Compostability of Co-Extruded Starch/Poly(Lactic Acid) Polymeric Material Degradation in an Activated Inert Solid Medium
Abstract
:1. Introduction
2. Experimental Section
2.1. Extruded Material
| Proportion (in weight) | Thickness (mm) | Humidity percentage | Elementary analysis (%) | |||
|---|---|---|---|---|---|---|
| Carbon | Hydrogen | Nitrogen | ||||
| A (PLA) | 19.4 ± 0.3 | 0.66 ± 0.05 | 5.4 ± 0.2 | 48.96 ± 0.10 | 5.00 ± 0.06 | 0.20 ± 0.002 |
| B (starch) | 80.6 ± 0.5 | 0.87 ± 0.06 | 10.5 ± 0.3 | 38.57 ± 0.15 | 6.74 ± 0.04 | 0.10 ± 0.01 |
2.2. Equipment
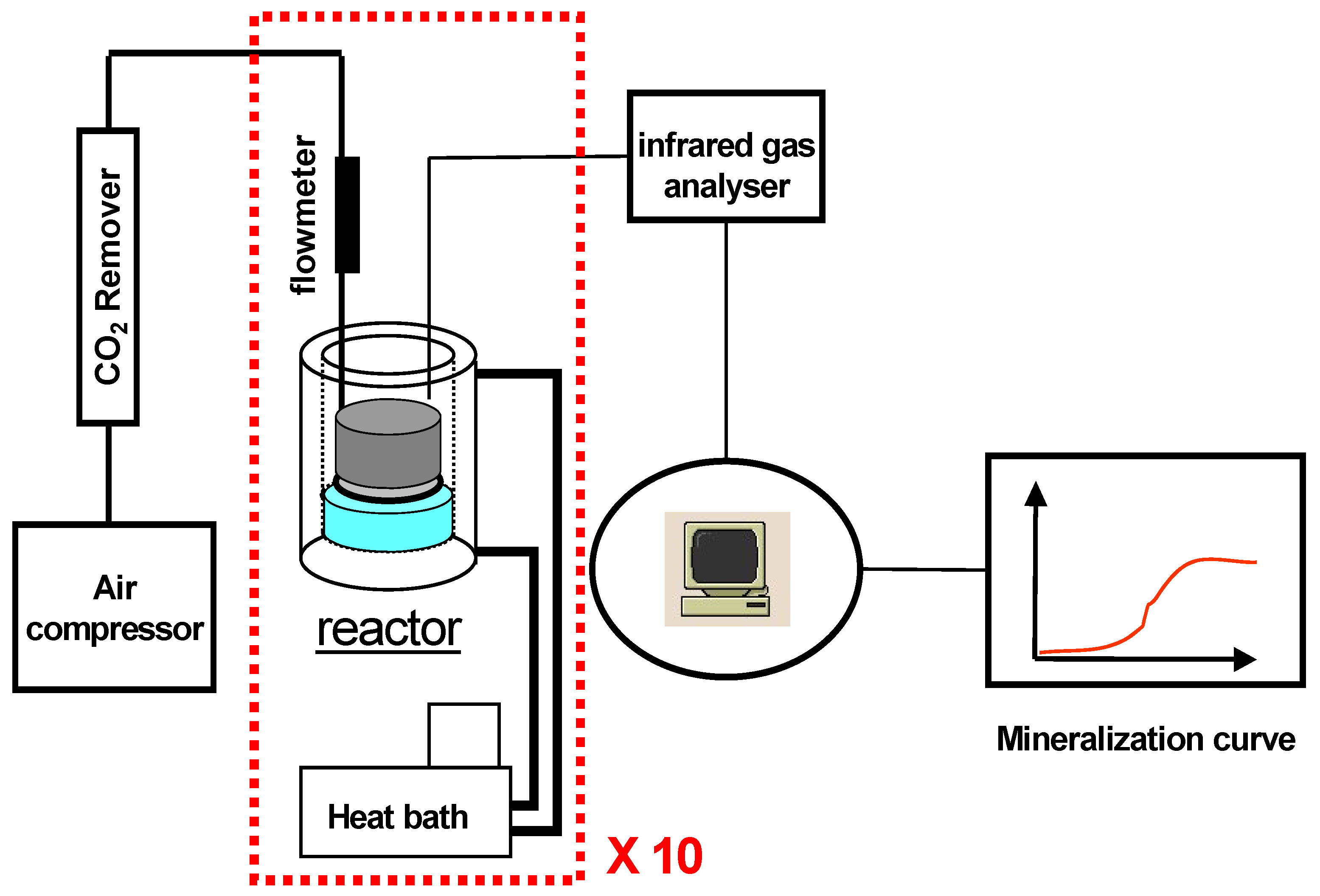
2.3. Degradation parameters
2.3.1. Compost test set-up
2.3.2. Inert solid medium tests set-up
2.3.2.1. Inoculum
2.3.2.2. Non activated medium tests
| Mineral salts | Composition for 5 L (g) |
|---|---|
| CaCl2,2H2O | 0.6500 |
| Na2HPO4,2H2O | 34.8500 |
| KH2PO4 | 18.7500 |
| (NH4)2SO4 | 20.0000 |
| MgSO4,7H2O | 1.0000 |
| FeSO4,7H2O | 0.0135 |
| MnSO4,7H2O | 0.0050 |
| ZnSO4,7H2O | 0.0050 |
| H3BO3 | 0.0050 |
| KI | 0.0050 |
| (NH4)6Mo7O2,4H2O | 0.0050 |
2.3.2.3. Activated medium tests
| Compound | Weight (g) |
|---|---|
| Wheat Starch | 1.5 |
| Cellulose | 1.5 |
| Nutrient Broth | 1.0 |
| Urea | 0.4 |
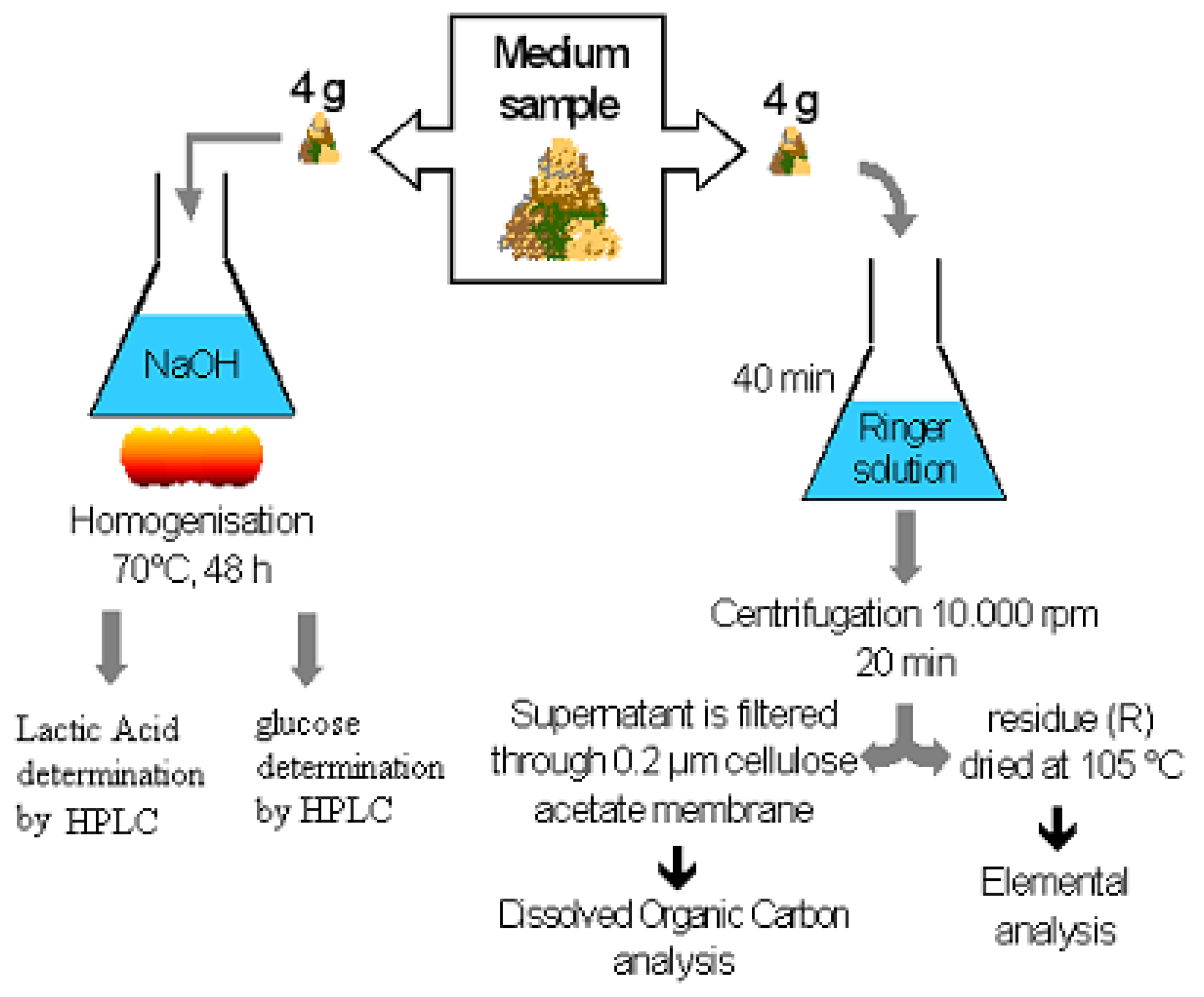
2.4. Sampling
2.5. Samples analysis
| Mineral salts | Composition for 1 L (g) |
|---|---|
| NaCl | 9 |
| KCl | 0.42 |
| CaCl | 0.48 |
| NaHCO3 | 0.2 |
2.6. Carbon balance
2.7. Extraction and analysis of remaining PLA material
2.7.1. Physico-chemical analysis of remaining PLA material
2.7.1.1. Molecular Weight Distribution of PLA Films
2.7.1.2. Differential scanning calorimetry analysis
3. Results and Discussion
3.1. Mineralization rate
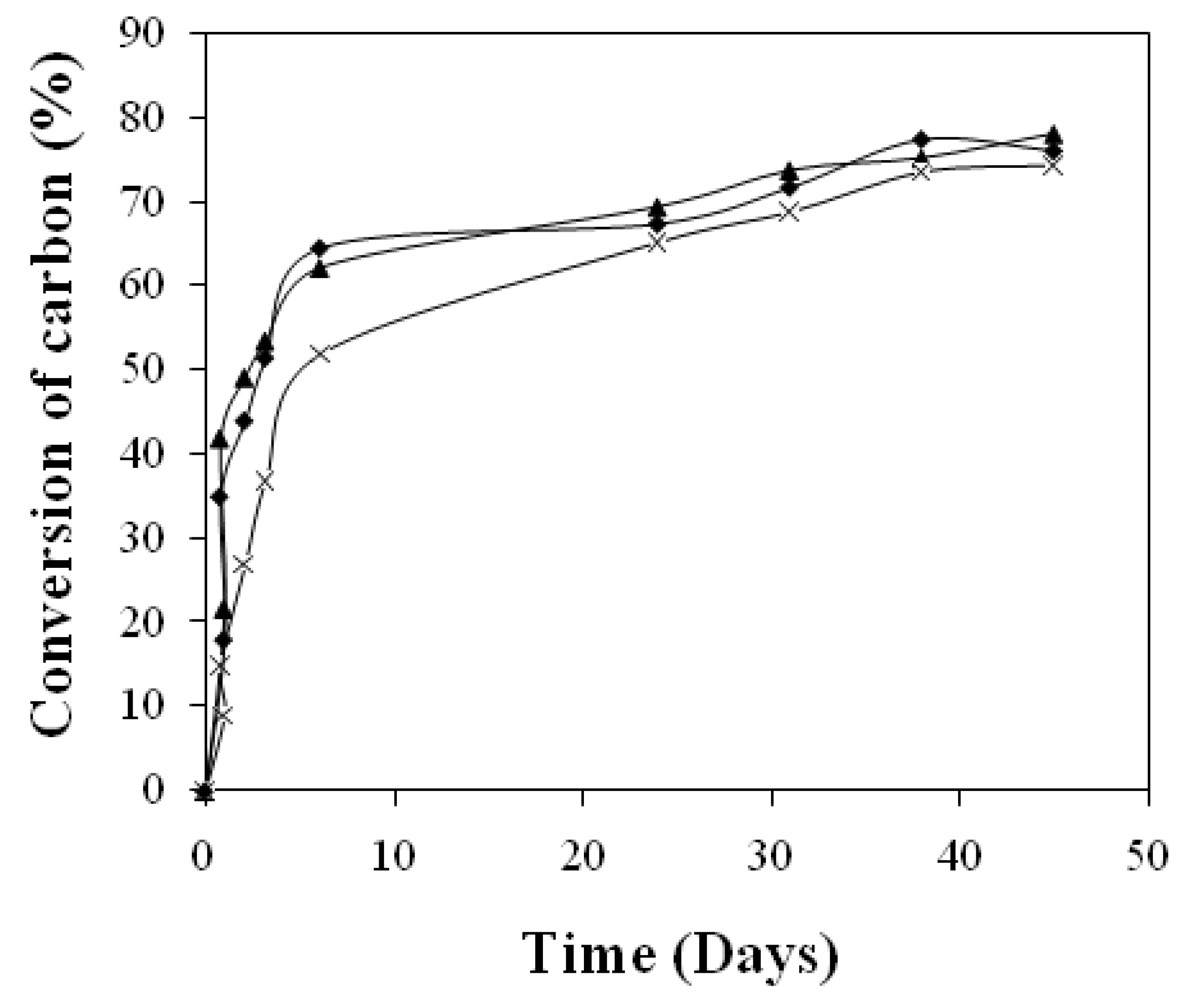
3.2. Carbon balance
3.2.1. Activated vermiculite medium

3.2.2. Non activated Vermiculite medium
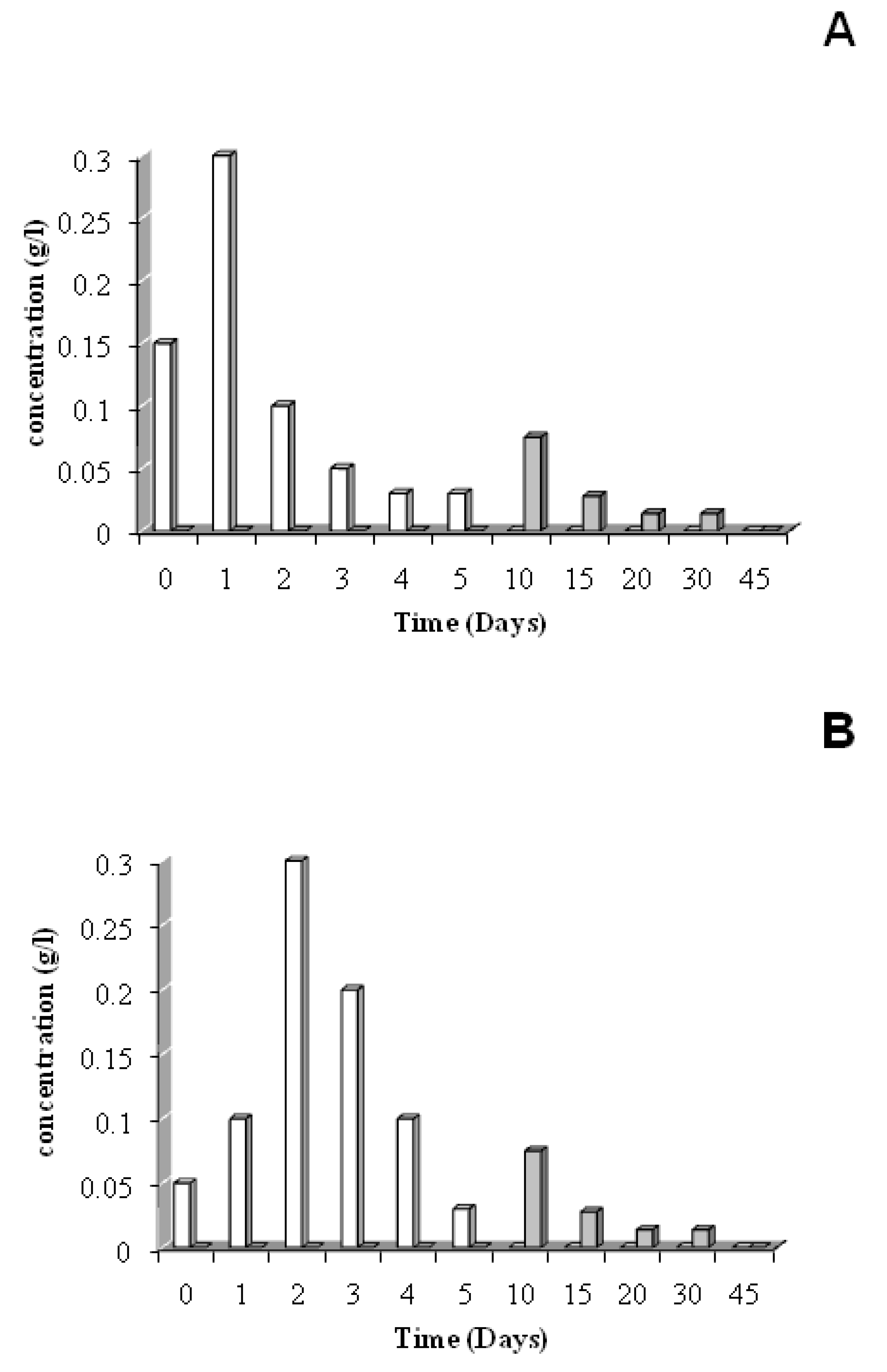
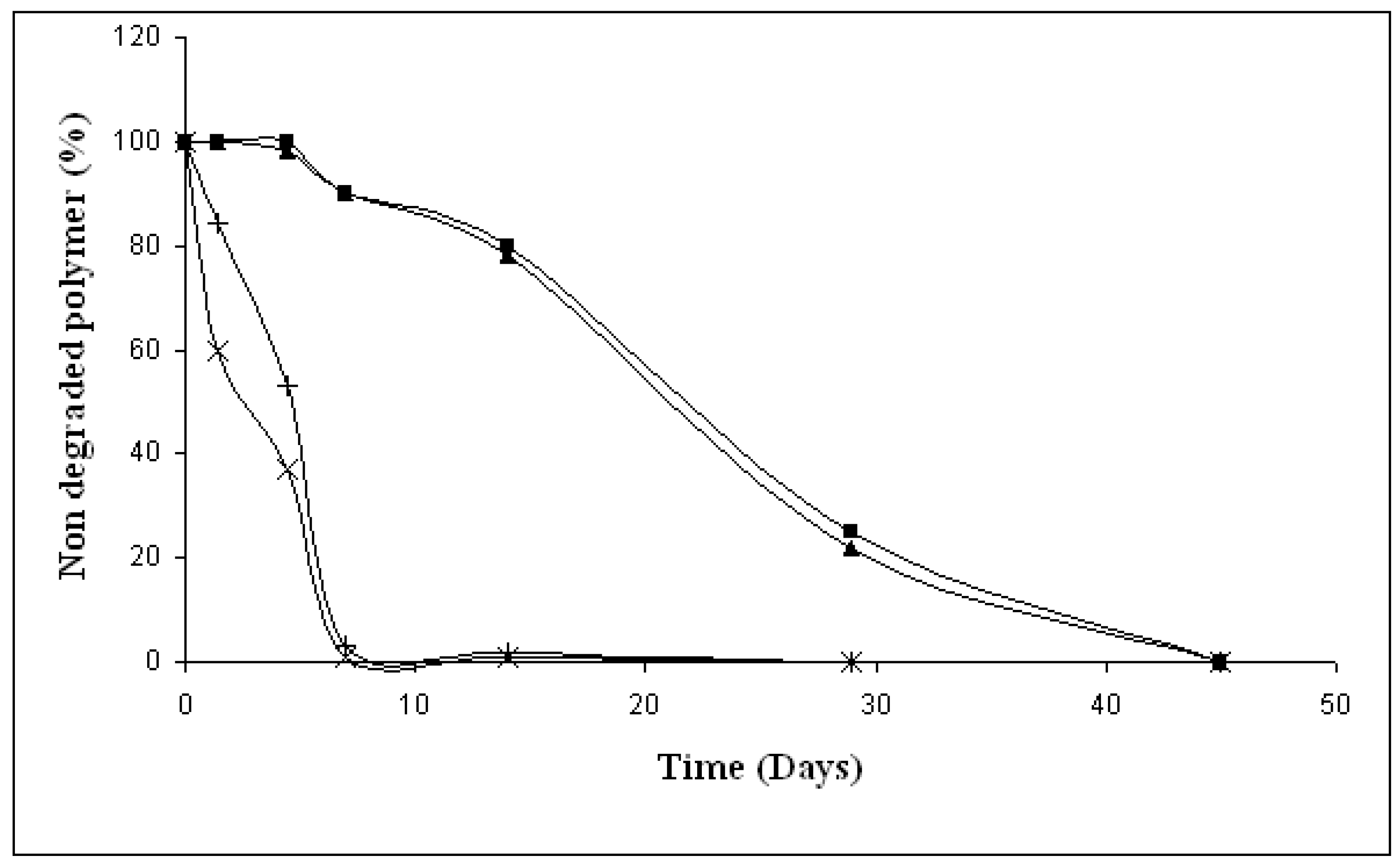
3.3. Residual material studies
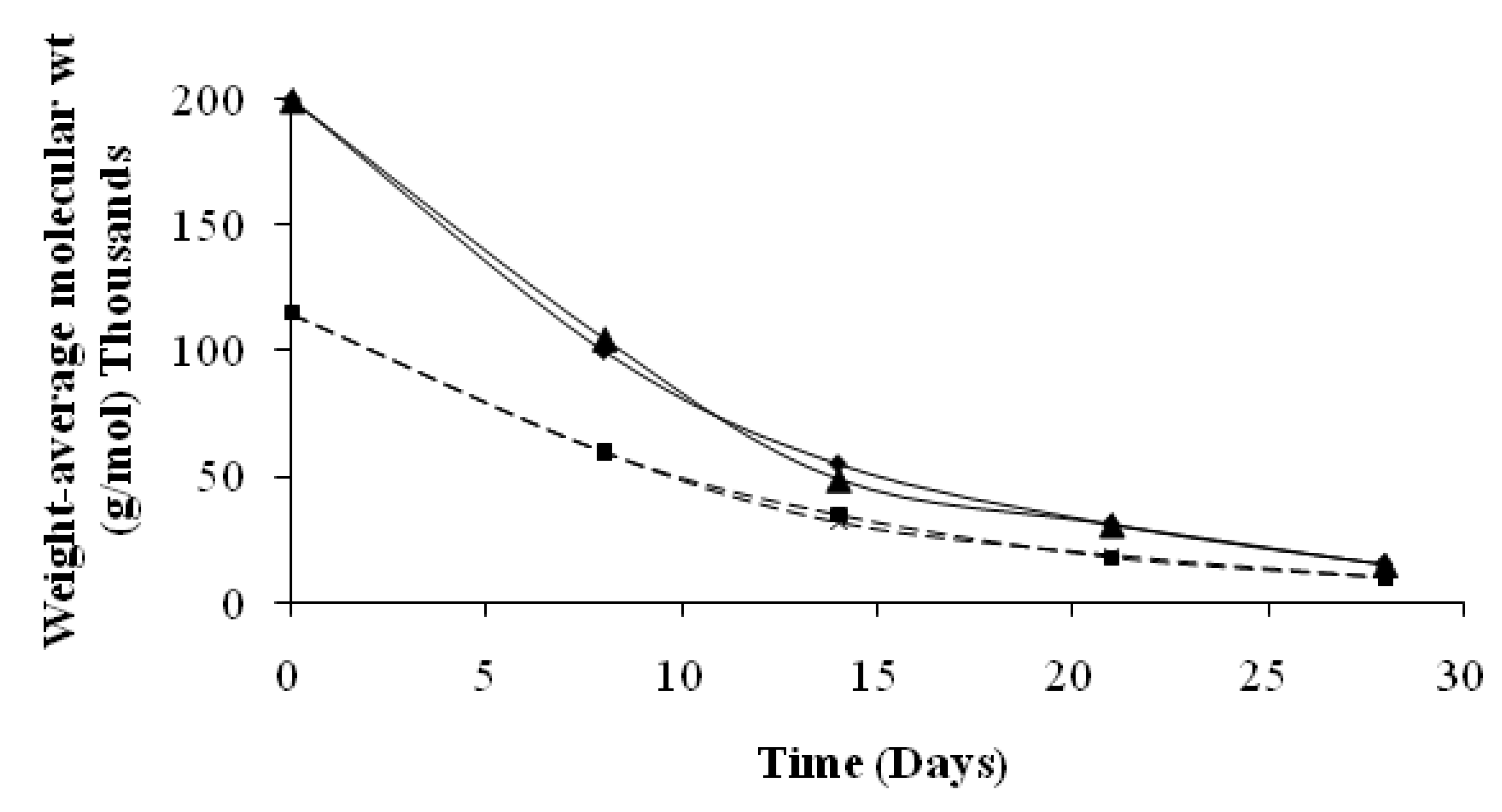
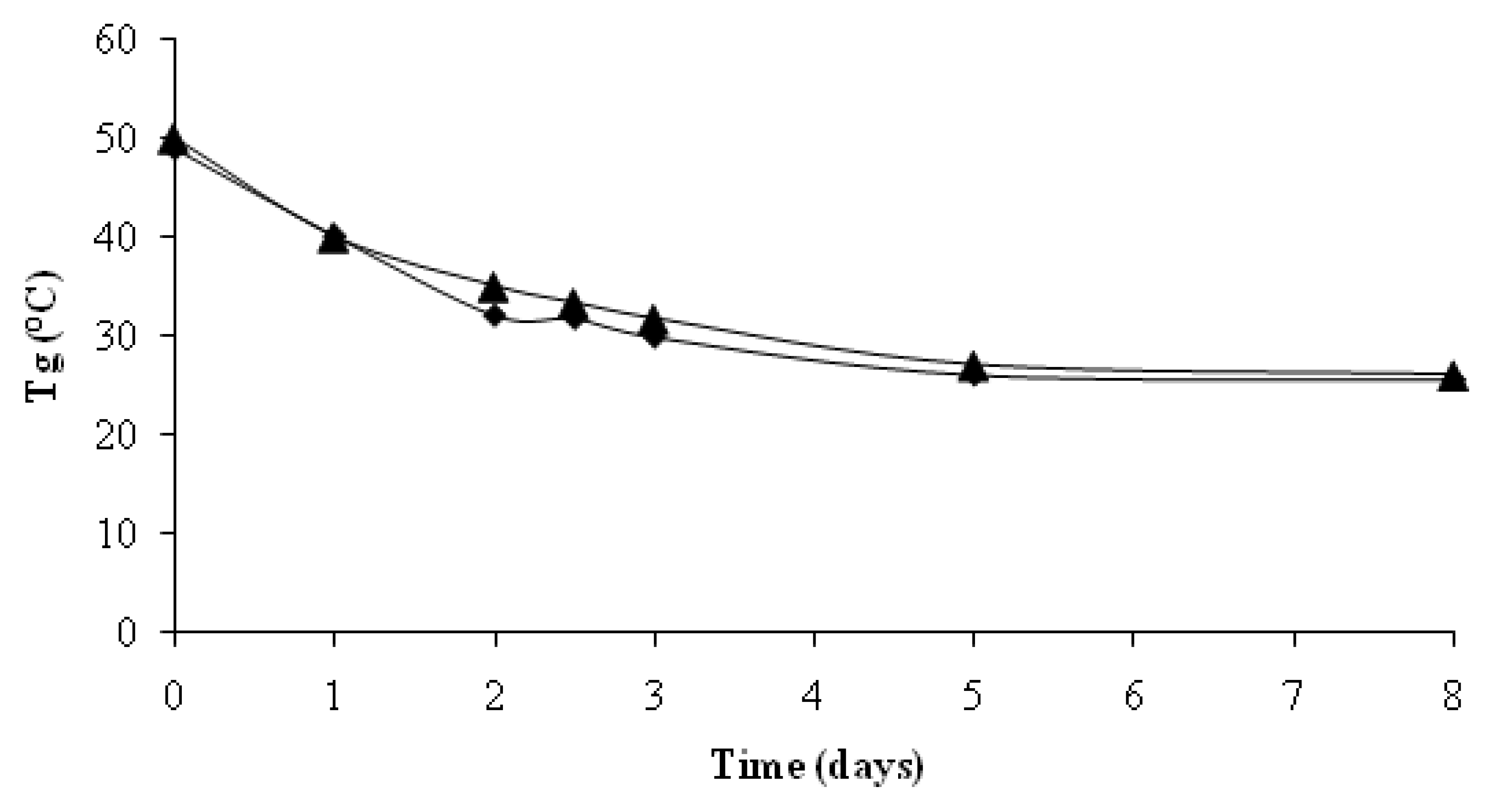
4. Conclusions
References and Notes
- Tosin, M.; Degli-Innocenti, F.; Bastioli, C. Effects of the composting substrate on biodegradation of solid materials under controlled composting conditions. J. Environ. Polym. Degrad 1996, 4, 55–63. [Google Scholar]
- Shen, J.; Bartha, J.R. Priming effect of subsrate addition in soil-based biodegradation tests. Appl. Environ. Microbiol. 1996, 62, 14–28. [Google Scholar]
- Bellia, G.; Tosin, G.; Floridi, M.; Degli-innocenti, F. Activated vermiculite, a solid bed for testing biodegradability under composting conditions. Polym. Degrad. Stab 1999, 66, 65–79. [Google Scholar] [CrossRef]
- Longieras, A.; Copinet, A.; Bureau, G.; Tighzert, L. An inert solid medium for simulation of material biodegradation in compost and achievement of carbon balance. Polym. Degrad. Stab 2004, 83, 187–194. [Google Scholar] [CrossRef]
- Copinet, A.; Coma, V.; Onteniente, J.P.; Couturier, Y. Enzymatic degradation of native and acetylated starch based extruded blends. Packag. Technol. Sci. 1998, 11, 69–74. [Google Scholar] [CrossRef]
- Gattin, R.; Poulet, C.; Copinet, A.; Couturier, Y. Comparison of mineralization of starch in liquid, inert solid and compost media according to ASTM and CEN norms for the composting of packaging materials. Biotech. Lett. 2000, 22, 1471–1475. [Google Scholar] [CrossRef]
- Gattin, R.; Copinet, A.; Bertrand, C.; Couturier, Y. Biodegradation study of a starch and poly(lactic acid) co-extruded material in liquid, composting and inert mineral media. J. Polym. Environ. 2001, 9, 11–17. [Google Scholar] [CrossRef]
- Gattin, R.; copinet, A.; Bertrand, C.; Couturier, Y. Biodegradation study of a starch and poly(lactic acid) co-extruded material in various media. J. Appl. Polym. Sci. 2003, 88, 825–831. [Google Scholar] [CrossRef]
- Longieras, A.; Copinet, A.; Bureau, G.; Tighzert, L. An inert solid medium for simulation of material biodegradation in compost and achievement of carbon balance. Polym. Degrad. 2004, 83, 187–194. [Google Scholar] [CrossRef]
- Li, S.M.; Garreau, H.; Vert, M. Structure property relationships in the case of degradation of massive poly (α-hydroxy acids) in aqueous media: Part 1: Poly (DL-lactid). J. Mater. Sci. – Mater. Med. 1990, 1, 198–206. [Google Scholar] [CrossRef]
- Torres, A.; Li, S.M.; Roussos, S.; Vert, M. Degradation of L-lactic acid and dl-lactic acid oligomers in the presence of fusarium moniliforme and pseudomonas putida. J. Appl. Polym. Sci. 1996, 62, 213–223. [Google Scholar]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly(DL-lactic acid) size dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Vert, M. Biodegradation of aliphatic polyester. In Degradable Polymers; Principles and Application; Scott, G., Gilead, D., Eds.; Chapman & Hall: London, UK, 1995; pp. 44–87. [Google Scholar]
- Agarwal, M.; Koelling, K.W.; Chalmers, J.J. Characterization of the degradation of polylactic acid polymer in a solid substrate environnement. Biotechnol. Prog. 1998, 14, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Longieras, A.; Garreau, H.; Tanchette, J.B.; Erre, D.; Copinet, A. Compostability of poly(lactide): degradation in an inert solid medium. J. Polym. Environ. 2007, 15, 200–206. [Google Scholar] [CrossRef]
- International Standard ISO/CEN 14855: Plastics. Evaluation of ultimate aerobic biodegradability and desintegration of plastic materials under controlled composting conditions. Method by analysis of released carbon dioxide, 2005.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Copinet, A.; Legin-Copinet, E.; Erre, D. Compostability of Co-Extruded Starch/Poly(Lactic Acid) Polymeric Material Degradation in an Activated Inert Solid Medium. Materials 2009, 2, 749-764. https://doi.org/10.3390/ma2030749
Copinet A, Legin-Copinet E, Erre D. Compostability of Co-Extruded Starch/Poly(Lactic Acid) Polymeric Material Degradation in an Activated Inert Solid Medium. Materials. 2009; 2(3):749-764. https://doi.org/10.3390/ma2030749
Chicago/Turabian StyleCopinet, Alain, Estelle Legin-Copinet, and Damien Erre. 2009. "Compostability of Co-Extruded Starch/Poly(Lactic Acid) Polymeric Material Degradation in an Activated Inert Solid Medium" Materials 2, no. 3: 749-764. https://doi.org/10.3390/ma2030749




