Synthetic Fabrication of Nanoscale MoS2-Based Transition Metal Sulfides
Abstract
:Contents
- 1.
- Introduction
- 2.
- General Synthesis of Transition Metal Sulfides Nanoparticles
- 2.1.
- Structure
- 2.2.
- General Synthetic Strategy
- 3.
- Synthetic Fabrication of Molybdenum Sulfide Nanoparticles with Organic Compounds
- 3.1.
- Synthetic Fabrication of Molybdenum Sulfide with Surfactants
- 3.1.1.
- Soft Templates
- 3.1.2.
- Structure Directors
- 3.1.3.
- Structure Stabilizers
- 3.2.
- Synthetic Fabrication of Molybdenum Sulfide with Polymers
- 4.
- Synthetic Fabrication of Molybdenum Sulfide Nanoparticles with Inorganic Compounds
- 4.1.
- Synthetic Fabrication of Molybdenum Sulfide with Support
- 4.2.
- Synthetic Fabrication of Molybdenum Sulfide with Promoter
- 4.3.
- Synthetic Fabrication of Molybdenum Sulfide with Doping
- 5.
- Synthetic Fabrication of Molybdenum Sulfide with Intercalation Chemistry
- 6.
- Concluding Remarks
1. Introduction
2. General Synthesis of Transition Metal Sulfides
2.1. Structure

2.2. General Synthetic Strategy


3. Synthetic Fabrication of Molybdenum Sulfide with Organic Compounds
3.1. Synthetic Fabrication of Molybdenum Sulfide with Surfactants
3.1.1. Soft Templates
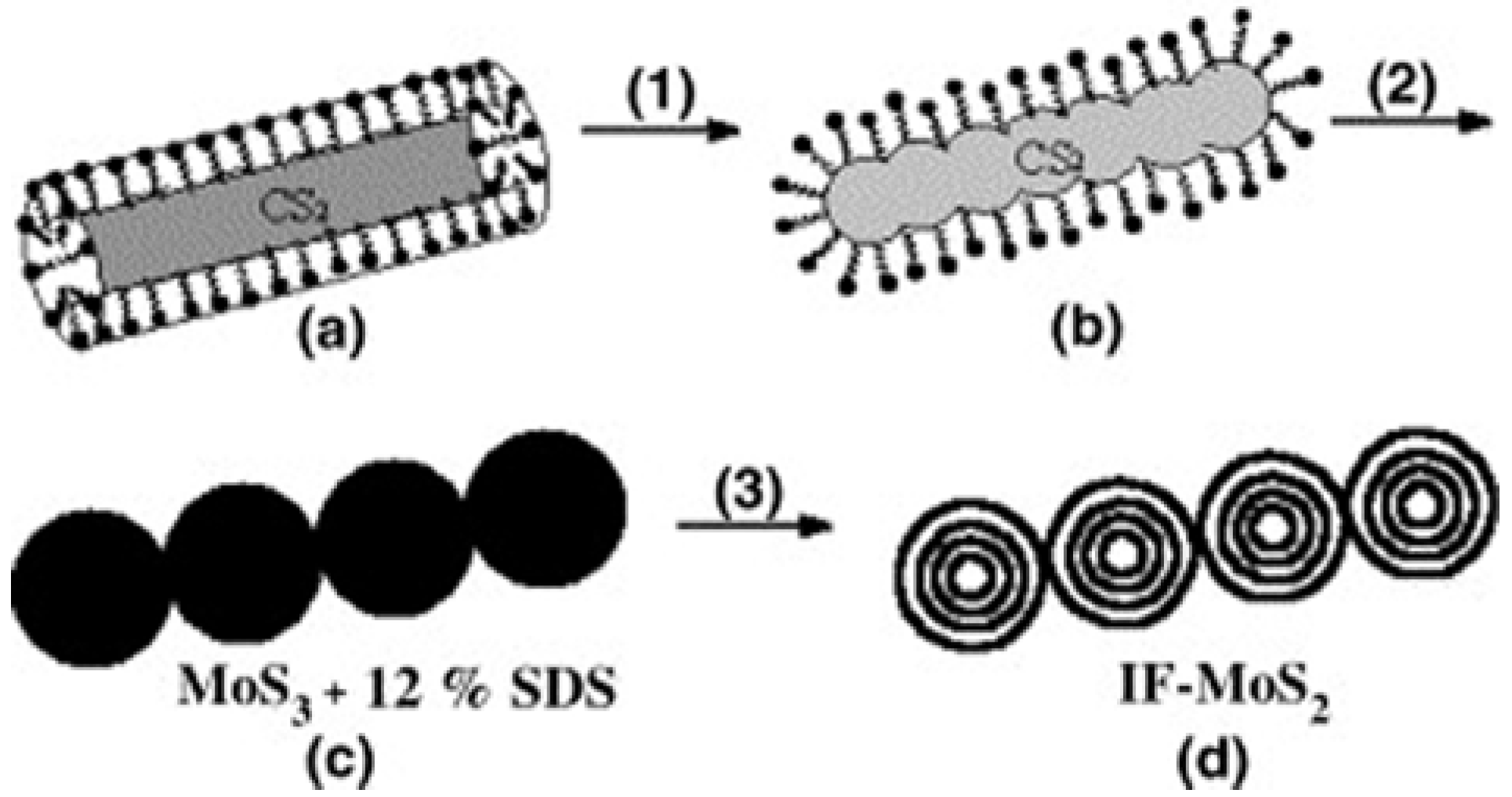
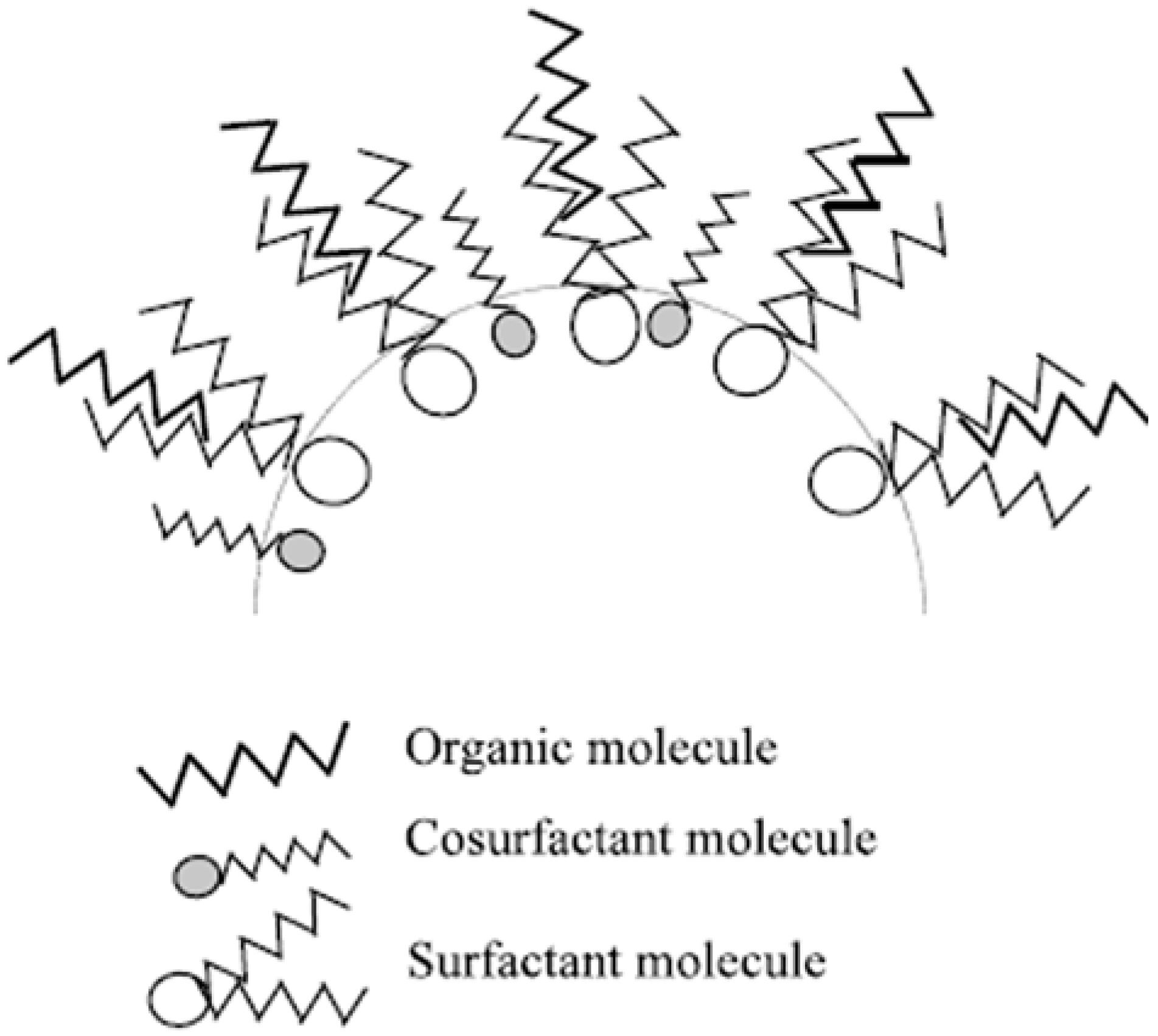
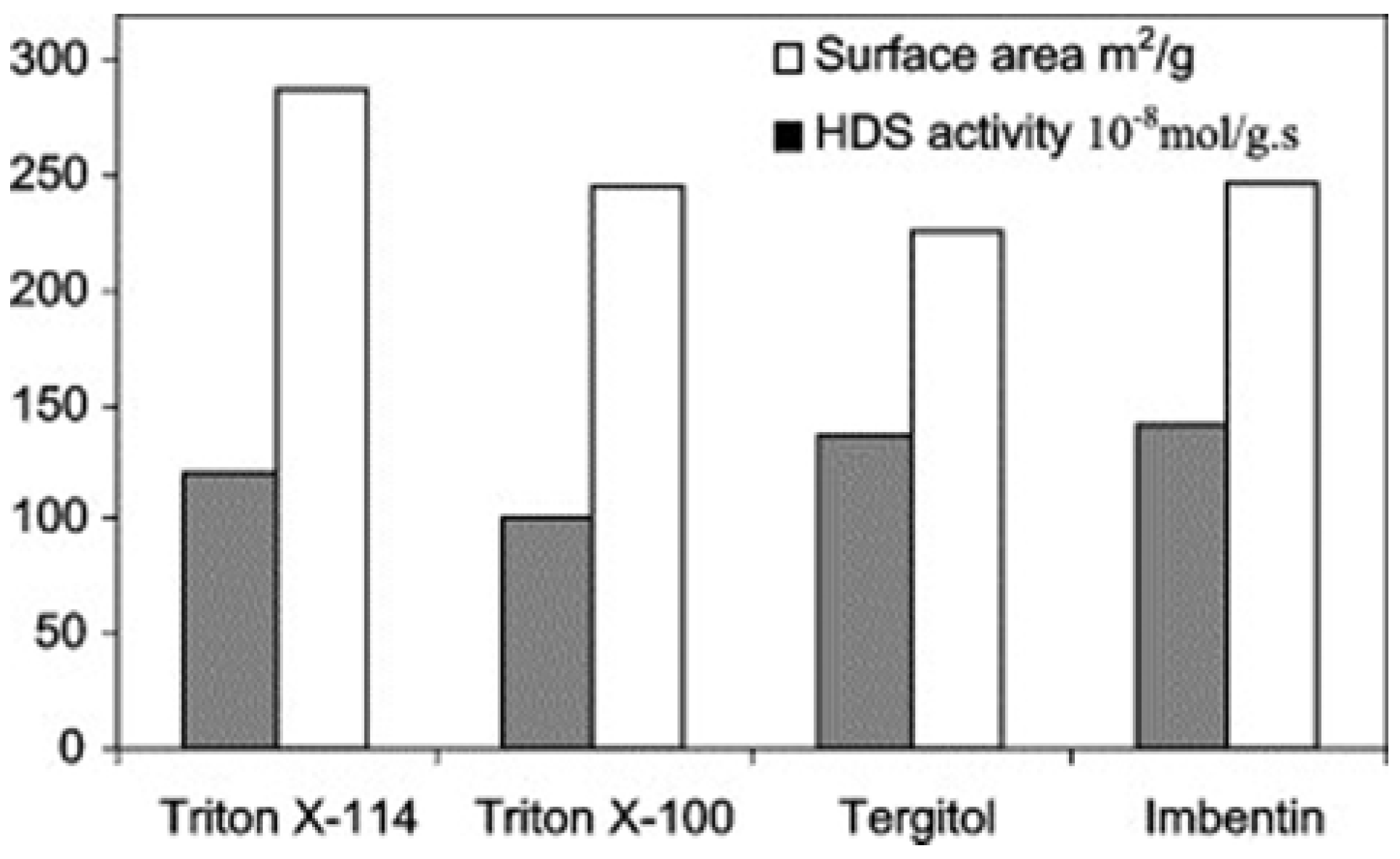
3.1.2. Structure Directors
3.1.3. Structure Stabilizers
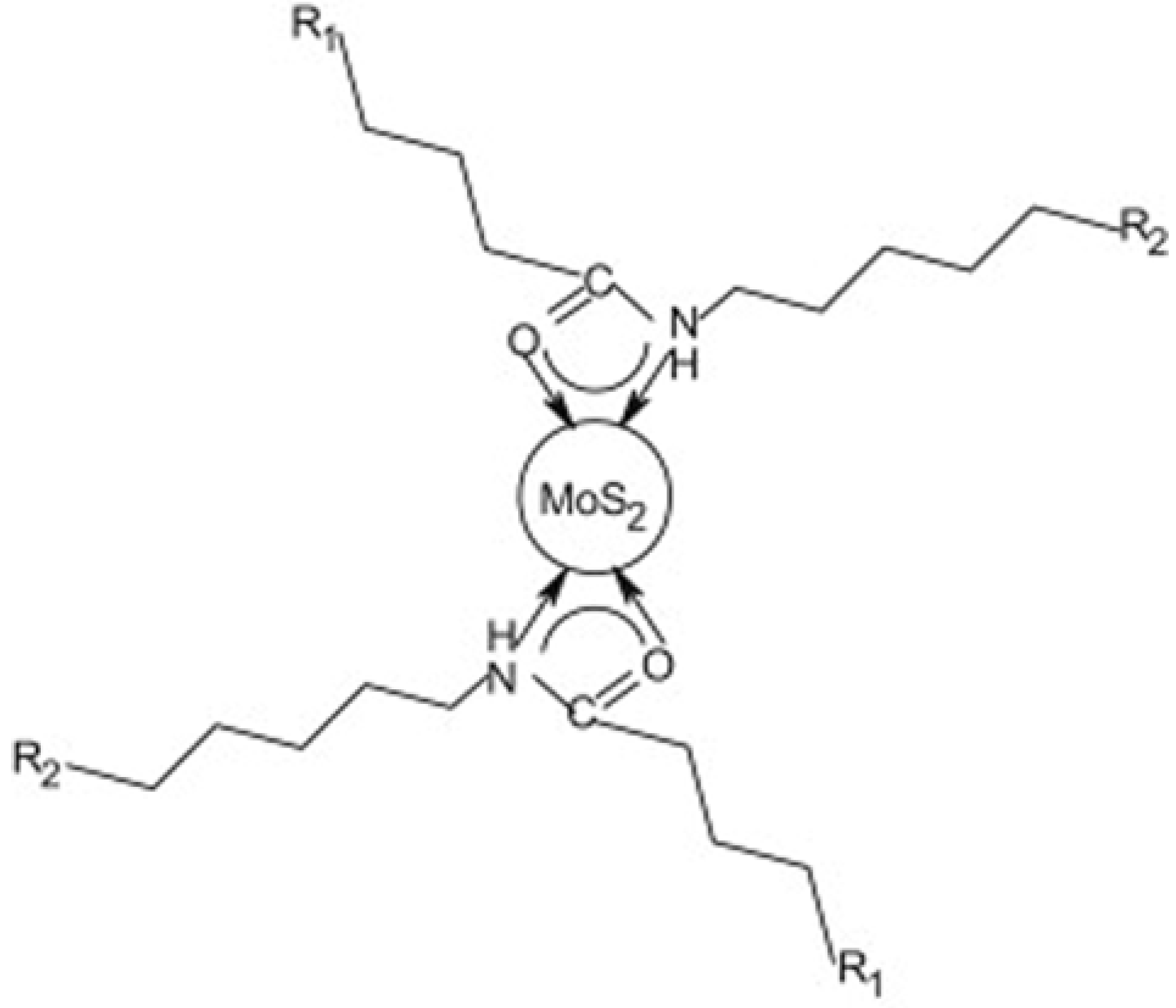
3.2. Synthetic Fabrication of Molybdenum Sulfide with Polymers
4. Synthetic Fabrication of Molybdenum Sulfide with Inorganic Compounds
4.1. Synthetic Fabrication of Molybdenum Sulfide with Supports
4.2. Synthetic Fabrication of Molybdenum Sulfide with Promotion

4.3. Synthetic Fabrication of Molybdenum Sulfide with Doping
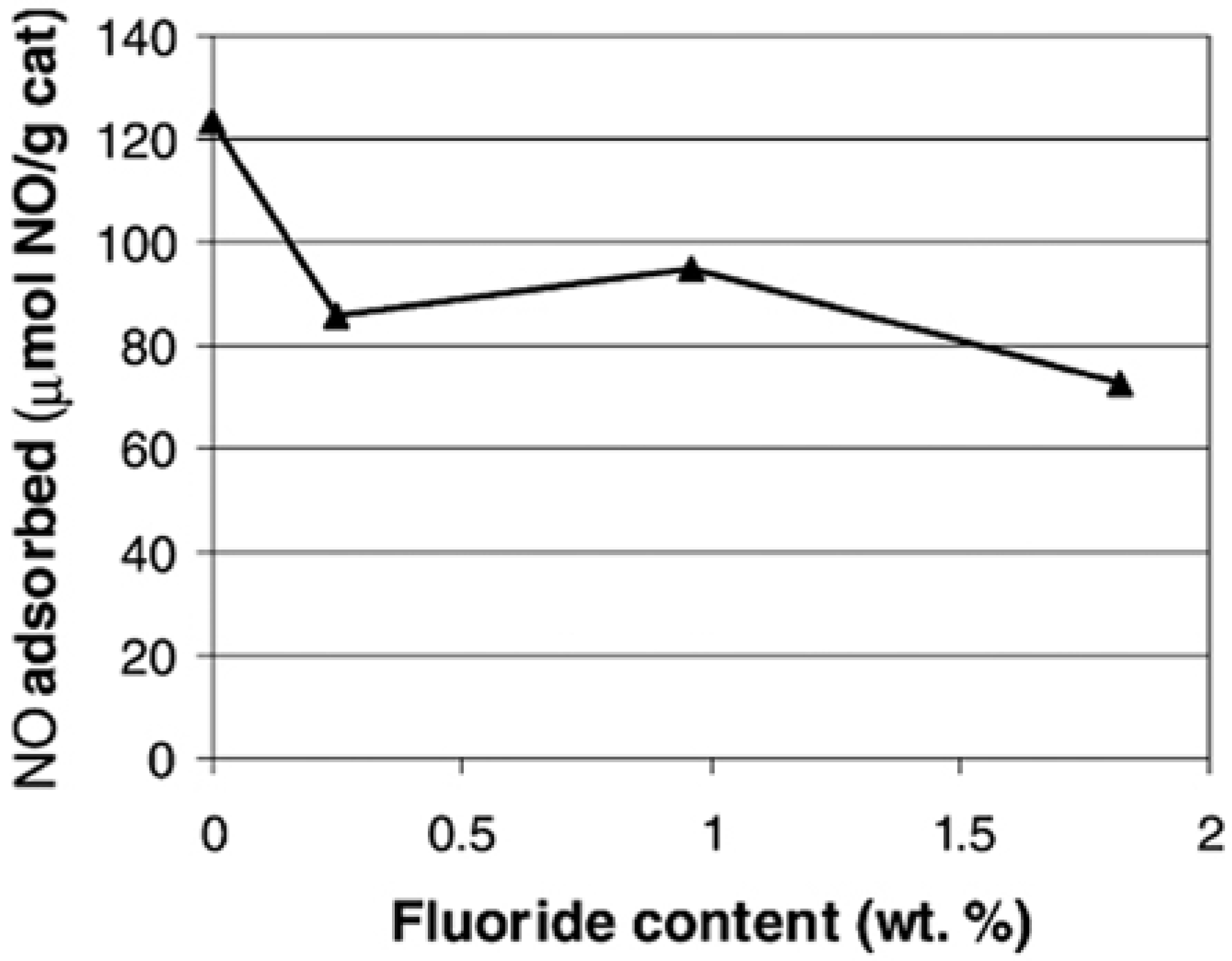
5. Synthetic Fabrication of Molybdenum Sulfide with Intercalation Chemistry
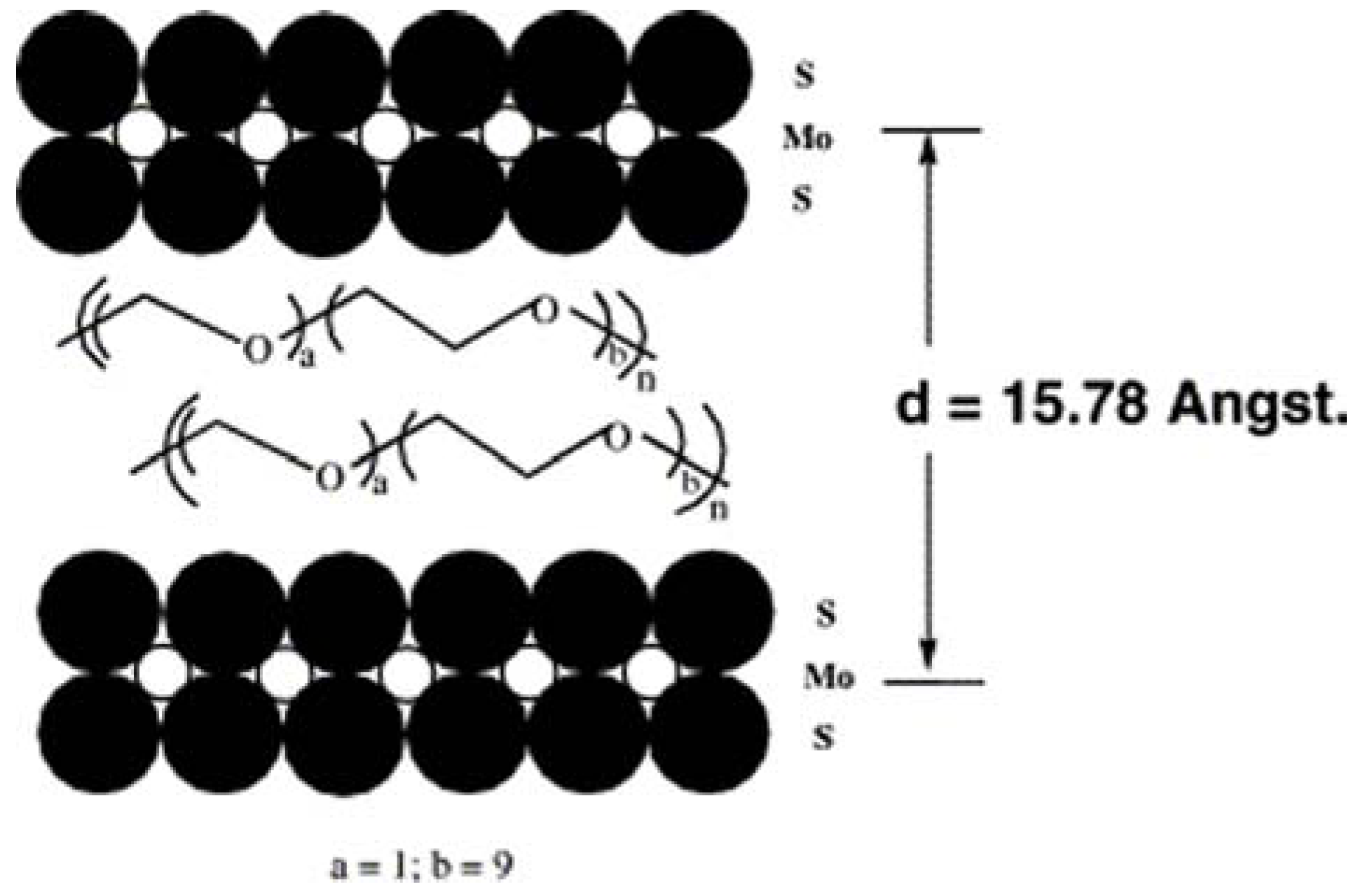
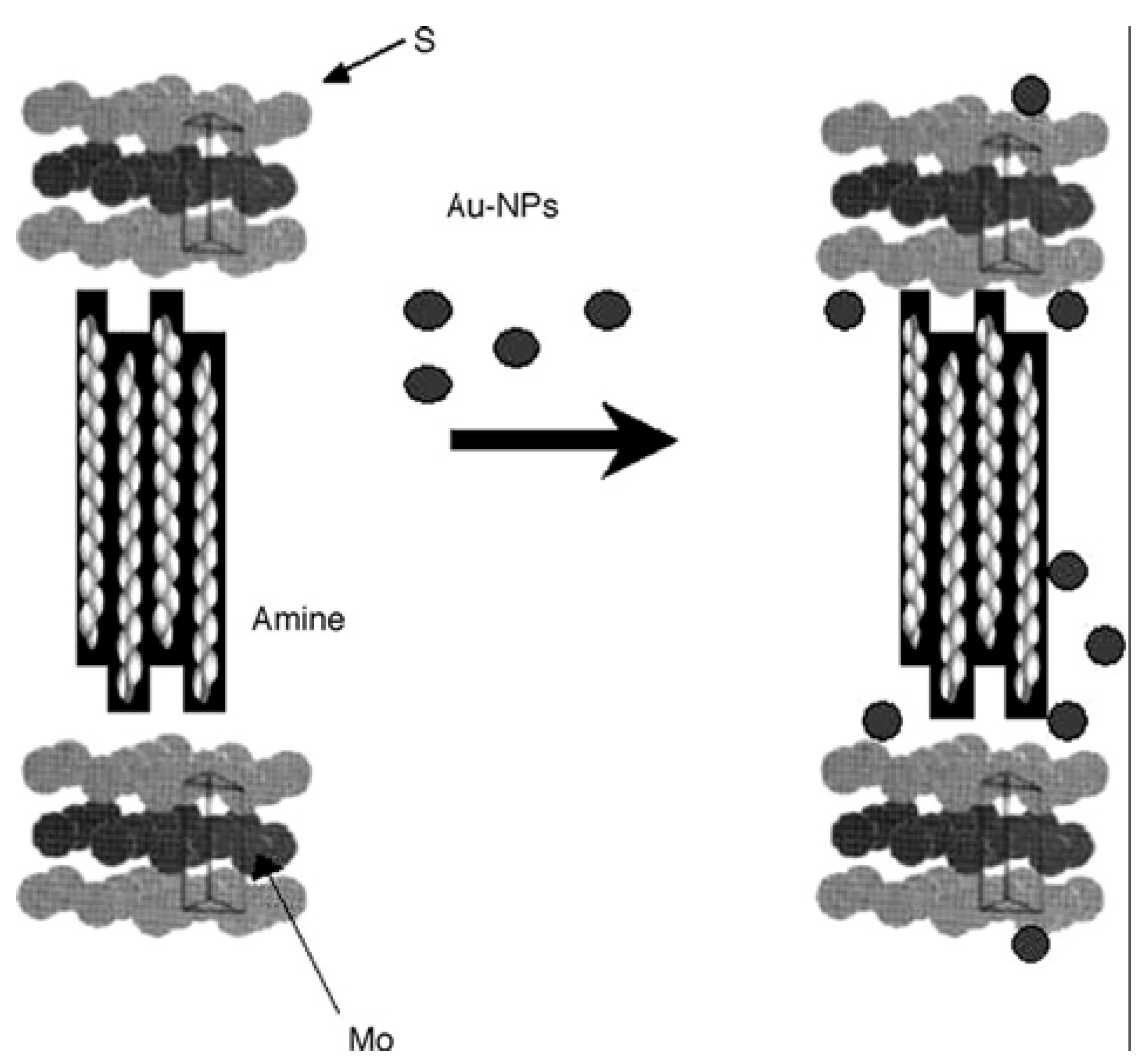
6. Concluding Remarks
Acknowledgements
References and Notes
- Dusheiko, V.A.; Lipkin, M.S. Synthesis of Sulfide Cathodic Materials and Study of Their Physicochemical Properties and Electrochemical Activity. J. Power Sources 1995, 54, 264–267. [Google Scholar] [CrossRef]
- Feng, C.; Ma, J.; Li, H.; Zeng, R.; Guo, Z.; Liu, H. Synthesis of Molybdenum Disulfide (MoS2) for Lithium Ion Battery Applications. Mater. Res. Bull. 2009, 44, 1811–1815. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, P.; Du, Z. Tribological Behavior and Structural Change of the LB Film of MoS2 Nanoparticles Coated with Dialkyldithiophosphate. Surf. Coat. Technol. 2000, 130, 110–115. [Google Scholar] [CrossRef]
- Tenne, R. Advances in the Synthesis of Inorganic Nanotubes and Fullerene-Like Nanoparticles. Angew. Chem. Int. Ed. 2003, 42, 5124–5132. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Hild, W.; Luo, J.; Schaefer, J.A. Friction and Adhesion in Boundary Lubrication Measured by Microtribometers. Tribol. Int. 2006, 39, 1674–1681. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, D.; Shi, H.; Fu, X.; Hu, Z.; Wang, X.; Yan, F. Study on the Tribological Properties of Surfactant-Modified MoS2 Micrometer Spheres as an Additive in Liquid Paraffin. Tribol. Int. 2007, 40, 863–868. [Google Scholar] [CrossRef]
- Rapoport, L.; Fleischer, N.; Tenne, R. Applications of WS2(MoS2) Inorganic Nanotubes and Fullerene-like Nanoparticles for Solid Lubrication and for Structural Nanocomposites. J. Mater. Chem. 2005, 15, 1782–1788. [Google Scholar] [CrossRef]
- Nicosia, D.; Prins, R. The Effect of Glycol on Phosphate-Doped CoMo/Al2O3 Hydrotreating Catalysts. J. Catal. 2005, 229, 424–438. [Google Scholar] [CrossRef]
- Pecoraro, T.A.; Chianelli, R.R. Hydrodesulfurization Catalysis by Transition Metal Sulfides. J. Catal. 1981, 67, 430–445. [Google Scholar] [CrossRef]
- Breysse, M.; Geantet, C.; Afanasiev, P.; Blanchard, J.; Vrinat, M. Recent Studies on the Preparation, Activation and Design of Active Phases and Supports of Hydrotreating Catalysts. Catal. Today 2008, 130, 3–13. [Google Scholar] [CrossRef]
- Dinter, N.; Rusanen, M.; Raybaud, P.; Kasztelan, S.; Silva, P.D.; Toulhoat, H. Temperature-Programed Reduction of Unpromoted MoS2-Based Hydrodesulfurization Catalysts: Experiments and Kinetic Modeling from First Principles. J. Catal. 2009, 267, 67–77. [Google Scholar] [CrossRef]
- Kosidowski, L.; Powell, A.V. Naphthalene Intercalation into Molybdenum Disulfide. Chem. Commun. 1998, 2201–2202. [Google Scholar]
- Bissessur, R.; Heising, J.; Hirpo, W. Toward Pillared Layered Metal Sulfides. Intercalation of the Chalcogenide Clusters Co6Q8(PR3)6 (Q = S, Se, and Te and R = Alkyl) into MoS2. Chem. Mater. 1996, 8, 318–320. [Google Scholar] [CrossRef]
- Benavente, E.; Santa Ana, M.A.; Mendizábal, F.; González, G. Intercalation Chemistry of Molybdenum Disulfide. Coord. Chem. Rev. 2002, 224, 87–109. [Google Scholar] [CrossRef]
- Bissessur, R.; Liu, P.K.Y. Direct Insertion of Polypyrrole into Molybdenum Disulfide. Solid State Ionics 2006, 177, 191–196. [Google Scholar] [CrossRef]
- Andrea, R.T.; Susan, H.; Peidong, Y. Shape Control of Colloidal Metal Nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Marchand, K.E.; Tarret, M.; Lechaire, J.P.; Normand, L.; Kasztelan, S.; Cseri, T. Investigation of AOT-Based Microemulsions for the Controlled Synthesis of MoSx Nanoparticles: An Electron Microscopy Study. Colloids Surf. A 2003, 214, 239–248. [Google Scholar] [CrossRef]
- Muhammad, N.T.; Marc, E.; Patrick, T.; Simon, F.; Andreas, J.; Tatiana, G.; Ute, K.; Wolfgang, T. Reactive Polymers: A Versatile Toolbox for the Immobilization of Functional Molecules on TiO2 Nanoparticles. Angew. Chem. Int. Ed. 2006, 45, 908–912. [Google Scholar] [CrossRef]
- Liu, T.; Burger, C.; Chu, B. Nanofabrication in Polymer Matrices. Prog. Polym. Sci. 2003, 28, 5–26. [Google Scholar] [CrossRef]
- Jun, Y.-w.; Choi, J.-s.; Cheon, J. Shape Control of Semiconductor and Metal Oxide Nanocrystals through Nonhydrolytic Colloidal Routes. Angew. Chem. Int. Ed. 2006, 45, 3414–3439. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A General Strategy for Nanocrystal Synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Puntes, V.F.; Krishnan, K.M.; Alivisatos, A.P. Colloidal Nanocrystal Shape and Size control: The Case of Cobalt. Science 2001, 291, 2115–2117. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Wagner, H.D.; Vaia, R.A. Nanocomposites: Issues at the Interface. Mater. Today 2004, 7, 38–42. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Sekine, T.; Li, Y.H.; Fay, M.W.; Zhao, Y.M.; Patrick Poa, C.H.; Wang, W.X.; Roe, M.J.; Brown, P.D.; Fleischer, N.; Tenne, R. Shock-Absorbing and Failure Mechanisms of WS2 and MoS2 Nanoparticles with Fullerene-like Structures under Shock Wave Pressure. J. Am. Chem. Soc. 2005, 127, 16263–16272. [Google Scholar] [CrossRef] [PubMed]
- Afanasiev, P. Synthetic Approaches to the Molybdenum Sulfide Materials. C R Chim. 2008, 11, 159–182. [Google Scholar] [CrossRef]
- Vollath, D.; Szab, D.V. Synthesis of Nanocrystalline MoS2 and WS2 in a Microwave Plasma. Mater. Lett. 1998, 35, 236–244. [Google Scholar] [CrossRef]
- Feldman, Y.; Wasserman, E.; Srolovitz, D.J.; Tenne, R. High-Rate, Gas-Phase Growth of MoS2 Nested Inorganic Fullerenes and Nanotubes. Science 1995, 267, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Zak, A.; Feldman, Y.; Alperovich, V.; Rosentsveig, R.; Tennne, R. Growth Mechanism of MoS2 Fullerene-Like Nanoparticles by Gas-Phase Synthesis. J. Am. Chem. Soc. 2000, 122, 11108–11116. [Google Scholar] [CrossRef]
- Bar-Sadan, M.; Enyashin, A.N.; Gemming, S.; Popovitz-Biro, R.; Hong, S.Y.; Prior, Y.; Tenne, R.; Seifert, G. Structure and Stability of Molybdenum Sulfide Fullerenes. J. Phys. Chem. B 2006, 110, 25399–25410. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, B.A.; Tenne, R. Polymer-Assisted Fabrication of Nanoparticles and Nanocomposites. Prog. Polym. Sci. 2008, 33, 40–112. [Google Scholar] [CrossRef]
- Castelvetro, V.; De Vita, C. Nanostructured Hybrid Materials from Aqueous Polymer Dispersions. Adv. Colloid Interface Sci. 2004, 108–109, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zeng, H.; Sahoo, Y.; Ohulchanskyy, T.Y.; Ding, Y.; Wang, Z.L.; Swihart, M.; Prasad, P.N. A General Approach to Binary and Ternary Hybrid Nanocrystals. Nano Lett. 2006, 6, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Eckert, H.; Ward, M. Controlling the Length Scale through "Soft" Chemistry: From Organic-Inorganic Nanocomposites to Functional Materials. Chem. Mater. 2001, 13, 3059–3060. [Google Scholar] [CrossRef]
- Huang, Z.-j.; Xiong, D.-S. MoS2 Coated with Al2O3 for Ni-MoS2/Al2O3 Composite Coatings by Pulse Electrodeposition. Surf. Coat. Technol. 2008, 202, 3208–3214. [Google Scholar] [CrossRef]
- Pan, D.; Wang, Q.; Jiang, S.; Ji, X.; An, L. Low-Temperature Synthesis of Oil-Soluble CdSe, CdS, and CdSe/CdS Core-Shell Nanocrystals by Using Various Water-Soluble Anion Precursors. J. Phys. Chem. C 2007, 111, 5661–5666. [Google Scholar] [CrossRef]
- Guo, F.; Matsui, F.; Fujikado, M.; Matsushita, T.; Daimon, H. Stereoscopic Photographs of Atomic Arrangements in MoS2 Single-Crystal. Appl. Surf. Sci. 2004, 237, 616–620. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.-S.; Xin, M.-H.; Xu, R.-R. Mesolamellar Molybdenum Sulfides with Intercalated Cetyltrimethylammonium Cations. Inorg. Chem. Commun. 2000, 3, 129–131. [Google Scholar] [CrossRef]
- Enyashin, A.; Gemming, S.; Seifert, G. Nanosized Allotropes of Molybdenum Disulfide. Eur. Phys. J. Special Topics 2007, 149, 103–125. [Google Scholar] [CrossRef]
- Orita, H.; Uchida, K.; Itoh, N. Ab Initio Density Functional Study of the Structural and Electronic Properties of an MoS2 Catalyst Model: a Real Size Mo27S54 Cluster. J. Mol. Catal. A Chem. 2003, 195, 173–180. [Google Scholar] [CrossRef]
- Topsøe, H.; Clause, B.S.; Candia, R.; Wivel, C.; Mørup, S. In situ Mossbauer Emission Spectroscopy Studies of Unsupported and Supported Sulfided Co-Mo Hydrodesulfurization Catalysts: Evidence for and Nature of a Co-Mo-S Phase. J. Catal. 1981, 68, 433–452. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Can, F.; Travert, A.; Maug, F. FTIR Study of Unsupported Molybdenum Sulfide—In Situ Synthesis and Surface Properties Characterization. Appl. Catal. A 2004, 268, 189–197. [Google Scholar] [CrossRef]
- Bélanger, D.; Laperrière, G.; Girard, F.; Guay, D.; Tourillon, G. Physicochemical Characteristics of Electrochemically Deposited Molybdenum Sulfide and Polypyrrole–Tetrathiomolybdate /Molybdenum Trisulfide Composite Electrodes. Chem. Mater. 2002, 5, 861–868. [Google Scholar] [CrossRef]
- Ponomarev, E.A.; Neumann-Spallart, M.; Hodes, G.; Lévy-Clément, C. Electrochemical Deposition of MoS2 Thin Films by Reduction of Tetrathiomolybdate. Thin Solid Films 1996, 280, 86–89. [Google Scholar] [CrossRef]
- Li, Q.; Newberg, J.T.; Walter, E.C.; Hemminger, J.C.; Penner, R.M. Polycrystalline Molybdenum Disulfide (2H-MoS2) Nano- and Microribbons by Electrochemical/Chemical Synthesis. Nano Lett. 2004, 4, 277–281. [Google Scholar] [CrossRef]
- Ouerfelli, J.; Srivastava, S.K.; Bernède, J.C.; Belgacem, S. Effect of Microwaves on Synthesis of MoS2 and WS2. Vacuum 2008, 83, 308–312. [Google Scholar] [CrossRef]
- Afanasiev, P.; Rawas, L.; Vrinat, M. Synthesis of Dispersed Mo Sulfides in the Reactive Fluxes Containing Liquid Sulfur and Alkali Metal Carbonates. Mater. Chem. Phys. 2002, 73, 295–300. [Google Scholar] [CrossRef]
- Vanchura, B.A.; He, P.; Antochshuk, V.; Jaroniec, M.; Ferryman, A.; Barbash, D.; Fulghum, J.E.; Huang, S.D. Direct Synthesis of Mesostructured Lamellar Molybdenum Disulfides Using a Molten Neutral n-Alkylamine as the Solvent and Template. J. Am. Chem. Soc. 2002, 124, 12090–12091. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Gozum, J.E.; Girolami, G.S. Chemical Vapor Deposition of MoS2 and TiS2 Films from the Metal-Organic Precursors Mo(S-t-Bu)4 and Ti(S-t-Bu)4. Chem. Mater. 1997, 9, 1847–1853. [Google Scholar] [CrossRef]
- Dhas, N.A.; Ekhtiarzadeh, A.; Suslick, K.S. Sonochemical Preparation of Supported Hydrodesulfurization Catalysts. J. Am. Chem. Soc. 2001, 123, 8310–8316. [Google Scholar] [CrossRef] [PubMed]
- Mdleleni, M.M.; Hyeon, T.; Suslick, K.S. Sonochemical Synthesis of Nanostructured Molybdenum Sulfide. J. Am. Chem. Soc. 1998, 120, 6189–6190. [Google Scholar] [CrossRef]
- Uzcanga, I.; Bezverkhyy, I.; Afanasiev, P.; Scott, C.; Vrinat, M. Sonochemical Preparation of MoS2 in Aqueous Solution: Replication of the Cavitation Bubbles in an Inorganic Material Morphology. Chem. Mater. 2005, 17, 3575–3577. [Google Scholar] [CrossRef]
- Rogach, A.L.; Talapin, D.V.; Shevchenko, E.V.; Weller, H. Organization of Matter on Different Size Scales: Monodisperse Nanocrystals and Their Superstructures. Adv. Funct. Mater. 2002, 12, 653–664. [Google Scholar] [CrossRef]
- Ma, L.; Chen, W.-X.; Li, H.; Xu, Z.-D. Synthesis and Characterization of MoS2 Nanostructures with Different Morphologies via an Ionic Liquid-Assisted Hydrothermal Route. Mater. Chem. Phys. 2009, 116, 400–405. [Google Scholar] [CrossRef]
- Nicole, B.; Tobias, G.; Lars, L.; John, R.G.; Ram, S.; Wolfgang, T. A Solvothermal Route to High-Surface-Area Nanostructured MoS2. Chem. Mater. 2003, 15, 4498–4502. [Google Scholar] [CrossRef]
- Flores-Ortiz, L.F.; Cortés-Jácome, M.A.; Ángeles-Chávez, C.; Toledo-Antonio, J.A. Synthesis and Structural Characterization of Molybdenum Sulfide Tubulenes. Sol. Energy Mater. Sol. Cells 2006, 90, 813–824. [Google Scholar] [CrossRef]
- Berhault, G.; Mehta, A.; Pavel, A.C.; Yang, J.; Rendon, L.; Yácaman, M.J.; Araiza, L.C.; Moller, A.D.; Chianelli, R.R. The Role of Structural Carbon in Transition Metal Sulfides Hydrotreating Catalysts. J. Catal. 2001, 198, 9–19. [Google Scholar] [CrossRef]
- Afanasiev, P.; Xia, G.-F.; Berhault, G.; Jouguet, B.; Lacroix, M. Surfactant-Assisted Synthesis of Highly Dispersed Molybdenum Sulfide. Chem. Mater. 1999, 11, 3216–3219. [Google Scholar] [CrossRef]
- Yoneyama, Y.; Song, C. A New Method for Preparing Highly Active Unsupported Mo Sulfide. Catalytic Activity for Hydrogenolysis of 4-(1-Naphthylmethyl)Bibenzyl. Catal. Today 1999, 50, 19–27. [Google Scholar] [CrossRef]
- Genuit, D.; Afanasiev, P.; Vrinat, M. Solution Syntheses of Unsupported Co(Ni)-Mo-S Hydrotreating Catalysts. J. Catal. 2005, 235, 302–317. [Google Scholar] [CrossRef]
- Zelenski, C.M.; Dorhout, P.K. Template Synthesis of Near-Monodisperse1 Microscale Nanofibers and Nanotubules of MoS2. J. Am. Chem. Soc. 1998, 120, 734–742. [Google Scholar] [CrossRef]
- Bezverkhy, I.; Afanasiev, P.; Lacroix, M. Aqueous Preparation of Highly Dispersed Molybdenum Sulfide. Inorg. Chem. 2000, 39, 5416–5417. [Google Scholar] [CrossRef] [PubMed]
- Lavayen, V.; Mirabal, N.; O'Dwyer, C.; Santa Ana, M.A.; Benavente, E.; Sotomayor Torres, C.M.; González, G. The Formation of Nanotubes and Nanocoils of Molybdenum Disulphide. Appl. Surf. Sci. 2007, 253, 5185–5190. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, X.; Shen, L.; Meng, F.; Tang, L.; Deng, Y.; Wang, Z. Synthesis of Amorphous MoS2 Nanospheres by Hydrothermal Reaction. Mater. Lett. 2006, 60, 527–529. [Google Scholar] [CrossRef]
- Rice, D.A.; Hibble, S.J.; Almond, M.J.; Mohammad, K.A.H.; Pearse, S.P. Novel Low-Temperature Route to Known (MnS and FeS2) and New (CrS3, MoS4 and WS5) Transition-Metal Sulfides. J. Mater. Chem. 1992, 2, 895–896. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Wang, Z.; Yu, W.; Qian, Y. Direct Sulfidization Synthesis of High-Quality Binary Sulfides (WS2, MoS2, and V5S8) from the Respective Oxides. Mater. Chem. Phys. 2004, 87, 327–331. [Google Scholar] [CrossRef]
- Afanasiev, P.; Bezverkhy, I. Genesis of Vesicle-Like and Tubular Morphologies in Inorganic Precipitates: Amorphous Mo Oxysulfides. J. Phys. Chem. B 2003, 107, 2678–2683. [Google Scholar] [CrossRef]
- Bezverkhyy, I.; Afanasiev, P.; Marhic, C.; Danot, M. Template-Free Solution Synthesis of Sulfur Microtubules. Chem. Mater. 2003, 15, 2119–2121. [Google Scholar] [CrossRef]
- Wang, S.; Song, Y.; Jiang, L. Photoresponsive Surfaces with Controllable Wettability. J. Photochem. Photobiol. C 2007, 8, 18–29. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-Induced Amphiphilic Surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Choi, J.-S.; Maugé, F.; Pichon, C.; Olivier-Fourcade, J.; Jumas, J.-C.; Petit-Clair, C.; Uzio, D. Alumina-Supported Cobalt–Molybdenum Sulfide Modified by Tin via Surface Organometallic Chemistry: Application to the Simultaneous Hydrodesulfurization of Thiophenic Compounds and the Hydrogenation of Olefins. Appl. Catal. A 2004, 267, 203–216. [Google Scholar] [CrossRef]
- Bakunin, V.N.; Suslov, A.Y.; Kuzmina, G.N.; Parenago, O.P.; Topchiev, A.V. Synthesis and Application of Inorganic Nanoparticles as Lubricant Components—A Review. J. Nanopart. Res. 2004, 6, 273–284. [Google Scholar] [CrossRef]
- Bezverkhyy, I.; Afanasiev, P.; Lacroix, M. Preparation and Chemical Transformation of Surfactant-Templated Hybrid Phase Containing MoS42- Anions. Mater. Res. Bull. 2002, 37, 161–168. [Google Scholar] [CrossRef]
- Khomane, R.B.; Manna, A.; Mandale, A.B.; Kulkarni, B.D. Synthesis and Characterization of Dodecanethiol-Capped Cadmium Sulfide Nanoparticles in a Winsor II Microemulsion of Diethyl Ether/AOT/Water. Langmuir 2002, 18, 8237–8240. [Google Scholar] [CrossRef]
- Xiong, Y.; Xie, Y.; Li, Z.; Li, X.; Zhang, R. Micelle-Assisted Fabrication of Necklace-Shaped Assembly of Inorganic Fullerene-Like Molybdenum Disulfide Nanospheres. Chem. Phys. Lett. 2003, 382, 180–185. [Google Scholar] [CrossRef]
- Zhang, X.; An, C.; Wang, S.; Wang, Z.; Xia, D. Green Synthesis of Metal Sulfide Nanocrystals Through a General Composite-Surfactants-Aided-Solvothermal Process. J. Cryst. Growth 2009, 311, 3775–3780. [Google Scholar] [CrossRef]
- Wu, Q.; Zheng, N.; Ding, Y.; Li, Y. Micelle-Template Inducing Synthesis of Winding ZnS Nanowires. Inorg. Chem. Commun. 2002, 5, 671–673. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, J.; Fu, W.; Liu, Y.; Zhu, Y.; Wang, Z. A Facile Route to Synthesis of MoS2 Nanorods. Mater. Lett. 2005, 59, 3452–3455. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Z.; Zhao, J.; Xue, Q. Study on the Structure of Surface-Modified MoS2 Nanoparticles. Mater. Res. Bull. 1996, 31, 345–349. [Google Scholar] [CrossRef]
- Trikalitis, P.N.; Kerr, T.A.; Kanatzidis, M.G. Mesostructured Cobalt and Nickel Molybdenum Sulfides. Micropor. Mesopor. Mater. 2006, 88, 187–190. [Google Scholar] [CrossRef]
- Shi, X.; Shen, M.; Möhwald, H. Polyelectrolyte Multilayer Nanoreactors toward the Synthesis of Diverse Nanostructured Materials. Prog. Polym. Sci. 2004, 29, 987–1019. [Google Scholar] [CrossRef]
- Chen, S.; Liu, W.; Yu, L. Preparation of DDP-coated PbS Nanoparticles and Investigation of the Antiwear Ability of the Prepared Nanoparticles as Additive in Liquid Paraffin. Wear 1998, 218, 153–158. [Google Scholar] [CrossRef]
- Liu, W.; Chen, S. An Investigation of the Tribological Behaviour of Surface-Modified ZnS Nanoparticles in Liquid Paraffin. Wear 2000, 238, 120–124. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhang, J.; Xue, Q.J. Synthesis and Characterization of a Molybdenum Disulfide Nanocluster. J. Phys. Chem. 1994, 98, 12973–12977. [Google Scholar] [CrossRef]
- Cates, E.; Pitcher, M.W.; Bianconi, P.A. Surfactant Nucleation and Orientation of Crystals in the Syntheses of Metal Sulfides in a Polymer Matrix. Mater. Chem. Phys. 2005, 94, 13–22. [Google Scholar] [CrossRef]
- Bissessur, R.; Schindler, J.L.; Kannewurf, C.R.; Kanatzidis, M. Nanoscale Composites Formed by Encapsulation of Polymers in MoS2. From Conjugated Polymers to Plastics. Detection of Metal to Insulator Transition. Mol. Cryst. Liquid Cryst. 1994, 245, 249–254. [Google Scholar] [CrossRef]
- Wang, L.; Schindler, J.; Thomas, J.A.; Kannewurf, C.R.; Kanatzidis, M.G. Entrapment of Polypyrrole Chains between MoS2 Layers via an In Situ Oxidative Polymerization Encapsulation Reaction. Chem. Mater. 2002, 7, 1753–1755. [Google Scholar] [CrossRef]
- Golodnitsky, D.; Nathan, M.; Yufit, V.; Strauss, E.; Freedman, K.; Burstein, L.; Gladkich, A.; Peled, E. Progress in Three-Dimensional (3D) Li-ion Microbatteries. Solid State Ionics 2006, 177, 2811–2819. [Google Scholar] [CrossRef]
- Costa, D.; Arrouvel, C.; Breysse, M.; Toulhoat, H.; Raybaud, P. Edge Wetting Effects of γ-Al2O3 and Anatase-TiO2 Supports by MoS2 and CoMoS Active Phases: A DFT Study. J. Catal. 2007, 246, 325–343. [Google Scholar] [CrossRef]
- Ramirez, J.; Fuentes, S.; Díaz, G.; Vrinat, M.; Breysse, M.; Lacroix, M. Hydrodesulphurization Activity and Characterization of Sulphided Molybdenum and Cobalt-Molybdenum Catalysts Comparison of Alumina-, Silica-Alumina- and Titania-Supported Catalysts. Appl. Catal. 1989, 52, 211–223. [Google Scholar]
- Kibsgaard, J.; Clausen, B.S.; Topsøe, H.; Læsgaard, E.; Lauritsen, J.V.; Besenbacher, F. Scanning Tunneling Microscopy Studies of TiO2-Supported Hydrotreating Catalysts: Anisotropic Particle Shapes by Edge-Specific MoS2-Support Bonding. J. Catal. 2009, 263, 98–103. [Google Scholar] [CrossRef]
- Sakashita, Y. Effects of Surface Orientation and Crystallinity of Alumina Supports on the Microstructures of Molybdenum Oxides and Sulfides. Surf. Sci. 2001, 489, 45–58. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Pawelec, B.; Rivera-Muñoz, E.; Nava, R.; Espino, J.; Fierro, J.L.G. Comparison of the Morphology and HDS Activity of Ternary Co-Mo-W Catalysts Supported on P-Modified SBA-15 and SBA-16 Substrates. Appl. Catal. B 2009, 92, 168–184. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kato, A.; Usman; Rinaldi, N.; Fujikawa, T.; Koshika, H.; Hiromitsu, I.; Kubota, T. Effect of Sulfidation Temperature on the Intrinsic Activity of Co-MoS2 and Co-WS2 Hydrodesulfurization Catalysts. J. Catal. 2009, 265, 216–228. [Google Scholar] [CrossRef]
- Dhas, N.A.; Suslick, K.S. Sonochemical Preparation of Hollow Nanospheres and Hollow Nanocrystals. J. Am. Chem. Soc. 2005, 127, 2368–2369. [Google Scholar] [CrossRef] [PubMed]
- Potapenko, D.V.; Horn, J.M.; Beuhler, R.J.; Song, Z.; White, M.G. Reactivity Studies with Gold-Supported Molybdenum Nanoparticles. Surf. Sci. 2005, 574, 244–258. [Google Scholar] [CrossRef]
- Raybaud, P. Understanding and Predicting Improved Sulfide Catalysts: Insights from First Principles Modeling. Appl. Catal. A 2007, 322, 76–91. [Google Scholar] [CrossRef]
- Joshi, Y.V.; Ghosh, P.; Daage, M.; Delgass, W.N. Support Effects in HDS Catalysts: DFT Analysis of Thiolysis and Hydrolysis Energies of Metal-Support Linkages. J. Catal. 2008, 257, 71–80. [Google Scholar] [CrossRef]
- Breysse, M.; Afanasiev, P.; Geantet, C.; Vrinat, M. Overview of Support Effects in Hydrotreating Catalysts. Catal. Today 2003, 86, 5–16. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J. Facilitated Lithium Storage in MoS2 Overlayers Supported on Coaxial Carbon Nanotubes. J. Phys. Chem. C 2007, 111, 1675–1682. [Google Scholar] [CrossRef]
- Iwamoto, R.; Inamura, K.; Nozaki, T.; Iino, A. Effect of Cobalt on the Sulfiding Temperature of CoO-MoO3/Al2O3 Studied by Temperature Programmed Sulfiding. Appl. Catal. A 1997, 163, 217–225. [Google Scholar] [CrossRef]
- Grimblot, J. Genesis, Architecture and Nature of Sites of Co(Ni)-MoS2 Supported Hydroprocessing Catalysts. Catal. Today 1998, 41, 111–128. [Google Scholar] [CrossRef]
- Genuit, D.; Bezverkhyy, I.; Afanasiev, P. Solution Preparation of the Amorphous Molybdenum Oxysulfide MoOS2 and Its Use for Catalysis. J. Solid State Chem. 2005, 178, 2759–2765. [Google Scholar] [CrossRef]
- Wang, W.-y.; Yang, Y.-q.; Bao, J.-g.; Luo, H.-a. Characterization and Catalytic Properties of Ni-Mo-B Amorphous Catalysts for Phenol Hydrodeoxygenation. Catal. Commun. 2009. [Google Scholar] [CrossRef]
- Lélias, M.A.; Kooyman, P.J.; Mariey, L.; Oliviero, L.; Travert, A.; van Gestel, J.; van Veen, J.A.R.; Maug, F. Effect of NTA Addition on the Structure and Activity of the Active Phase of Cobalt-Molybdenum Sulfide Hydrotreating Catalysts. J. Catal. 2009, 267, 14–23. [Google Scholar] [CrossRef]
- Vogelaar, B.M.; Kagami, N.; van der Zijden, T.F.; van Langeveld, A.D.; Eijsbouts, S.; Moulijn, J.A. Relation between Sulfur Coordination of Active Sites and HDS Activity for Mo and NiMo Catalysts. J. Mol. Catal. A Chem. 2009, 309, 79–88. [Google Scholar] [CrossRef]
- Surisetty, V.R.; Tavasoli, A.; Dalai, A.K. Synthesis of Higher Alcohols from Syngas over Alkali Promoted MoS2 Catalysts Supported on Multi-Walled Carbon Nanotubes. Appl. Catal. A 2009, 365, 243–251. [Google Scholar] [CrossRef]
- Cattenot, M.; Geantet, C.; Glasson, C.; Breysse, M. Promoting Effect of Ruthenium on NiMo/Al2O3 Hydrotreating Catalysts. Appl. Catal. A 2001, 213, 217–224. [Google Scholar] [CrossRef]
- Rueda, N.; Bacaud, R.; Vrinat, M. Highly Dispersed, Nonsupported Molybdenum Sulfides. J. Catal. 1997, 169, 404–406. [Google Scholar] [CrossRef]
- Glasson, C.; Geantet, C.; Lacroix, M.; Labruyere, F.; Dufresne, P. Beneficial Effect of Carbon on Hydrotreating Catalysts. J. Catal. 2002, 212, 76–85. [Google Scholar] [CrossRef]
- Iwamoto, R.; Grimblot, J. Genesis, Characterizations and HDS Activity of Mo-P-Alumina based Hydrotreating Catalysts Prepared by a Sol-Gel Method. Studies Surf. Sci. Catal. 1997, 106, 195–210. [Google Scholar]
- Nava, H.; Espino, J.; Berhault, G.; Alonso-Nunez, G. Effect of Phosphorus Addition on Unsupported Ni-Mo-W Sulfide Catalysts Prepared by the In Situ Activation of Nickel/Tetramethylammonium Thiomolybdotungstate. Appl. Catal. A 2006, 302, 177–184. [Google Scholar] [CrossRef]
- Cuevas, R.; Ramírez, J.; Busca, G. Fluoride Modification of Mo/Al2O3 Catalysts: Characterization of the Changes Induced in Support and Mo Phases. J. Fluorine Chem. 2003, 122, 151–158. [Google Scholar] [CrossRef]
- Klimova, T.; Vara, P.M.; Lee, I.P. Development of New NiMo/γ-alumina Catalysts Doped with Noble Metals for Deep HDS. Catal. Today 2009. [Google Scholar] [CrossRef]
- Homyonfer, M.; Alperson, B.; Rosenberg, Y.; Sapir, L.; Cohen, S.R.; Hodes, G.; Tennne, R. Intercalation of Inorganic Fullerene like Structures Yields Photosensitive Films and New Tips for Scanning Probe Microscopy. J. Am. Chem. Soc. 1997, 119, 2693–2698. [Google Scholar] [CrossRef]
- Bissessur, R.; Gallant, D.; Brüning, R. Novel Nanocomposite Material Consisting of Poly[oxymethylene-(oxyethylene)] and Molybdenum Disulfide. Mater. Chem. Phys. 2003, 82, 316–320. [Google Scholar] [CrossRef]
- Julien, C.; Sekine, T.; Balkanski, M. Lattice Dynamics of Lithium Intercalated MoS2. Solid State Ionics 1991, 48, 225–229. [Google Scholar] [CrossRef]
- Benavente, E.; González, G. Microwave Activated Lithium Intercalation in Transition Metal Sulfides. Mater. Res. Bull. 1997, 32, 709–717. [Google Scholar] [CrossRef]
- Gonzalez, G.; Santa Ana, M.A.; Benavente, E.; Donoso, J.P.; Bonagamba, T.J.; Mello, N.C.; Panepucci, H. Electrical Conductivity and Lithium Diffusion in Molybdenum Disulfide Intercalated with Poly(ethylene oxide). Solid State Ionics 1996, 85, 225–230. [Google Scholar] [CrossRef]
- Lemaux, S.; Golub, A.S.; Gressier, P.; Ouvrard, G. Synthesis and Characterization of a Mercury-Intercalated Molybdenum Disulfide. J. Solid State Chem. 1999, 147, 336–340. [Google Scholar] [CrossRef]
- Lavayen, V.; O'Dwyer, C.; Ana, M.A.S.; Mirabal, N.; Benavente, E.; Cardenas, G.; Gonzalez, G.; Torres, C.M.S. Functionalization of Lamellar Molybdenum Disulphide Nanocomposite with Gold Nanoparticles. Appl. Surf. Sci. 2007, 253, 3444–3449. [Google Scholar] [CrossRef]
- Golub, A.S.; Shumilova, I.B.; Novikov, Y.; Mansot, J.L.; Danot, M. Phenanthroline Intercalation into Molybdenum Disulfide. Solid State Ionics 1996, 91, 307–314. [Google Scholar] [CrossRef]
- Li, X.-L.; Li, Y.-D. MoS2 Nanostructures: Synthesis and Electrochemical Mg2+ Intercalation. J. Phys. Chem. B 2004, 108, 13893–13900. [Google Scholar] [CrossRef]
- Divigalpitiya, W.M.R.; Frindt, R.F.; Morrison, R.S. Inclusion Systems of Organic Molecules in Restacked Single-Layer Molybdenum Disulfide. Science 1989, 246, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Hideyuki, T.; Tohru, H.; Masa, K.; Taeko, I.; Koji, C. Inclusion of Substituted Ferrocenes and Aromatic Compounds into MoS2 Layers as New Intercalation Compounds. Chem. Lett. 1991, 20, 2113. [Google Scholar]
- Dines, M.B. Intercalation of Metallocenes in the Layered Transition-Metal Dichalcogenides. Science 1975, 188, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Heising, J.; Bonhomme, F.; Kanatzidis, M.G. Toward Pillared Metal Sulfides: Encapsulation and Rietveld Structural Characterization of the Al13O4(OH)24(H2O)127+ Cluster into MoS2 and WS2. J. Solid State Chem. 1998, 139, 22–26. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, S.; An, C.; Yuan, J. Synthetic Fabrication of Nanoscale MoS2-Based Transition Metal Sulfides. Materials 2010, 3, 401-433. https://doi.org/10.3390/ma3010401
Wang S, An C, Yuan J. Synthetic Fabrication of Nanoscale MoS2-Based Transition Metal Sulfides. Materials. 2010; 3(1):401-433. https://doi.org/10.3390/ma3010401
Chicago/Turabian StyleWang, Shutao, Changhua An, and Jikang Yuan. 2010. "Synthetic Fabrication of Nanoscale MoS2-Based Transition Metal Sulfides" Materials 3, no. 1: 401-433. https://doi.org/10.3390/ma3010401



