Chicago/Turabian StyleLiens, Alethea, Aurélien Etiemble, Pascaline Rivory, Sandra Balvay, Jean-Marc Pelletier, Sandrine Cardinal, Damien Fabrègue, Hidemi Kato, Philippe Steyer, Tais Munhoz, and et al. 2018. "On the Potential of Bulk Metallic Glasses for Dental Implantology: Case Study on Ti40Zr10Cu36Pd14" Materials 11, no. 2: 249. https://doi.org/10.3390/ma11020249






