Structural, Electronic, and Thermodynamic Properties of Tetragonal t-SixGe3−xN4
Abstract
:1. Introduction
2. Calculation Methods
3. Results and Discussion
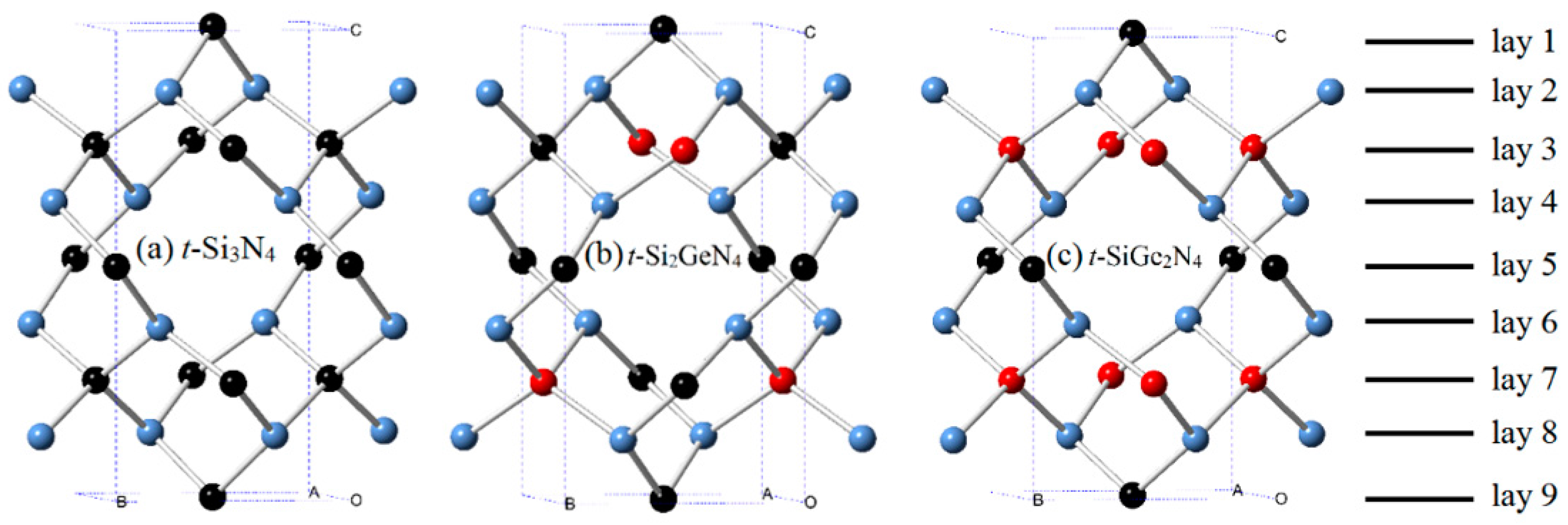
3.1. Structural Properties
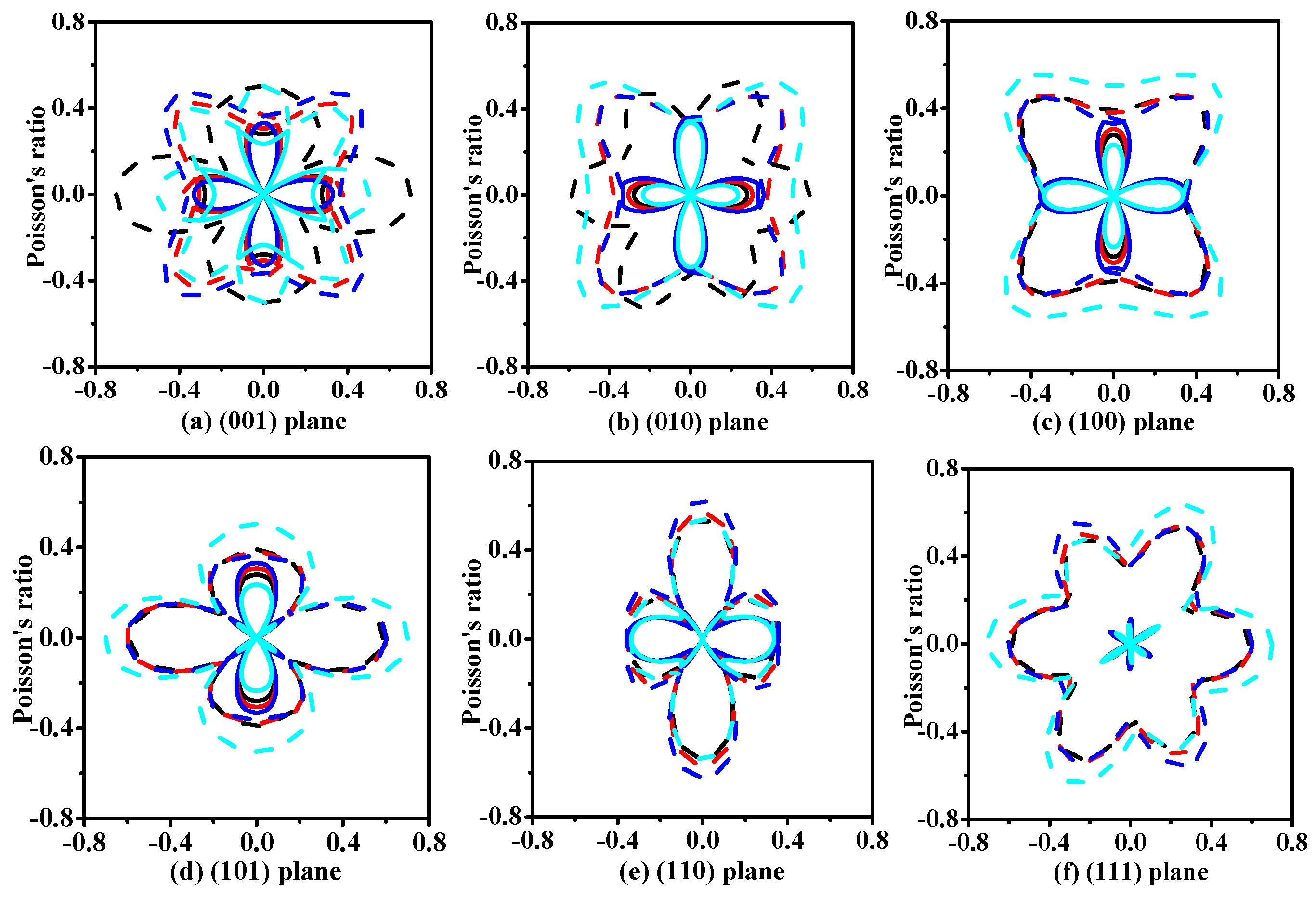
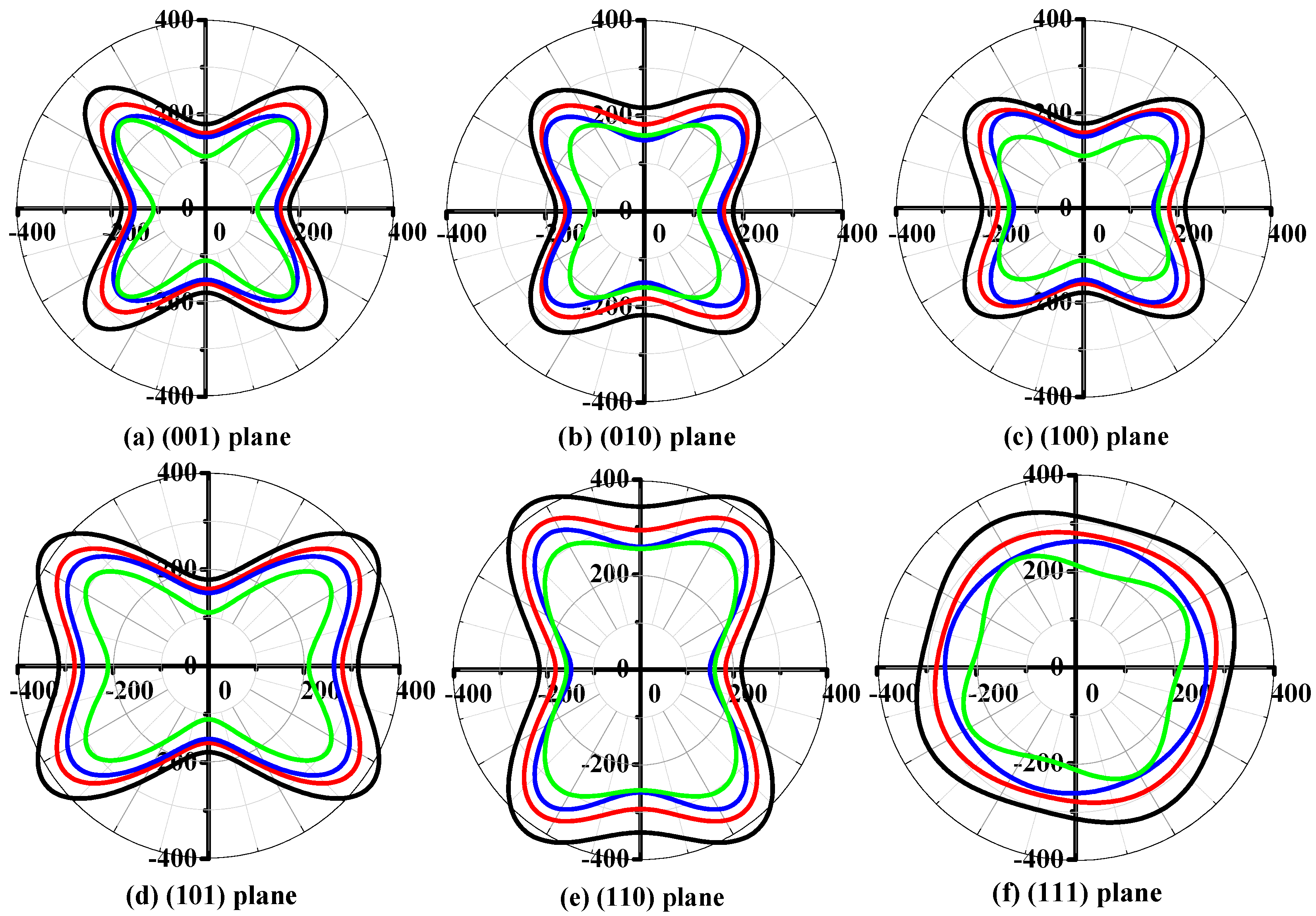
3.2. Mechanical and Anisotropic Properties
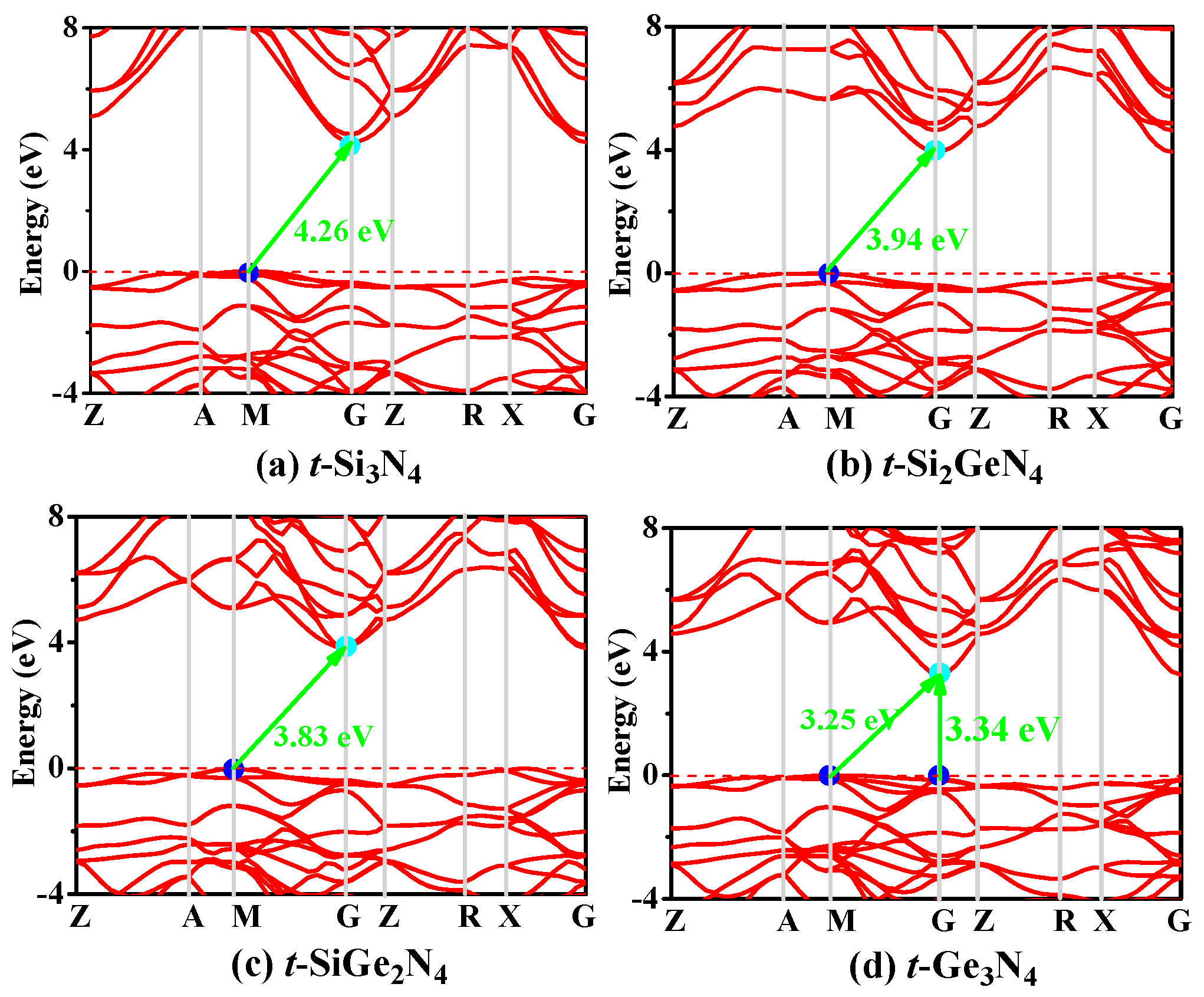
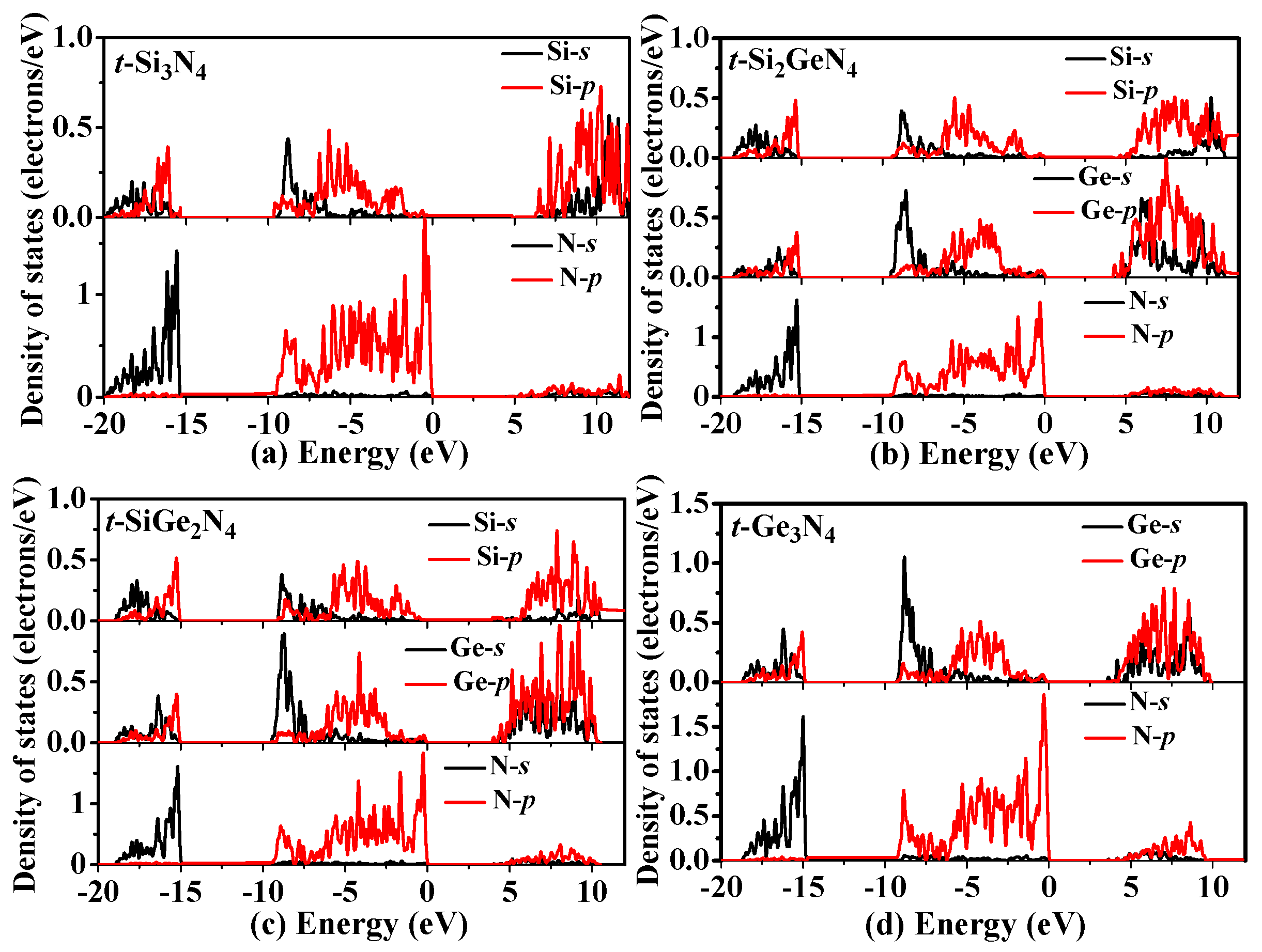
3.3. Electronic Properties
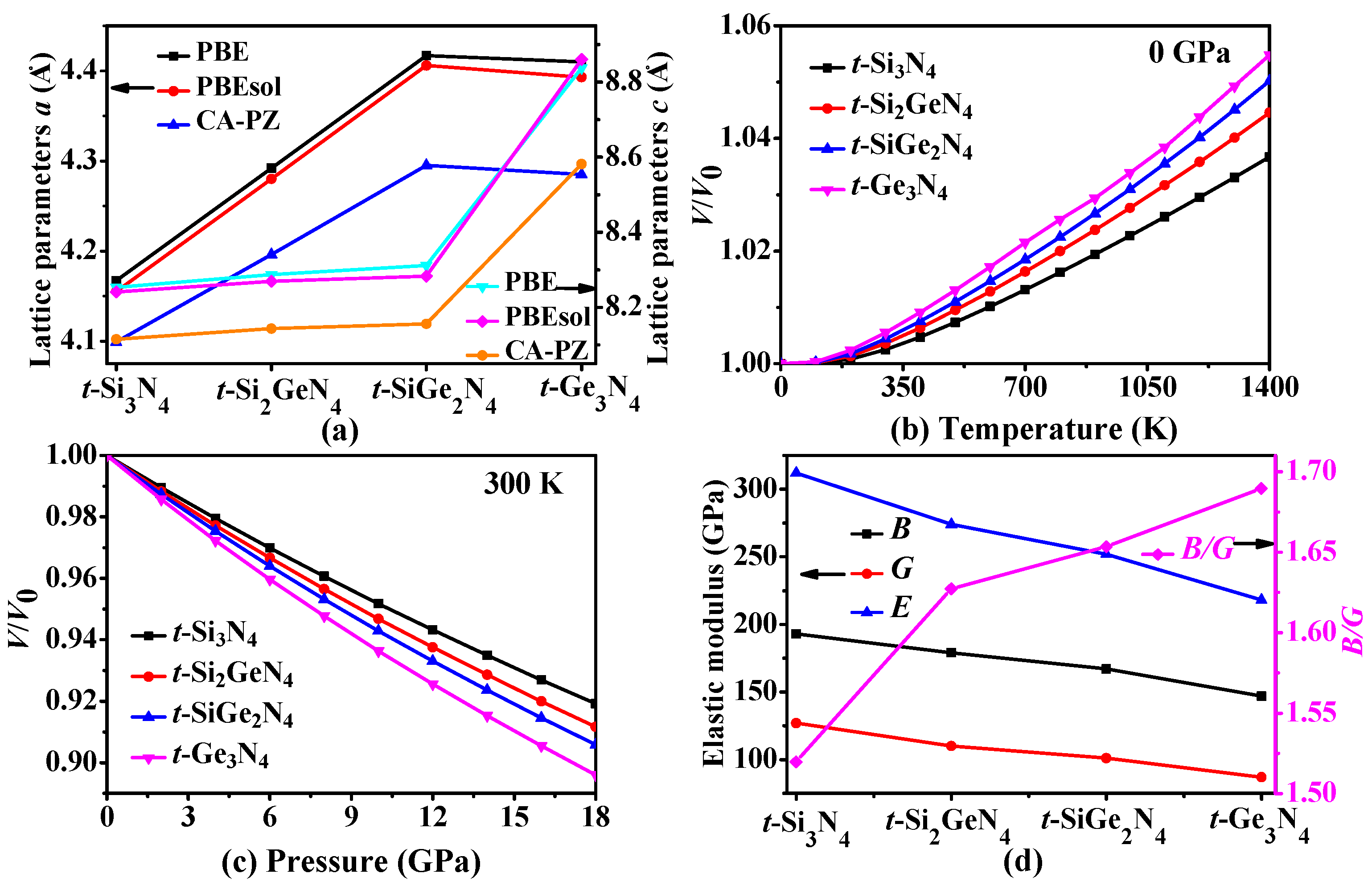
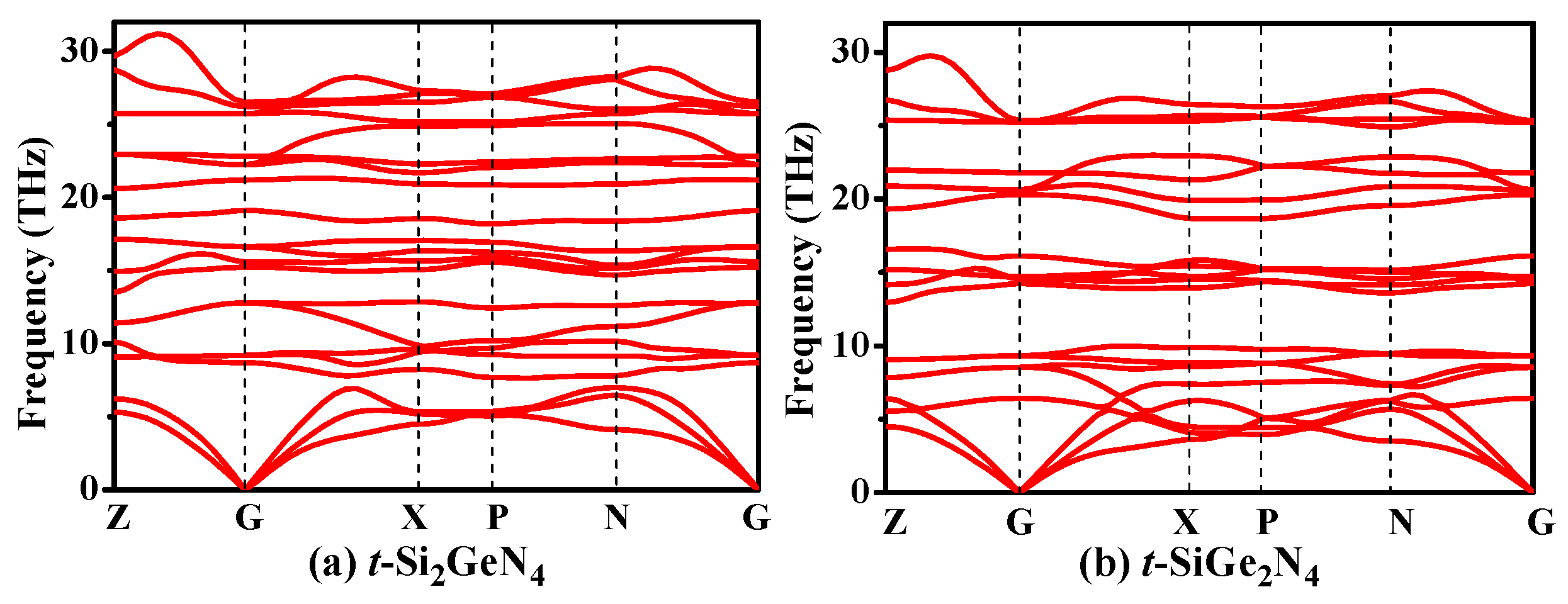
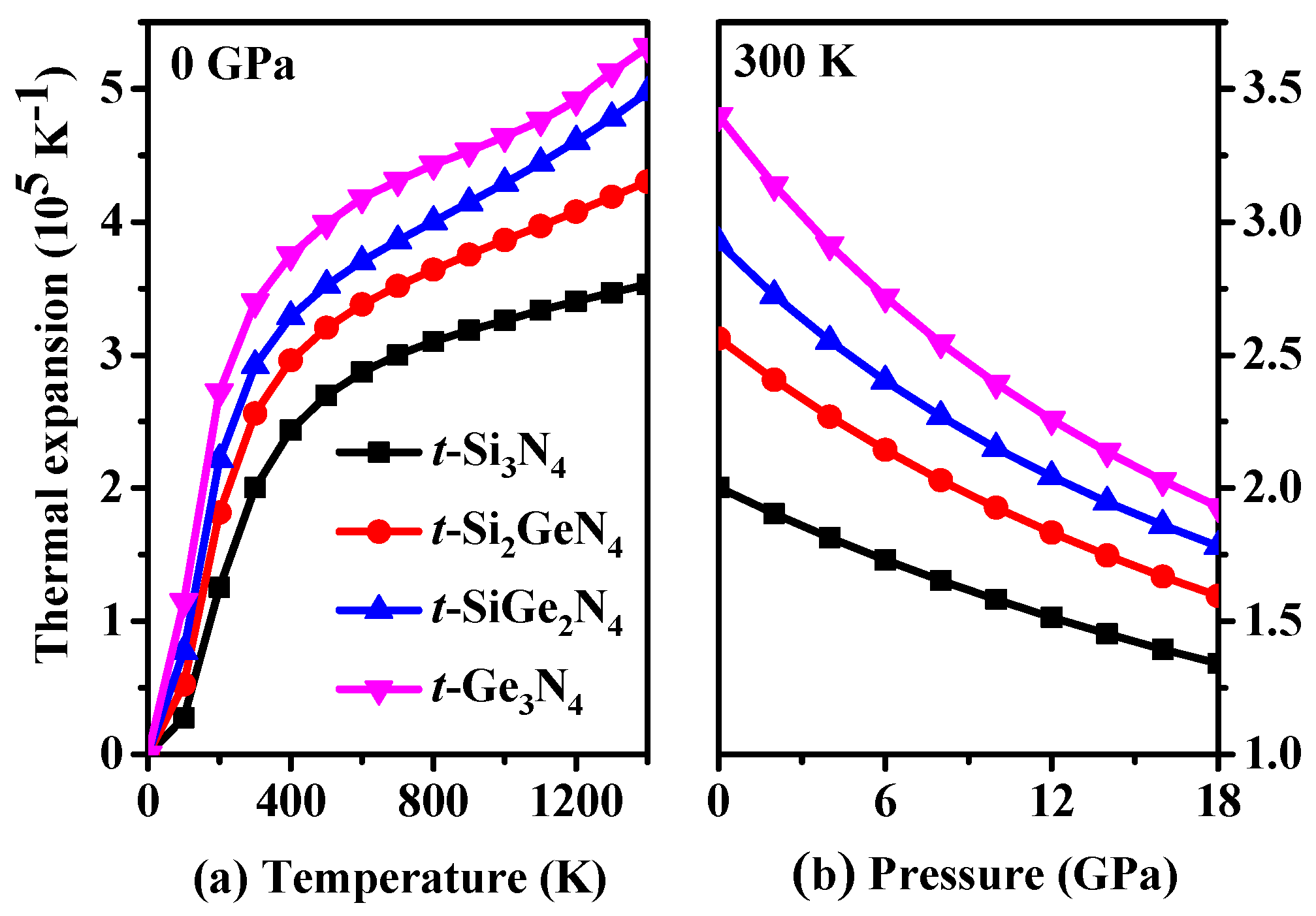
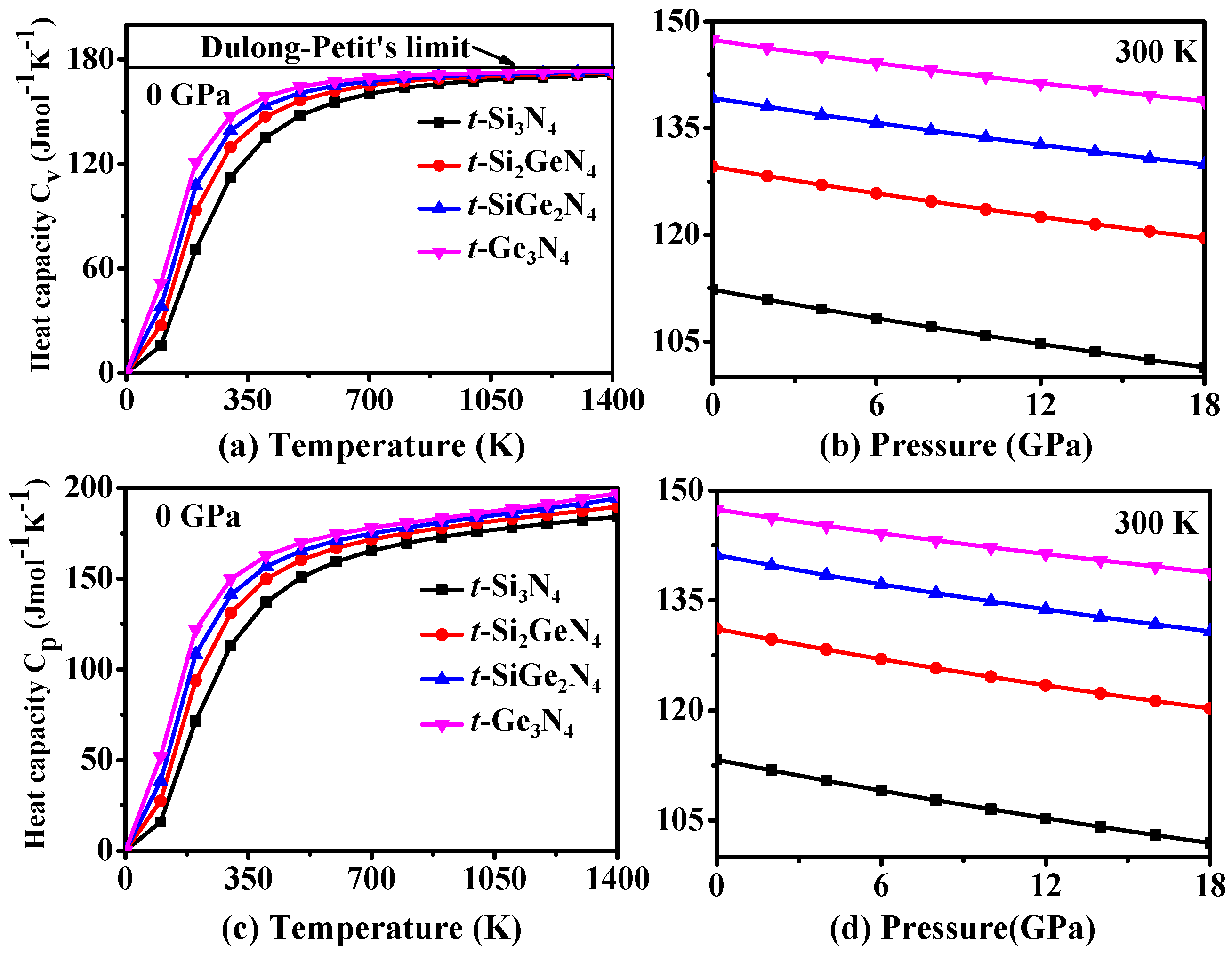
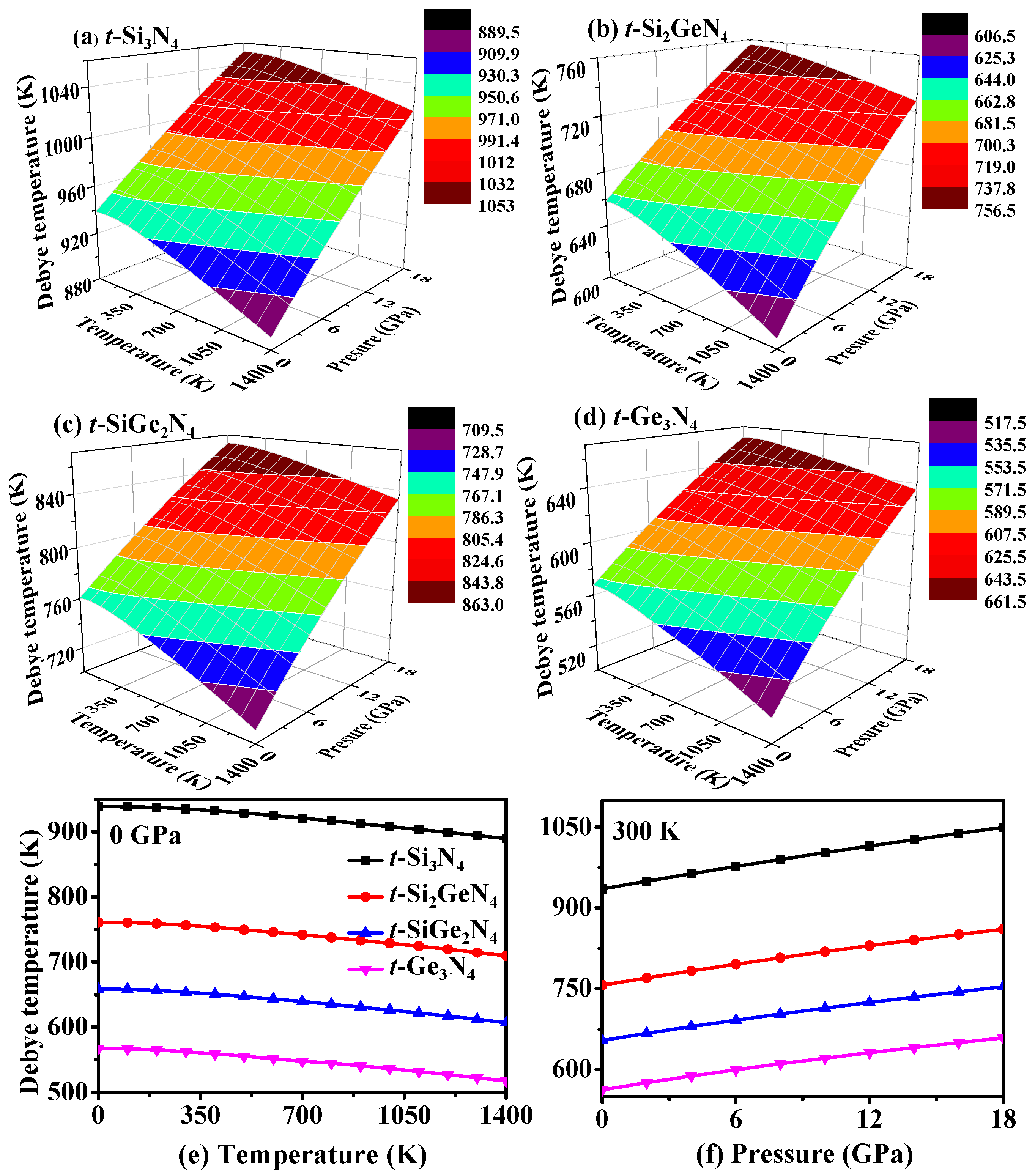
3.4. Thermodynamic Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reshak, A.H.; Khan, S.A.; Auluck, S. Linear and nonlinear optical properties for AA and AB stacking of carbon nitride polymorph (C3N4). RSC Adv. 2014, 4, 11967–11974. [Google Scholar] [CrossRef]
- Jack, K.H. Sialons and related nitrogen ceramics. J. Mater. Sci. 1976, 11, 1135–1158. [Google Scholar] [CrossRef]
- Salamat, A.; Hector, A.L.; Kroll, P.; McMillan, P.F. Nitrogen-rich transition metal nitrides. Coord. Chem. Rev. 2013, 257, 2063–2072. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M.; Wu, W. Theoretical calculations of stability, mechanical and thermodynamic properties of IVA group Willemite-II nitrides. J. Theor. Comput. Chem. 2015, 14, 1550024. [Google Scholar] [CrossRef]
- Xu, B.; Dong, J.; McMillan, P.F.; Shebanova, O.; Salamatet, A. Equilibrium and metastable phase transitions in silicon nitride at high pressure: A first-principles and experimental study. Phys. Rev. B 2011, 84, 014113. [Google Scholar] [CrossRef]
- Yu, B.-H.; Chen, D. First-principles study on the electronic structure and phase transition of α-, β- and γ-Si3N4. Acta Phys. Sin. 2012, 61, 197102. [Google Scholar] [CrossRef]
- Zerr, A.; Miehe, G.; Serghiou, G.; Schwarz, M.; Kroke, E.; Riedel, R.; Fueß, H.; Kroll, P.; Boehler, R. Synthesis of cubic silicon nitride. Nature 1999, 400, 340–342. [Google Scholar] [CrossRef]
- Togo, A.; Kroll, P. First-principles lattice dynamics calculations of the phase boundary between β-Si3N4 and γ-Si3N4 at elevated temperatures and pressures. J. Comput. 2008, 29, 2255–2259. [Google Scholar] [CrossRef]
- Kruger, M.B.; Nguyen, J.H.; Li, Y.M.; Caldwell, W.A.; Manghnani, M.H.; Jeanloz, R. Equation of state of α-Si3N4s. Phys. Rev. B 1997, 55, 3456. [Google Scholar] [CrossRef]
- Kroll, P.; von Appen, J. Post-Spinel Phases of Silicon Nitride. Phys. Status Solidi B 2001, 226. [Google Scholar] [CrossRef]
- Kroll, P. Pathways to metastable nitride structures. J. Solid State Chem. 2003, 176, 530–537. [Google Scholar] [CrossRef]
- Yu, B.-H.; Chen, D. Investigations of meta-stable and post-spinel silicon nitrides. Phys. B Condens. Matter 2012, 407, 4660–4664. [Google Scholar] [CrossRef]
- Johnson, W.C. Nitrogen compounds of germanium. I. The preparation and properties of Germanic nitride. J. Am. Chem. Soc. 1930, 52, 5160–5165. [Google Scholar] [CrossRef]
- Ruddlesden, S.N.; Popper, P. On the crystal structure of the nitrides of silicon and germanium. Acta Cryst. 1958, 11, 465–468. [Google Scholar] [CrossRef]
- He, H.; Sekine, T.; Kobayashi, T.; Kimoto, K. Phase transformation of germanium nitride (Ge3N4) under shock wave compression. J. Appl. Phys. 2001, 90, 4403–4406. [Google Scholar] [CrossRef]
- McMillan, P.F.; Deb, S.K.; Dong, J.-J. High-pressure metastable phase transitions in β-Ge3N4 studied by Raman spectroscopy. J. Raman Spectrosc. 2003, 34, 567–577. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Schiferl, D.; Qian, J. Threshold pressure for disappearance of size-induced effect in spinel-structure Ge3N4 nanocrystals. J. Phys. Chem. B 2003, 107. [Google Scholar] [CrossRef]
- Luo, Y.; Cang, Y.; Chen, D. Determination of the finite-temperature anisotropic elastic and thermal properties of Ge3N4: A first-principles study. Comput. Condens. Matter 2014, 1, 1–7. [Google Scholar] [CrossRef]
- Molina, B.; Sansores, L.E. Electronic structure of Ge3N4 possible structures. Int. J. Quantum Chem. 2000, 80, 249–257. [Google Scholar] [CrossRef]
- Huang, M.; Feng, Y.; Lim, A.T.L.; Zheng, J. Structural and electronic properties of Si3P4. Phys. Rev. B 2004, 69, 054112. [Google Scholar] [CrossRef]
- Gopal, K.G.; Clark, S.J.; Bandyopadhyay, N.R. Electronic, mechanical and optical properties of Si3P4 and Ge3P4: AN AB initio study. Int. J. Mod. Phys. B 2010, 24, 5487–5494. [Google Scholar] [CrossRef]
- Brazhkin, V.V.; Lyapin, A.G.; Hemley, R.J. Harder than diamond: Dreams and reality. Philos. Mag. A 2002, 82, 231–253. [Google Scholar] [CrossRef]
- Wang, S.; Yu, X.; Lin, Z.; Zhang, R.; He, D.; Qin, J.; Zhu, J.; Han, J.; Wang, L.; Mao, H.; et al. Synthesis, crystal structure, and elastic properties of novel tungsten nitrides. Chem. Mater. 2012, 24, 3023–3028. [Google Scholar] [CrossRef]
- Liu, K.; Wang, S.; Zhou, X.; Chang, J. Theoretical calculations for structural, elastic, and thermodynamic properties of c-W3N4 under high pressure. J. Appl. Phys. 2013, 114, 063512. [Google Scholar] [CrossRef]
- He, D.; Zhao, Y.; Daemen, L.; Qian, J.; Shen, T. Boron suboxide: As hard as cubic boron nitride. Appl. Phys. Lett. 2002, 81, 643–645. [Google Scholar] [CrossRef]
- Zhang, R.; Veprek, S.; Argon, A.S. Anisotropic ideal strengths and chemical bonding of wurtzite BN in comparison to zincblende BN. Phys. Rev. B 2008, 77, 172103. [Google Scholar] [CrossRef]
- Zhang, R.; Lin, Z.; Mao, H.; Zhao, Y. Thermodynamic stability and unusual strength of ultra-incompressible rhenium nitrides. Phys. Rev. B 2011, 83, 060101. [Google Scholar] [CrossRef]
- Fan, Q.; Wei, Q.; Yan, H.; Zhang, M.; Zhang, Z.; Zhang, J.; Zhang, D. Elastic and electronic properties of Pbca-BN: First-principles calculations. Comput. Mater. Sci. 2014, 85, 80–87. [Google Scholar] [CrossRef]
- Ma, Z.; Han, Z.; Liu, X.; Yu, X.; Wang, D.; Tian, Y. Pnma-BN: Another boron nitride polymorph with interesting physical properties. Nanomaterials 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Pournamdari1, E.; Akbarzadeh, E. AB initio and DFT study of energetic, stability, and nuclear magnetic resonance of BN nanotube. Med. Chem. (Los Angel.) 2017, 7, 8. [Google Scholar] [CrossRef]
- Teter, D.M.; Hemley, R.J. Low-compressibility carbon nitrides. Science 1996, 271, 53. [Google Scholar] [CrossRef]
- Fang, C.; Wijs, G.A. Lattice vibrations and thermal properties of carbon nitride with defect ZnS structure from first-principles calculations. J. Phys. Condens. Matter 2004, 16, 3027–3034. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, C. First-principles study on hardness of five polymorphs of C3N4. Phys. B Condens. Matter 2008, 403, 1956–1959. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Zhou, D.; Zheng, W.; Wu, Z.; Ma, Y. A novel low compressible and superhard carbon nitride: Body-centered tetragonal CN2. Phys. Chem. Chem. Phys. 2012, 14, 13081–13087. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Chai, C.; Wei, Q.; Yang, Y. Two novel C3N4 phases: Structural, mechanical and electronic properties. Materials 2016, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Hu, M.; Wang, Q.; Yang, Y. Prediction of novel hard phases of Si3N4: First-principles calculations. J. Solid State Chem. 2015, 228, 20–26. [Google Scholar] [CrossRef]
- Yao, X.; Dong, C. Structural, electronic and thermodynamic properties of tetragonal, monoclinic and orthorhombic Ge3N4. Chin. J. Comput. Phys. 2017, 34, 89–98. [Google Scholar]
- Fan, Q.; Chai, C.; Wei, Q.; Zhou, P.; Yang, Y. Elastic anisotropy and electronic properties of Si3N4 under pressures. AIP Adv. 2016, 6, 085207. [Google Scholar] [CrossRef]
- Cang, Y.; Lian, S.; Yang, H.; Chen, D. Predicting physical properties of tetragonal, monoclinic and orthorhombic M3N4 (M = C, Si, Sn) polymorphs via first-principles calculations. Chin. Phys. Lett. 2016, 33, 066301. [Google Scholar] [CrossRef]
- Tatsumi, K.; Tanaka, I.; Adachi, H. Theoretical prediction of post-spinel phases of silicon nitride. J. Am. Ceram. Soc. 2002, 85, 7–10. [Google Scholar] [CrossRef]
- Yang, M.; Wang, S.; Feng, Y.; Peng, G.; Sun, Y. Electronic structure of germanium nitride considered for gate dielectrics. J. Appl. Phys. 2007, 102, 013507. [Google Scholar] [CrossRef]
- Hart, J.N.; Allan, N.L.; Claeyssens, F. Ternary silicon germanium nitrides: A class of tunable band gap materials. Phys. Rev. B 2011, 84, 245209. [Google Scholar] [CrossRef]
- Ching, W.Y.; Mo, S.D.; Ouyang, L. Electronic and optical properties of the cubic spinel phase of c-Si3N4, c-Ge3N4, c-SiGe2N4, and c-GeSi2N4. Phys. Rev. B 2001, 63, 245110. [Google Scholar] [CrossRef]
- Moakafi, M.; Khenata, R.; Bouhemadou, A.; Benkhettou, N.; Rachen, D.; Reshak, A.H. Elastic, electronic and optical properties of SiGe2N4 under pressure: An ab initio study. Phys. Lett. A 2009, 373, 2393–2398. [Google Scholar] [CrossRef]
- Ma, Z.; Yan, F.; Wang, S.; Jia, Q.; Yu, X.; Shi, C. Mechanical, elastic anisotropy and electronic properties of the monoclinic phase of m-SixGe3−xN4. Chin. Phys. B 2017, 26, 126105. [Google Scholar] [CrossRef]
- Ceperley, D.M.; Alder, B.J. Ground state of the electron gas by a stochastic method. Phys. Rev. Lett. 1980, 45, 566. [Google Scholar] [CrossRef]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5048. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Shanno, D.F.; Kettler, P.C. Optimal conditioning of quasi-newton methods. Math. Comput. 1970, 24, 657–664. [Google Scholar] [CrossRef]
- Blanco, M.A.; Francisco, E.; Luana, V. GIBBS: Isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic Debye model. Comput. Phys. Commun. 2004, 158, 57–72. [Google Scholar] [CrossRef]
- Fan, Q.; Wei, Q.; Chai, C.; Yang, Y.; Yu, X.; Liu, Y.; Zheng, J.; Zhou, P.; Zhang, D. The elastic anisotropic and thermodynamic properties of I4mm-B3C. Acta Phys. Pol. A 2016, 129, 103–108. [Google Scholar] [CrossRef]
- Zerr, A.; Kempf, M.; Schwarz, M.; Kroke, E.; Göken, M.; Riedel, R. Elastic moduli and hardness of cubic silicon nitride. J. Am. Ceram. Soc. 2002, 85, 86–90. [Google Scholar] [CrossRef]
- Serghiou, G.; Miehe, G.; Tschauner, O.; Zerr, A.; Boehler, R. Synthesis of a cubic Ge3N4 phase at high pressures and temperatures. J. Chem. Phys. 1999, 111, 4659–4662. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals: Their Representation by Tensors and Matrices; Oxford University Press: New York, NY, USA, 1985. [Google Scholar]
- Bouhemadou, A.; Al-Douri, Y.; Khenata, R.; Haddadi, K. Structural, elastic, electronic, optical and thermal properties of c-SiGe2N4. Eur. Phys. J. B 2009, 71, 185–194. [Google Scholar] [CrossRef]
- Soignard, E.; Somayazulu, M.; Dong, J.; Sankey, O.F.; McMillan, P.F. High pressure-high temperature synthesis and elasticity of the cubic nitride spinel γ-Si3N4. J. Phys. Condens. Matter 2001, 13, 557–563. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, Y.; Peng, M.; Zhou, S. Anisotropic elastic properties of the Ca–Pb compounds. J. Alloys Compd. 2014, 595, 14–21. [Google Scholar] [CrossRef]
- Marmier, A.; Lethbridge, Z.A.D.; Walton, R.I.; Smith, C.W.; Parker, S.C.; Evans, K.E. ElAM: A computer program for the analysis and representation of anisotropic elastic properties. Comput. Phys. Commun. 2010, 181, 2102–2115. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.; Li, B.; Yu, Z.; Chen, Q. C2/m-carbon: Structural, mechanical, and electronic properties. J. Mater. Sci. 2015, 50, 7104–7114. [Google Scholar] [CrossRef]
- Fan, Q.; Chai, C.; Wei, Q.; Yan, H.; Zhao, Y.; Yang, Y.; Yu, X.; Liu, Y.; Xing, M.; Zhang, J.; et al. Novel silicon allotropes: Stability, mechanical, and electronic properties. J. Appl. Phys. 2015, 118, 185704. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Chai, C.; Wei, Q.; Yang, Y. Two novel silicon phases with direct band gaps. Phys. Chem. Chem. Phys. 2016, 18, 12905–12913. [Google Scholar] [CrossRef] [PubMed]
- Debye, P. Zur theorie der spezifischen wärmen. Ann. Phys. 1912, 344, 789–839. [Google Scholar] [CrossRef]
| Materials | PBE | PBEsol | CA–PZ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| a | c | Eg | a | c | Eg | a | c | Eg | |
| t-Si3N4 | 4.167 | 8.253 | 3.05 | 4.155 | 8.241 | 2.89 | 4.099 | 8.115 | 2.99 |
| - | 4.131 1 | 8.168 | 3.15 | - | - | - | - | - | - |
| - | 4.166 2 | 8.249 | - | - | - | - | - | - | - |
| t-Si2GeN4 | 4.292 | 8.287 | 2.74 | 4.280 | 8.269 | 2.60 | 4.196 | 8.143 | 2.71 |
| t-SiGe2N4 | 4.417 | 8.312 | 2.31 | 4.406 | 8.283 | 2.33 | 4.295 | 8.156 | 2.59 |
| t-Ge3N4 | 4.410 | 8.838 | 1.79 | 4.393 | 8.86 | 1.80 | 4.285 | 8.582 | 2.67 |
| c-Si3N4 | 7.763 | - | - | 7.751 | - | - | 7.639 | - | - |
| - | 7.773 3 | - | - | - | - | - | - | - | |
| - | 7.770 4 | - | - | - | - | - | - | - | |
| c-Si2GeN4 | 7.934 | - | - | 7.918 | - | - | 7.767 | - | - |
| - | 8.001 5 | - | - | - | - | - | - | - | |
| c-SiGe2N4 | 8.111 | - | - | 8.095 | - | - | 7.909 | - | - |
| - | 8.182 6 | - | - | - | - | - | - | - | |
| c-Ge3N4 | 8.296 | - | - | 8.289 | - | - | 8.040 | - | - |
| - | 8.288 7 | - | - | - | - | - | - | - | |
| - | 8.300 8 | - | - | - | - | - | - | - | |
| Materials | C11 | C12 | C13 | C33 | C44 | C66 | BH | GH | E | BH/GH | v |
|---|---|---|---|---|---|---|---|---|---|---|---|
| t-Si3N4 | 277 | 148 | 144 | 314 | 176 | 206 | 193 | 127 | 312 | 1.52 | 0.230 |
| - | 277 1 | 152 | 145 | 312 | 178 | 207 | 194 | 126 | 311 | 1.54 | - |
| t-Si2GeN4 | 254 | 138 | 137 | 278 | 158 | 171 | 179 | 110 | 274 | 1.63 | 0.245 |
| t-SiGe2N4 | 241 | 127 | 131 | 243 | 150 | 151 | 167 | 101 | 252 | 1.65 | 0.248 |
| t-Ge3N4 | 200 | 126 | 110 | 233 | 127 | 148 | 147 | 87 | 218 | 1.69 | 0.253 |
| c-Si3N4 | 524 | 177 | - | - | 333 | - | 293 | 256 | 595 | 1.14 | 0.161 |
| - | 512 2 | 177 | - | - | 331 | - | 289 | - | - | - | - |
| - | - | - | - | - | - | - | 290 7 | - | - | - | - |
| c-Si2GeN4 | 453 | 167 | - | - | 298 | - | 263 | 222 | 520 | 1.18 | 0.170 |
| - | - | - | - | 258 8 | - | - | - | - | |||
| c-SiGe2N4 | 440 | 151 | - | - | 251 | - | 247 | 201 | 474 | 1.229 | 0.179 |
| - | 441 3 | 150 | - | - | 129 | - | 247 | - | - | - | - |
| - | 494 4 | 172 | - | - | 288 | - | 283 | - | - | - | - |
| c-Ge3N4 | 375 | 140 | - | - | 223 | - | 218 | 172 | 409 | 1.27 | 0.187 |
| - | 368 5 | 145 | - | - | 223 | - | 220 | - | - | - | - |
| - | - | - | - | - | - | - | 296 9 | - | - | - | - |
| m-Si3N4 6 | 257 | 36 | 131 | 353 | 93 | 92 | 165 | 110 | 270 | 1.51 | 0.228 |
| m-Si2GeN4 6 | 242 | 37 | 126 | 316 | 76 | 85 | 154 | 97 | 241 | 1.59 | 0.239 |
| m-SiGe2N4 6 | 235 | 42 | 164 | 363 | 60 | 42 | 170 | 78 | 202 | 2.20 | 0.303 |
| m-Ge3N4 6 | 180 | 34 | 110 | 274 | 57 | 61 | 126 | 73 | 184 | 1.73 | 0.258 |
| Materials | BV | BR | AB | GV | GR | AG | AU |
|---|---|---|---|---|---|---|---|
| t-Si3N4 | 193.22 | 193.74 | 0.123 | 140.60 | 112.87 | 10.940 | 1.231 |
| t-Si2GeN4 | 179.17 | 178.88 | 0.081 | 122.27 | 98.38 | 10.827 | 1.216 |
| t-SiGe2N4 | 167.22 | 167.20 | 0.006 | 112.57 | 89.59 | 11.367 | 1.283 |
| t-Ge3N4 | 147.15 | 146.98 | 0.058 | 99.47 | 74.95 | 14.058 | 1.637 |
| c-Si3N4 | 292.98 | 292.98 | 0 | 269.29 | 243.56 | 5.017 | 0.528 |
| c-Si2GeN4 | 262.92 | 262.92 | 0 | 236.36 | 207.84 | 6.421 | 0.686 |
| c-SiGe2N4 | 247.09 | 247.09 | 0 | 208.72 | 194.06 | 3.640 | 0.378 |
| c-Ge3N4 | 218.24 | 218.24 | 0 | 180.81 | 164.07 | 4.854 | 0.510 |
| m-Si3N4 1 | 168.41 | 162.83 | 1.685 | 118.92 | 101.12 | 8.089 | 0.968 |
| m-Si2GeN4 1 | 156.16 | 152.44 | 1.205 | 105.18 | 89.59 | 8.004 | 0.905 |
| m-SiGe2N4 1 | 176.94 | 165.26 | 3.130 | 93.00 | 62.06 | 19.954 | 2.557 |
| m-Ge3N4 1 | 129.33 | 123.55 | 2.286 | 82.36 | 63.68 | 12.791 | 1.513 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, C.; Chai, C.; Fan, Q.; Yang, J.; Yang, Y. Structural, Electronic, and Thermodynamic Properties of Tetragonal t-SixGe3−xN4. Materials 2018, 11, 397. https://doi.org/10.3390/ma11030397
Han C, Chai C, Fan Q, Yang J, Yang Y. Structural, Electronic, and Thermodynamic Properties of Tetragonal t-SixGe3−xN4. Materials. 2018; 11(3):397. https://doi.org/10.3390/ma11030397
Chicago/Turabian StyleHan, Chenxi, Changchun Chai, Qingyang Fan, Jionghao Yang, and Yintang Yang. 2018. "Structural, Electronic, and Thermodynamic Properties of Tetragonal t-SixGe3−xN4" Materials 11, no. 3: 397. https://doi.org/10.3390/ma11030397




