Influence of Particle Size on Compressive Strength of Alkali Activated Refractory Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of Precursor and Its Alkali Activated Counterpart
2.2. Preparation of Precursors, Activator and Alkali Activated Samples
3. Results and Discussion
3.1. Analysis of Precursor
3.2. Analysis of Alkali Activated Samples Prepared from Different Precursor’s Fractions
3.3. Analysis of Alkali Activated Sample Cured at Different Temperatures
3.4. Proposed Mechanism behind AAM Structure from Different Precursor’s Fractions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Škvára, F. Alkali Activated Material-Geopolymer; ICT Prague: Prague, Czech Republic, 2007; p. 16. [Google Scholar]
- Glasby, T.; Day, J.; Genrich, R.; Aldred, D.J. EFC Geopolymer Concrete Aircraft Pavements at Brisbane West Wellcamp Airport. Concrete 2015, 2015, 9. [Google Scholar]
- Sanjayan, J.G.; Nazari, A.; Chen, L.; Nguyen, G.H. Physical and mechanical properties of lightweight aerated geopolymer. Constr. Build. Mater. 2015, 79, 236–244. [Google Scholar] [CrossRef]
- Ducman, V.; Kramar, S.; Šajna, A. Alkali activated repair mortars based on different precursors. In Eco-Efficient Repair and Rehabilitation of Concrete Infrastructures; Elsevier: Duxford, UK, 2018; pp. 263–292. ISBN 978-0-08-102181-1. [Google Scholar]
- Kargin, A.; Baev, V.; Mashkin, N. Fly-Ash Geo-Polymer Foamed Concrete. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; p. 020005. [Google Scholar] [CrossRef]
- Ahmari, S.; Allahverdi, A.; Alonso, M.M.; Baklouti, S.; Balomenos, E.; Barbieri, L.; Barroso de Aguiar, J.; Baščarević, Z.; Bernal, S.A.; Borrachero, M.V.; et al. Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-1-78242-276-1. [Google Scholar]
- Fernandez-Jimenez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Al Bakri, A.M.M.; Kamarudin, H.; Binhussain, M.; Khairul Nizar, I.; Rafiza, A.R.; Zarina, Y. Comparison of geopolymer fly ash and ordinary portland cement to the strength of concret. Adv. Sci. Lett. 2013, 19, 3592–3595. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Shi, C.; Qian, J. High performance cementing materials from industrial slags—A review. Resour. Conserv. Recycl. 2000, 29, 195–207. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Pacheco-Torgal, F.; Félix, T.; Tahri, W.; Barroso Aguiar, J. Mix design, properties and cost analysis of fly ash-based geopolymer foam. Constr. Build. Mater. 2015, 80, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Furlani, E.; Maschio, S.; Magnan, M.; Aneggi, E.; Andreatta, F.; Lekka, M.; Lanzutti, A.; Fedrizzi, L. Synthesis and characterization of geopolymers containing blends of unprocessed steel slag and metakaolin: The role of slag particle size. Ceram. Int. 2018, 44, 5226–5232. [Google Scholar] [CrossRef]
- Isabella, C.; Lukey, G.C.; Xu, H. The Effect of Aggregate Particle Size on Formation of Geopolymeric Gel. In Engineering Conferences International; ECI Digital Archives: New York, NY, USA, 2003. [Google Scholar]
- Traven, K.; Češnovar, M.; Ducman, V. Particle size manipulation as an influential parameter in the development of mechanical properties in electric arc furnace slag-based AAM. Ceram. Int. 2019, 45, 22632–22641. [Google Scholar] [CrossRef]
- Petrakis, E.; Karmali, V.; Bartzas, G.; Komnitsas, K. Grinding Kinetics of Slag and Effect of Final Particle Size on the Compressive Strength of Alkali Activated Materials. Minerals 2019, 9, 714. [Google Scholar] [CrossRef] [Green Version]
- Petrakis, E.; Karmali, V.; Komnitsas, K. Effect of Particle Size on Alkali-Activation of Slag. Int. J. Mater. Metall. Eng. 2019, 13, 4. [Google Scholar]
- Assi, L.N.; Eddie Deaver, E.; Ziehl, P. Effect of source and particle size distribution on the mechanical and microstructural properties of fly Ash-Based geopolymer concrete. Constr. Build. Mater. 2018, 167, 372–380. [Google Scholar] [CrossRef]
- Azevedo, A.G.S.; Strecker, K.; Barros, L.A.; Tonholo, L.F.; Lombardi, C.T. Effect of Curing Temperature, Activator Solution Composition and Particle Size in Brazilian Fly-Ash Based Geopolymer Production. Mater. Res. 2019, 22. [Google Scholar] [CrossRef] [Green Version]
- Wiyono, D.; Vianthi, A.; Putra, P.; Kartadinata, G.; Hardjito, D. Effect of Particle Size on Properties of Sidoarjo Mud-Based Geopolymer. Mater. Sci. Forum 2014, 803, 44–48. [Google Scholar] [CrossRef]
- Beghoura, I.; Castro-Gomes, J. Design of alkali-activated aluminium powder foamed materials for precursors with different particle sizes. Constr. Build. Mater. 2019, 224, 682–690. [Google Scholar] [CrossRef]
- Szabó, R.; Gombkötő, I.; Svéda, M.; Mucsi, G. Effect of Grinding Fineness of Fly Ash on the Properties of Geopolymer Foam. Arch. Metall. Mater. 2017, 62, 1257–1261. [Google Scholar] [CrossRef] [Green Version]
- Type of Monolithic Refractories. Available online: http://www.idc-online.com/technical_references/pdfs/mechanical_engineering/Type_of_Monolithic_Refractories.pdf (accessed on 26 February 2020).
- Guo, J.-W.; Lin, Z.-Y.; Huang, B.-R.; Lu, C.-H.; Chen, J.-K. Antigen detection with thermosensitive hydrophilicity of poly(N -isopropylacrylamide)-grafted poly(vinyl chloride) fibrous mats. J. Mater. Chem. B 2018, 6, 3486–3496. [Google Scholar] [CrossRef] [PubMed]
- Polyvinyl Chloride PVC. Available online: https://www.bpf.co.uk/plastipedia/polymers/pvc.aspx (accessed on 5 March 2020).
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Horvat, B.; Ducman, V. Potential of Green Ceramics Waste for Alkali Activated Foams. Materials 2019, 30, 3563. [Google Scholar] [CrossRef] [PubMed]
- Eremenko, B.V.; Shumovskaya, E.F. Reactions of aqueous silicon carbide suspensions with an acid and an alkali. Sov. Powder Metall. Metal Ceram. 1972, 11, 815–818. [Google Scholar] [CrossRef]




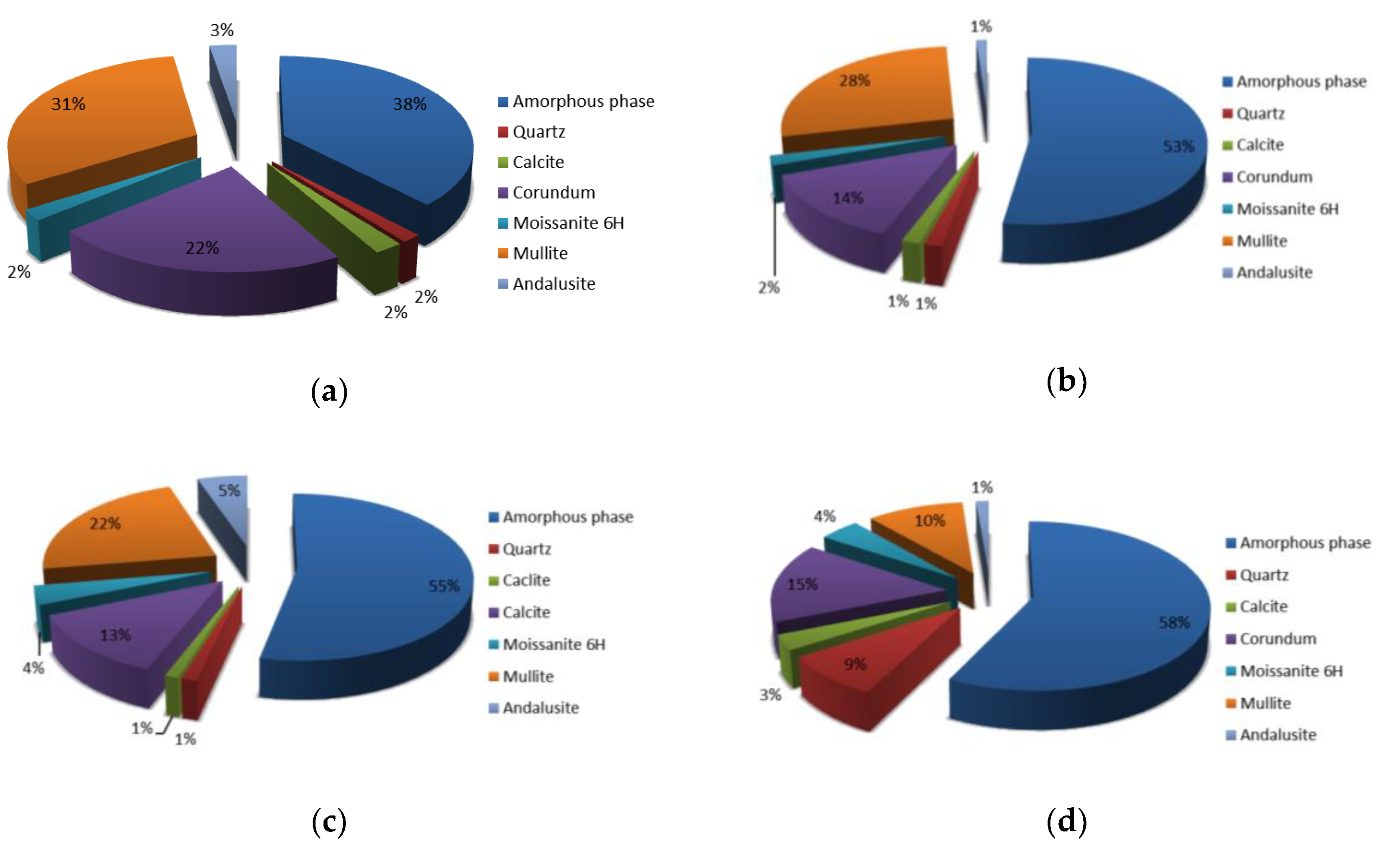

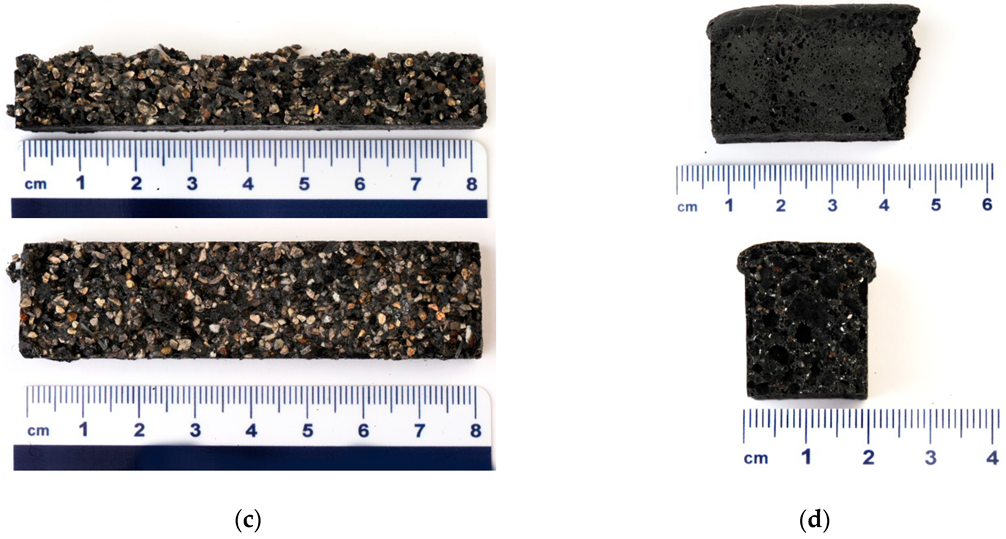


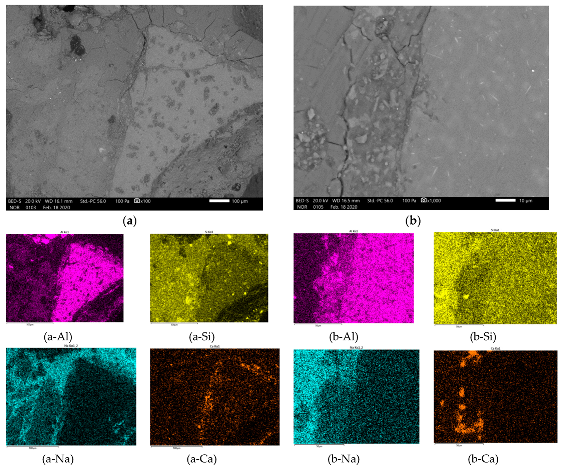

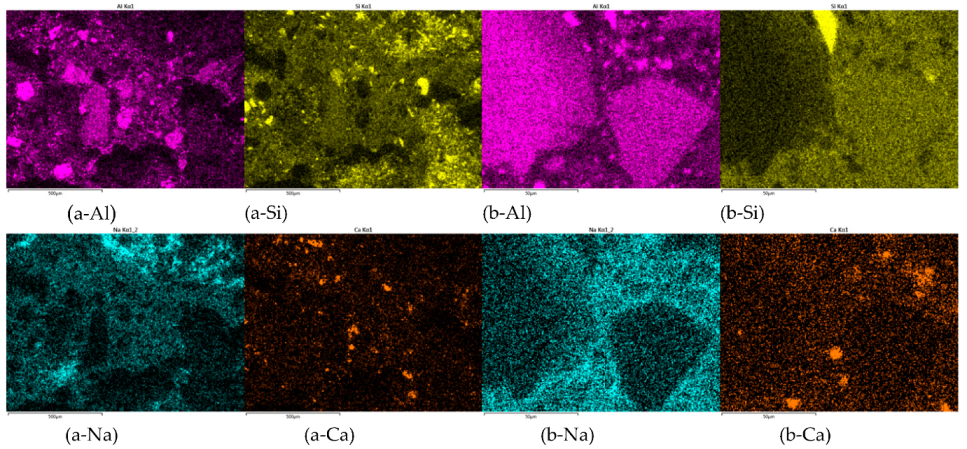




| Precursor’s Fraction | Elements [m%] | Na | K | Cs | Mg | Ca | Sr | Ba | Al | Si |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 mm < x | XRF | 0.20 | 0.21 | 0 | 0.62 | 2.19 | 0.04 | 0.20 | 32.71 | 12.61 |
| XRD | 0 | 0 | 0 | 0 | 0.88 | 0 | 0 | 22.59 | 5.56 | |
| XRF-XRD | 0.20 | 0.21 | 0 | 0.62 | 1.31 | 0.04 | 0.20 | 10.12 | 7.05 | |
| 2 mm < x ≤ 4 mm | XRF | 0.25 | 0.27 | 0 | 0.45 | 1.69 | 0.03 | 0.10 | 30.46 | 15.27 |
| XRD | 0 | 0 | 0 | 0 | 0.60 | 0 | 0 | 18.44 | 5.50 | |
| XRF-XRD | 0.25 | 0.27 | 0 | 0.45 | 1.09 | 0.03 | 0.10 | 12.02 | 9.77 | |
| 1 mm < x ≤ 2 mm | XRF | 0.24 | 0.29 | 0 | 0.40 | 2.04 | 0.03 | 0.13 | 30.05 | 16.83 |
| XRD | 0 | 0 | 0 | 0 | 0.44 | 0 | 0 | 17.56 | 6.56 | |
| XRF-XRD | 0.24 | 0.29 | 0 | 0.40 | 1.60 | 0.03 | 0.13 | 12.49 | 10.27 | |
| x ≤ 1 mm | XRF | 0.48 | 0.24 | 0 | 0.46 | 2.63 | 0.03 | 0.22 | 25.79 | 20.01 |
| XRD | 0 | 0 | 0 | 0 | 1.20 | 0 | 0 | 12.36 | 8.42 | |
| XRF-XRD | 0.48 | 0.24 | 0 | 0.46 | 1.43 | 0.03 | 0.22 | 13.43 | 11.59 |
| Precursor’s Fraction | Compressive Strength [MPa] | Bending Strength [MPa] | Density [kg/L] |
|---|---|---|---|
| 4 mm < x | 3.9 | 2.2 | 1.2 |
| 2 mm < x ≤ 4 mm | 14.1 | 5.3 | 1.3 |
| 1 mm < x ≤ 2 mm | 21.3 | 8.6 | 1.5 |
| x ≤ 1 mm | 6.4 | 3.5 | 0.9 |
| Curing Temperature for 7 Days | Compressive Strength after 7 Days [MPa] | Compressive Strength after 28 Days [MPa] | Bending Strength after 7 Days [MPa] | Bending Strength after 28 Days [MPa] |
|---|---|---|---|---|
| Room conditions | 0 | 1.8 | 0 | 1.9 |
| 40 °C | 2.4 | 4.1 | 0.05 | 3.6 |
| 70 °C | 17.4 | 20.4 | 7.6 | 9.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvat, B.; Ducman, V. Influence of Particle Size on Compressive Strength of Alkali Activated Refractory Materials. Materials 2020, 13, 2227. https://doi.org/10.3390/ma13102227
Horvat B, Ducman V. Influence of Particle Size on Compressive Strength of Alkali Activated Refractory Materials. Materials. 2020; 13(10):2227. https://doi.org/10.3390/ma13102227
Chicago/Turabian StyleHorvat, Barbara, and Vilma Ducman. 2020. "Influence of Particle Size on Compressive Strength of Alkali Activated Refractory Materials" Materials 13, no. 10: 2227. https://doi.org/10.3390/ma13102227





