Electrospun F18 Bioactive Glass/PCL—Poly (ε-caprolactone)—Membrane for Guided Tissue Regeneration
Abstract
:1. Introduction
2. Results
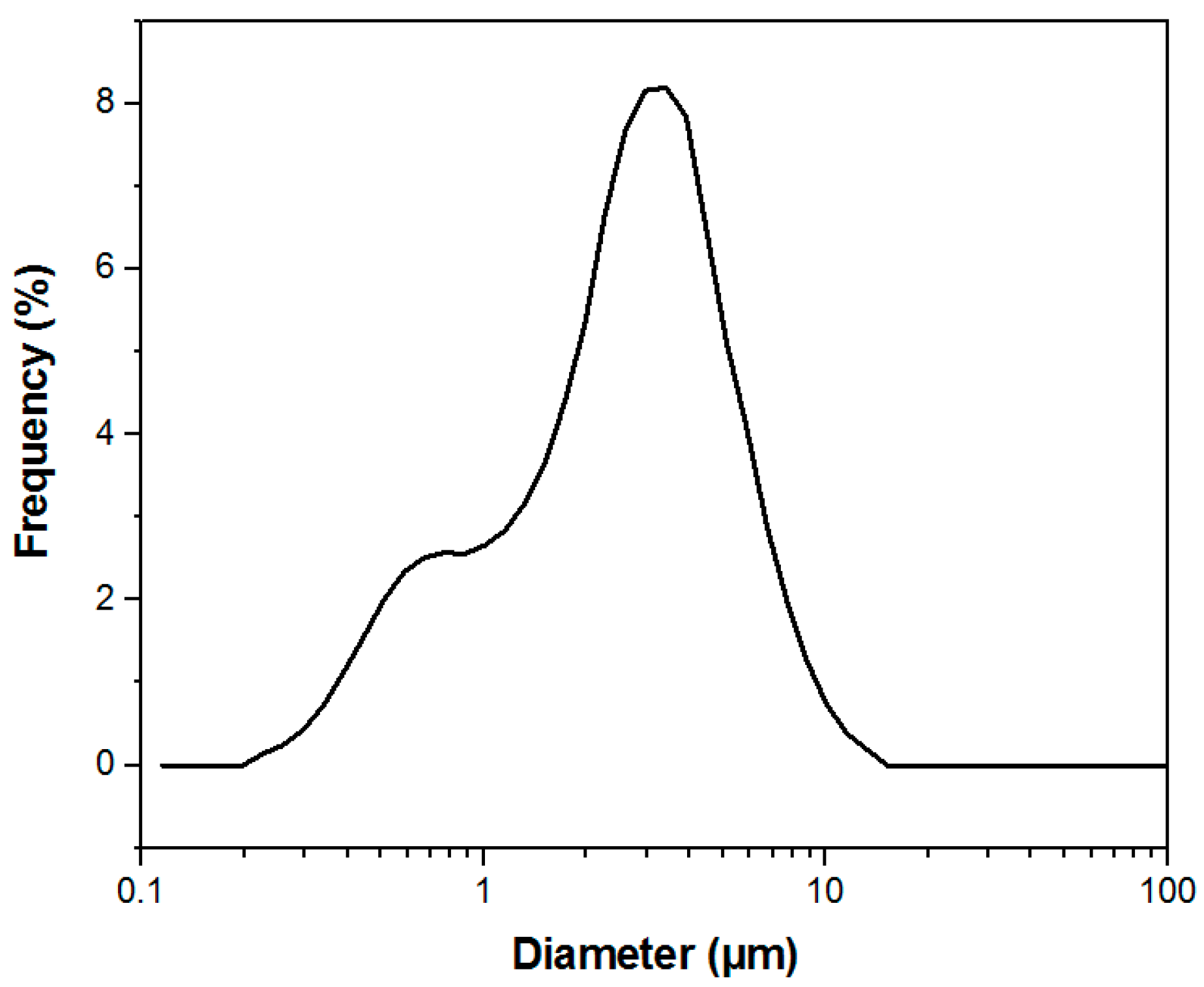
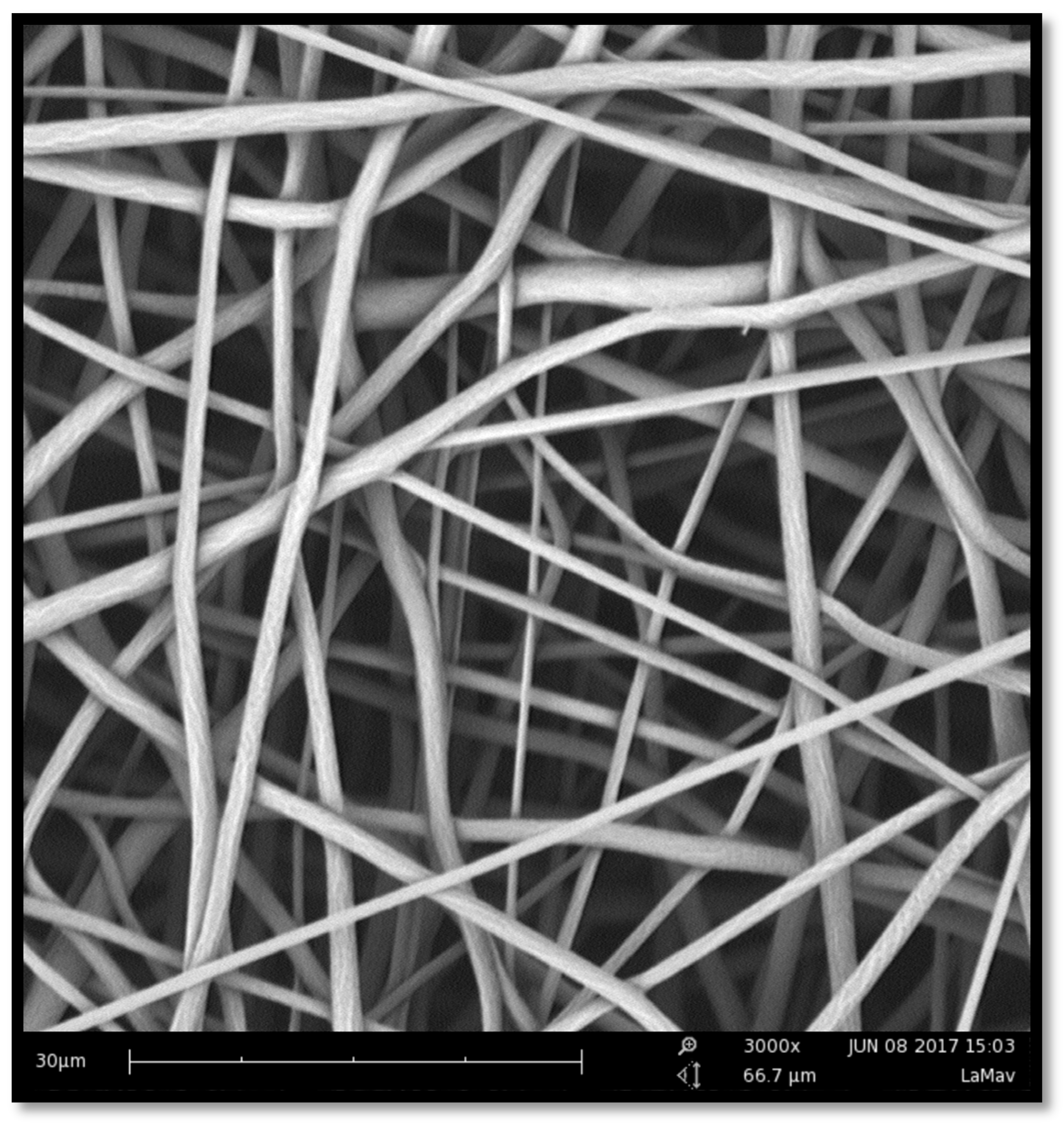
2.1. Manufacture and Characterization of Electrospun Membranes
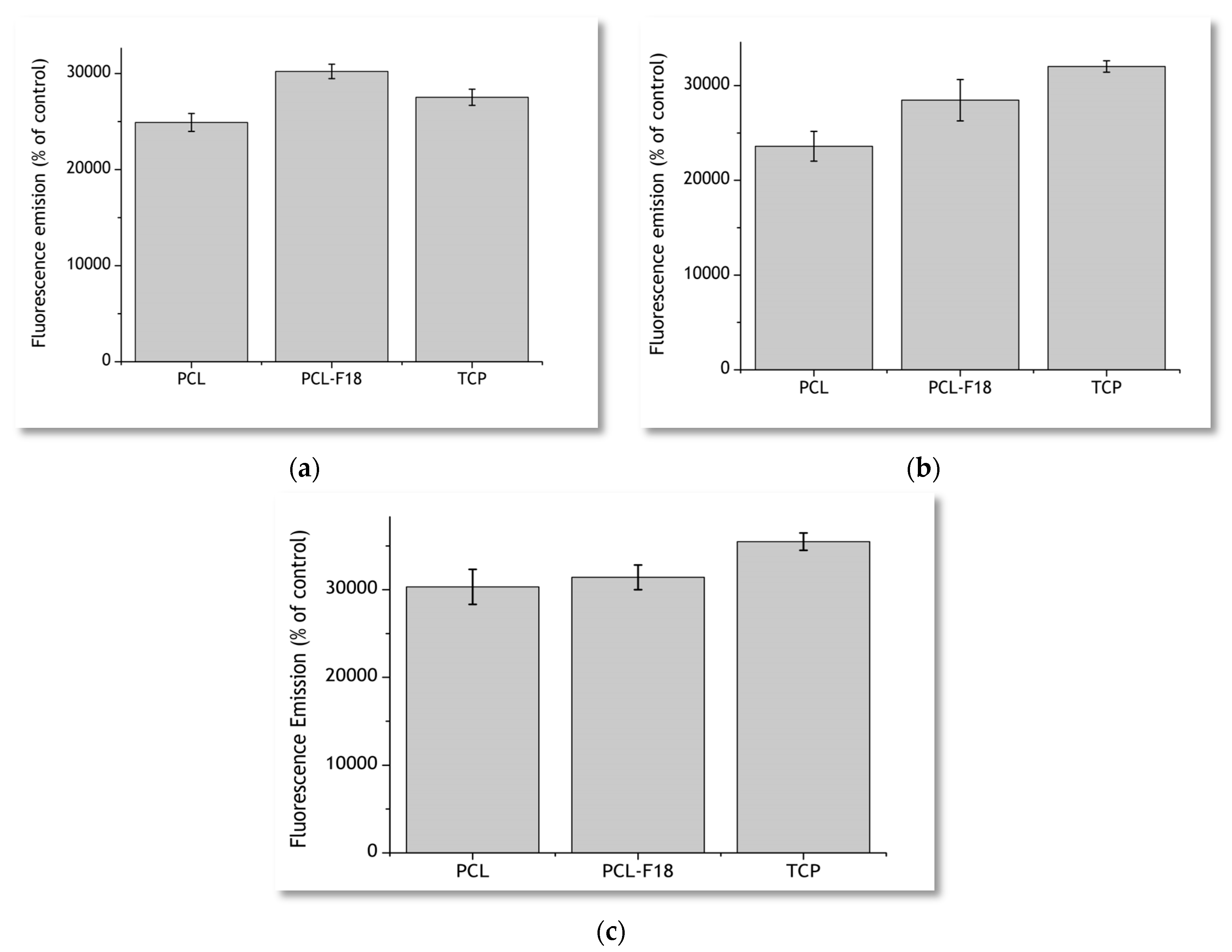
2.2. Cytoxicity Studies
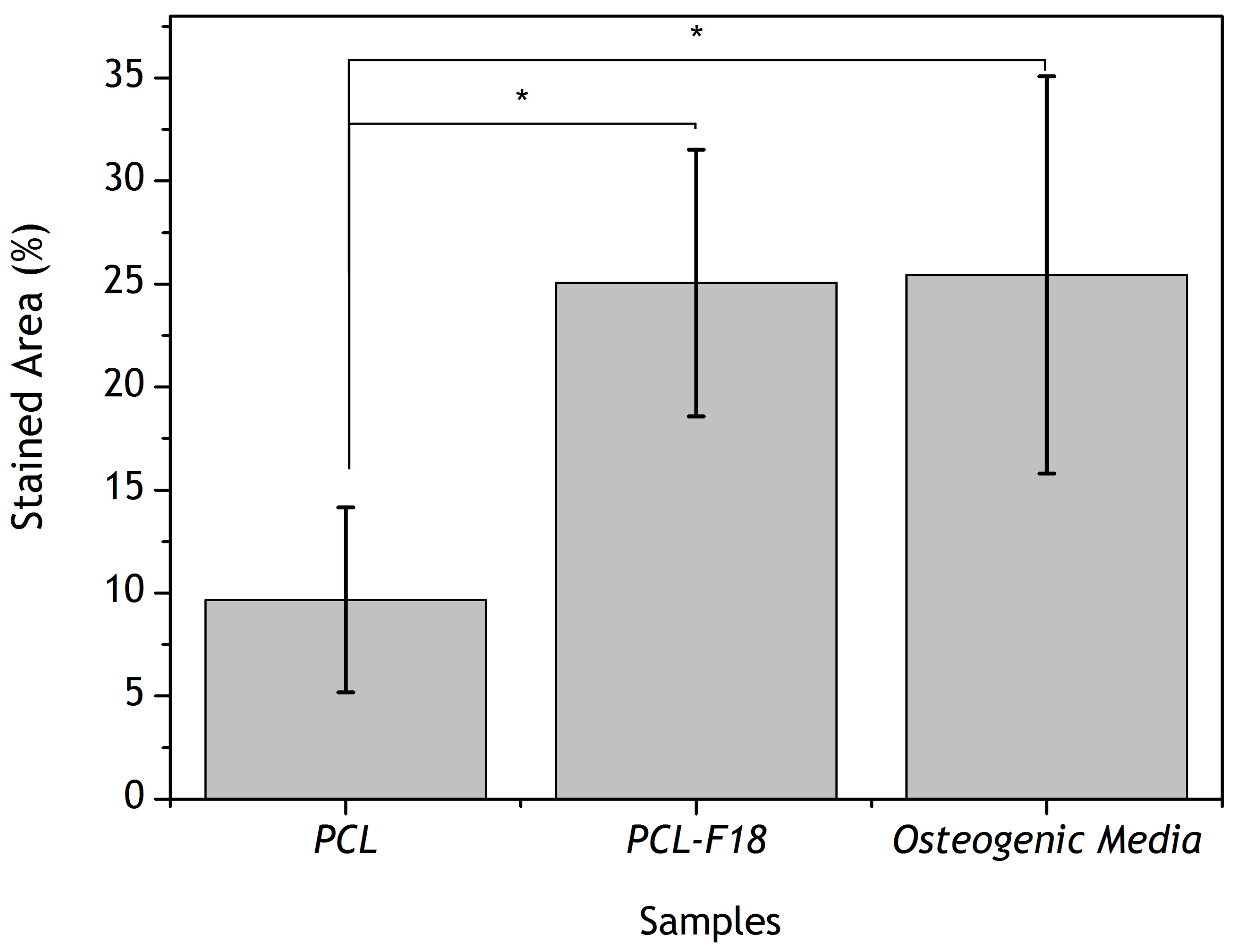
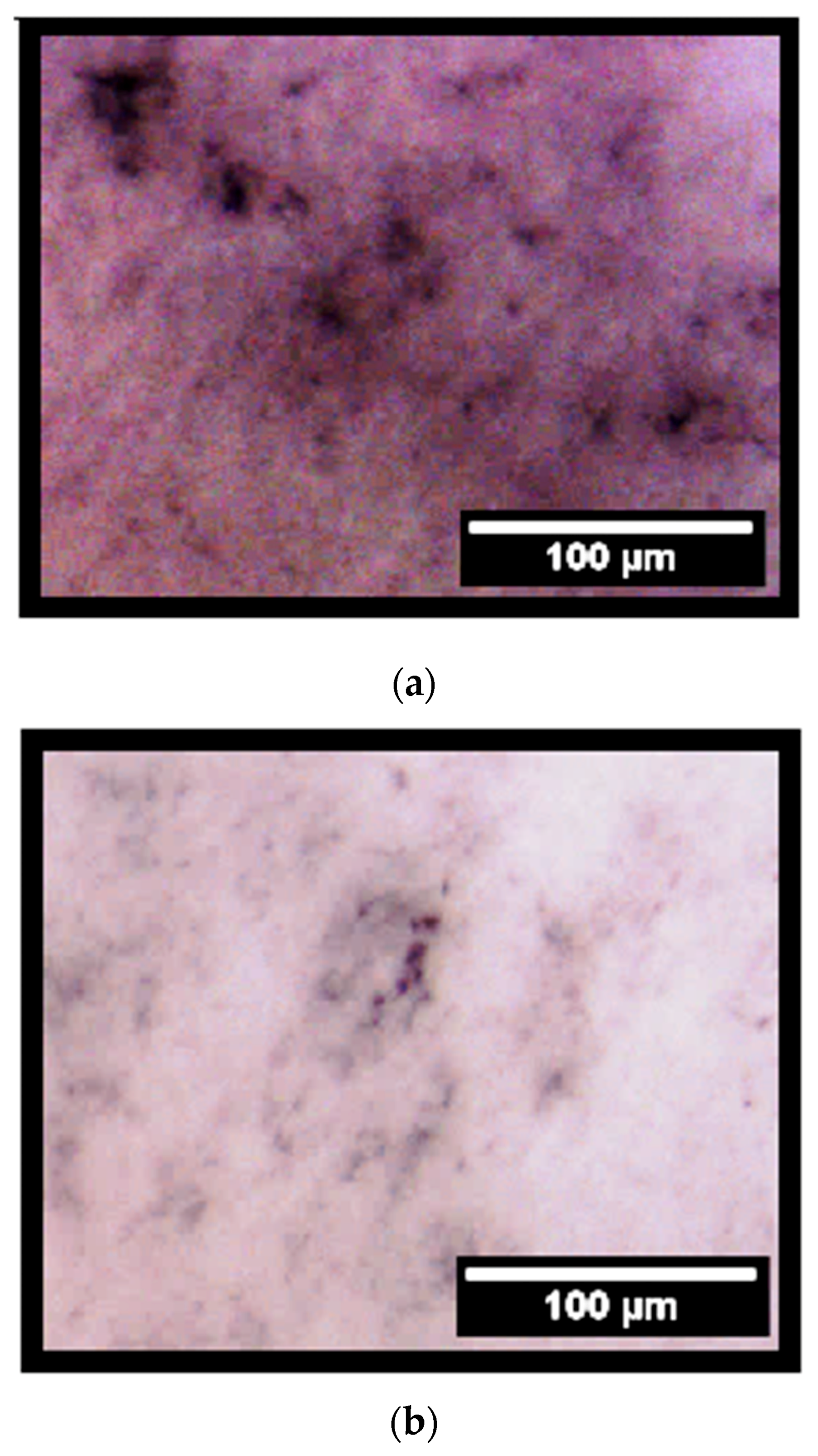
2.3. Effect of F18 Bioactive Glass on MG-63 Cells Osteogenesis
2.4. Mechanical Properties
3. Discussion
4. Materials and Methods
4.1. Bioactive Glass Manufacturing
4.2. Glass/Polymer Solution Preparation
4.3. Electrospinning Process
4.4. Cell Viability
4.5. Alkaline Phophatase Assay
4.6. Scanning Electronic Microscope (SEM) Imaging
4.7. Fibre Diameter Measurement
4.8. Mechanical Tests
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meyer, U. The History of Tissue Engineering and Regenerative Medicine in Perspective. In Fundamentals of Tissue Engineering and Regenerative Medicine; Springer: Berlin Heidelberg, Germany, 2009; pp. 5–12. [Google Scholar] [CrossRef]
- Mano, J.F.; Sousa, R.A.; Boesel, L.F.; Neves, N.M.; Reis, R.L. Bioinert, biodegradable and injectable polymeric matrix composites for hard tissue replacement: State of the art and recent developments. Compos. Sci. Technol. 2004, 64, 789–817. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, M.; Koyama, Y.; Takakuda, K.; Miyairi, H.; Shirahama, N.; Tanaka, J. In vitro change in mechanical strength of beta-tricalcium phosphate/copolymerized poly-l-lactide composites and their application for guided bone regeneration. J. Biomed. Mater. Res. Part A 2002, 62, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.I.; Caridade, S.G.; Ma, J.; Yu, N.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, X.F.; Mano, J.F. Asymmetric PDLLA membranes containing Bioglass® for guided tissue regeneration: Characterization and in vitro biological behavior. Dent. Mater. 2017, 29, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santocildes-Romero, M.E.; Goodchild, R.L.; Hatton, P.V.; Crawford, A.; Reaney, I.M.; Miller, C.A. Preparation of Composite Electrospun Membranes Containing Strontium-Substituted Bioactive Glasses for Bone Tissue Regeneration. Macromol. Mater. Eng. 2016, 301, 972–981. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C 2017, 78, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanism of Guided Bone Regeneration : A Review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Downes, S. Physicochemical characterisation of novel ultra-thin biodegradable scaffolds for peripheral nerve repair. J. Mater. Sci. Mater. Med. 2009, 20, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, Y.; Hillmyer, M.A.; Francis, L.F. Processing and properties of porous poly(l-lactide)/bioactive glass composites. Biomaterials 2004, 25, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, E.L.; Shih, C.K.; Lemoine, J.J.; Timmer, M.D.; Liebschner, M.A.; Jansen, J.A.; Mikos, A.G. In vitro degradation of porous poly(propylene fumarate)/poly(dl-lactic-co-glycolic acid) composite scaffolds. Biomaterials 2005, 26, 3215–3225. [Google Scholar] [CrossRef] [PubMed]
- Boccaccini, A.R.; Blaker, J.J.; Maquet, V.; Day, R.M.; Jéróme, R. Preparation and characterisation of poly(lactide-co-grycolide) (PLGA) and PLGA/Bioglass® composite tubular foam scaffolds for tissue engineering applications. Mater. Sci. Eng. C 2005, 25, 23–31. [Google Scholar] [CrossRef]
- Kim, H.W.; Knowles, J.C.; Kim, H.E. Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 2004, 25, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Meszaros, R.; Stähli, C.; Romeis, S.; Schmidt, J.; Peukert, W.; Marelli, B.; Nazhat, S.N.; Wondraczek, L.; Lao, J.; et al. In vitro reactivity of Cu doped 45S5 Bioglass® derived scaffolds for bone tissue engineering. J. Mater. Chem. B 2013, 1, 5659–5674. [Google Scholar] [CrossRef]
- Crovace, M.C.; Souza, M.T.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D. Biosilicate®—A multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J. Non-Cryst. Solids 2016, 432, 90–110. [Google Scholar] [CrossRef]
- Van Gestel, N.A.P.; Geurts, J.; Hulsen, D.J.W.; Van Rietbergen, B.; Hofmann, S.; Arts, J.J. Clinical Applications of S53P4 Bioactive Glass in Bone Healing and Osteomyelitic Treatment: A Literature Review. BioMed Res. Int. 2015, 2015, 684826. [Google Scholar] [CrossRef] [PubMed]
- McAndrew, J.; Efrimescu, C.; Sheehan, E.; Niall, D. Through the looking glass; Bioactive glass S53P4 (BonAlive®) in the treatment of chronic osteomyelitis. Ir. J. Med. Sci. 2013, 182, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Hulsen, D.J.; van Gestel, N.A.; Geurts, J.A.P.; Arts, J.J. 4-S53P4 bioactive glass. In Management of Periprosthetic Joint Infections (PJIs); Woodhead Publishing: Cambridge, UK, 2017; pp. 69–80. ISBN 9780081002056. [Google Scholar]
- Souza, M.T.; Rennó, A.C.M.; Peitl, O.; Zanotto, E.D. New highly bioactive crystallization-resistant glass for tissue engineering applications. Trans. Mater. Res. 2017, 4, 14002. [Google Scholar] [CrossRef]
- Gabbai-Armelin, P.R.; Souza, M.T.; Kido, H.W.; Tim, C.R.; Bossini, P.S.; Magri, A.M.P.; Fernandes, K.R.; Pastor, F.A.C.; Zanotto, E.D.; Parizotto, N.A.; et al. Effect of a new bioactive fibrous glassy scaffold on bone repair. J. Mater. Sci. Mater. Med. 2015, 26, 177. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.T.; Peitl, O.; Zanotto, E.D.; Boccaccini, A.R. Novel Double-Layered Conduit Containing Highly Bioactive Glass Fibers for Potential Nerve Guide Application. Int. J. Appl. Glass Sci. 2016, 7, 183–194. [Google Scholar] [CrossRef]
- Souza, M.T.; Campanini, L.A.; Chinaglia, C.R.; Peitl, O.; Zanotto, E.D.; Souza, C.W.O. Broad-Spectrum Bactericidal Activity of a New Bioactive Grafting Material (F18) Against Clinically Important Bacterial Strains. Int. J. Antimicrob. Agents 2017, 50, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.C.; Dey, M.; Dutta, A.K.; Basu, B. Competent processing techniques for scaffolds in tissue engineering. Biotechnol. Adv. 2017, 35, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Nyman, S. Bone regeneration using the principle of guided tissue regeneration. J. Clin. Periodontol. 1991, 18, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.; Tansaz, S.; Zanotto, E.; Boccaccini, A. Bioactive Glass Fiber-Reinforced PGS Matrix Composites for Cartilage Regeneration. Materials 2017, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Yu, N.; Caridade, S.G.; Luz, G.M.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Frank Walboomers, X.; Mano, J.F. Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 2012, 8, 4173–4180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.; Blackwood, K.A.; Doustgani, A.; Poh, P.P.; Steck, R.; Stevens, M.M.; Woodruff, M.A. Melt-electrospun polycaprolactone strontium-substituted bioactive glass scaffolds for bone regeneration. J. Biomed. Mater. Res. Part A 2014, 102, 3140–3153. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.S.; Gentile, P.; Pires, R.A.; Reis, R.L.; Hatton, P.V. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Both, S.K.; Yang, X.; Walboomers, X.F.; Jansen, J.A. Development of an electrospun nano-apatite/PCL composite membrane for GTR/GBR application. Acta Biomater. 2009, 5, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, P.; Cannillo, V.; Sola, A.; Dorigato, A.; Chiellini, F. Highly porous polycaprolactone-45S5 Bioglass® scaffolds for bone tissue engineering. Compos. Sci. Technol. 2010, 70, 1869–1878. [Google Scholar] [CrossRef]
- Tamjid, E.; Bagheri, R.; Vossoughi, M.; Simchi, A. Effect of particle size on the in vitro bioactivity, hydrophilicity and mechanical properties of bioactive glass-reinforced polycaprolactone composites. Mater. Sci. Eng. C 2011, 31, 1526–1533. [Google Scholar] [CrossRef]
- Poh, P.S.P.; Hutmacher, D.W.; Stevens, M.M.; Woodruff, M. A Fabrication and in vitro characterization of bioactive glass composite scaffolds for bone regeneration. Biofabrication 2013, 5, 45005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Y.; Du, Y.; Chen, X.; Lei, B.; Xue, Y.; Ma, P.X. A highly bioactive and biodegradable poly(glycerol sebacate)-silica glass hybrid elastomer with tailored mechanical properties for bone tissue regeneration. J. Mater. Chem. B 2015, 3, 3222–3233. [Google Scholar] [CrossRef]
- Rowe, M.J.; Kamocki, K.; Pankajakshan, D.; Li, D.; Bruzzaniti, A.; Thomas, V.; Blanchard, S.B.; Bottino, M.C. Dimensionally stable and bioactive membrane for guided bone regeneration: An in vitro study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, Y.; Yu, S.; Yao, Q.; Boccaccini, A.R. Multifunctional Chitosan-45S5 Bioactive Glass-Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Microsphere Composite Membranes for Guided Tissue/Bone Regeneration. ACS Appl. Mater. Interfaces 2015, 7, 20845–20854. [Google Scholar] [CrossRef] [PubMed]
- Kaw, A.K. Mechanics of Composite Materials; CRC Press: Boca Raton, FL, USA, 2006; Volume 29, ISBN 9780849313431. [Google Scholar]
- Li, J.; Zuo, Y.; Cheng, X.; Yang, W.; Wang, H.; Li, Y. Preparation and characterization of nano-hydroxyapatite/polyamide 66 composite GBR membrane with asymmetric porous structure. J. Mater. Sci. Mater. Med. 2009, 20, 1031–1038. [Google Scholar] [CrossRef] [PubMed]



| Sample | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|
| PCL | 3.7 ± 0.5 | 230.0 ± 39.1 |
| PCL-F18 | 4.8 ± 0.9 | 170.4 ± 34.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo Pitaluga, L.; Trevelin Souza, M.; Dutra Zanotto, E.; Santocildes Romero, M.E.; Hatton, P.V. Electrospun F18 Bioactive Glass/PCL—Poly (ε-caprolactone)—Membrane for Guided Tissue Regeneration. Materials 2018, 11, 400. https://doi.org/10.3390/ma11030400
Hidalgo Pitaluga L, Trevelin Souza M, Dutra Zanotto E, Santocildes Romero ME, Hatton PV. Electrospun F18 Bioactive Glass/PCL—Poly (ε-caprolactone)—Membrane for Guided Tissue Regeneration. Materials. 2018; 11(3):400. https://doi.org/10.3390/ma11030400
Chicago/Turabian StyleHidalgo Pitaluga, Lucas, Marina Trevelin Souza, Edgar Dutra Zanotto, Martin Eduardo Santocildes Romero, and Paul V. Hatton. 2018. "Electrospun F18 Bioactive Glass/PCL—Poly (ε-caprolactone)—Membrane for Guided Tissue Regeneration" Materials 11, no. 3: 400. https://doi.org/10.3390/ma11030400





