Polymeric Microspheres for Medical Applications
Abstract
:1. Introduction
- 1)
- 2)
- bulking agents in soft tissues to augment the efficiency of opening-closing systems, e.g., for treatment of stress urinary incontinence (SUI), vesicoureteral reflux, or for vocal cord augmentation. The injected biomaterial enhances tissue stiffness and gives support to the muscles responsible for opening and closing tubular structures [3,6,7,8].
- 3)
- particles for embolization therapies that purposefully occlude blood vessels. Embolization therapy can be applied to combat the growth and development of solid tumors by blocking the feeding artery. Occlusion of blood vessels can also be required in case of arterio-venous malformations or hemoptysis (severe bleeding) [9,10,11].
- 4)
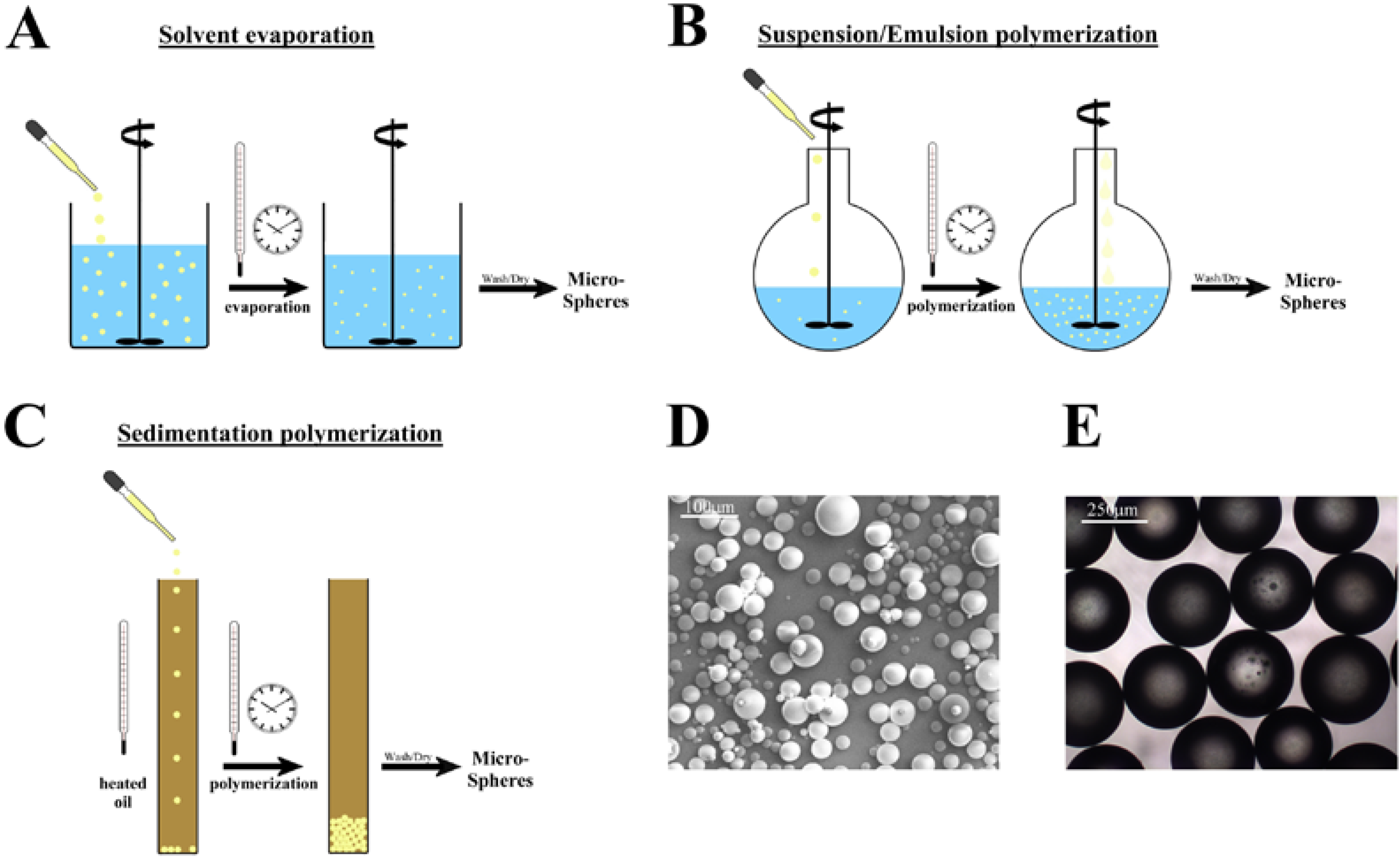
2. Microsphere Synthesis
2.1. Microspheres from linear polymers
2.2. Microsphere synthesis from radical polymerization
| Stirred solution | Added solution | |
| Suspension polymerization | Surfactant | Monomers |
| Emulsion polymerization | Surfactant | Monomers |
| Dispersion polymerization | Surfactant | - |
3. Clinical Applications of Microspheres
3.1. Microspheres as fillers and bulking agents
3.1.1. Microspheres in minimally-invasive treatment of stress-urinary-incontinence (SUI)
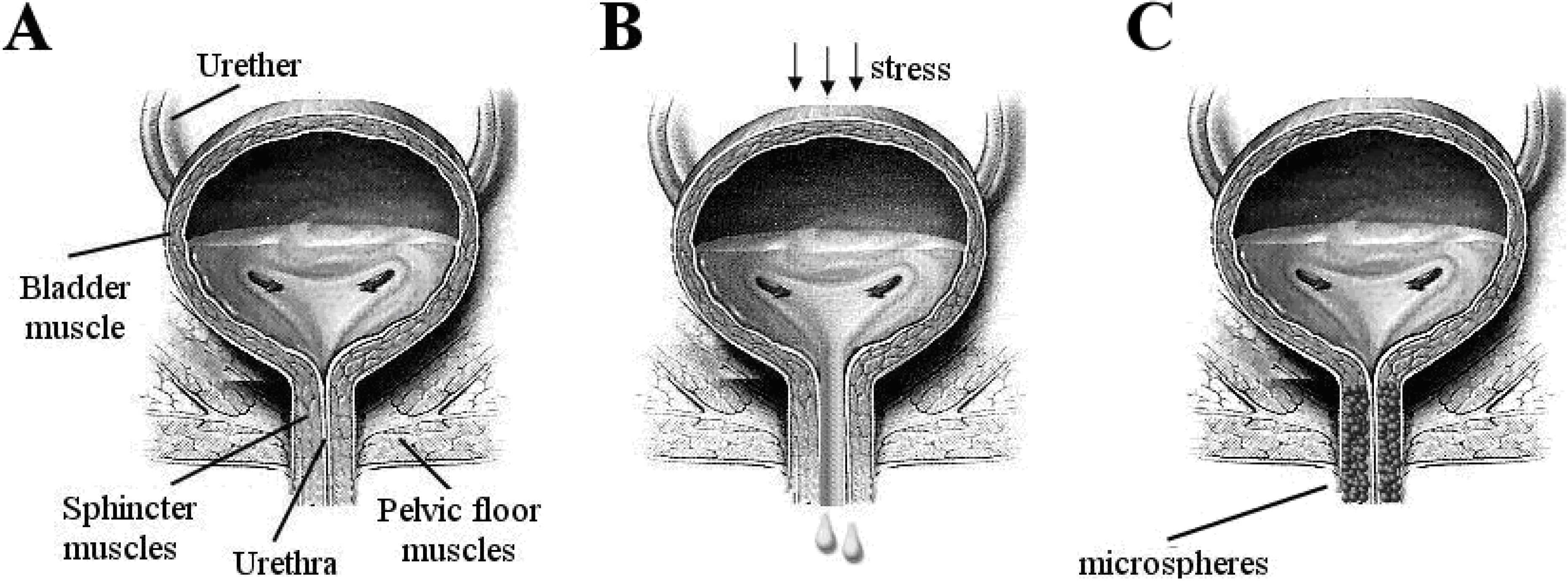
3.1.2. Treatment of SUI
3.1.3. Modern minimally invasive therapies for SUI
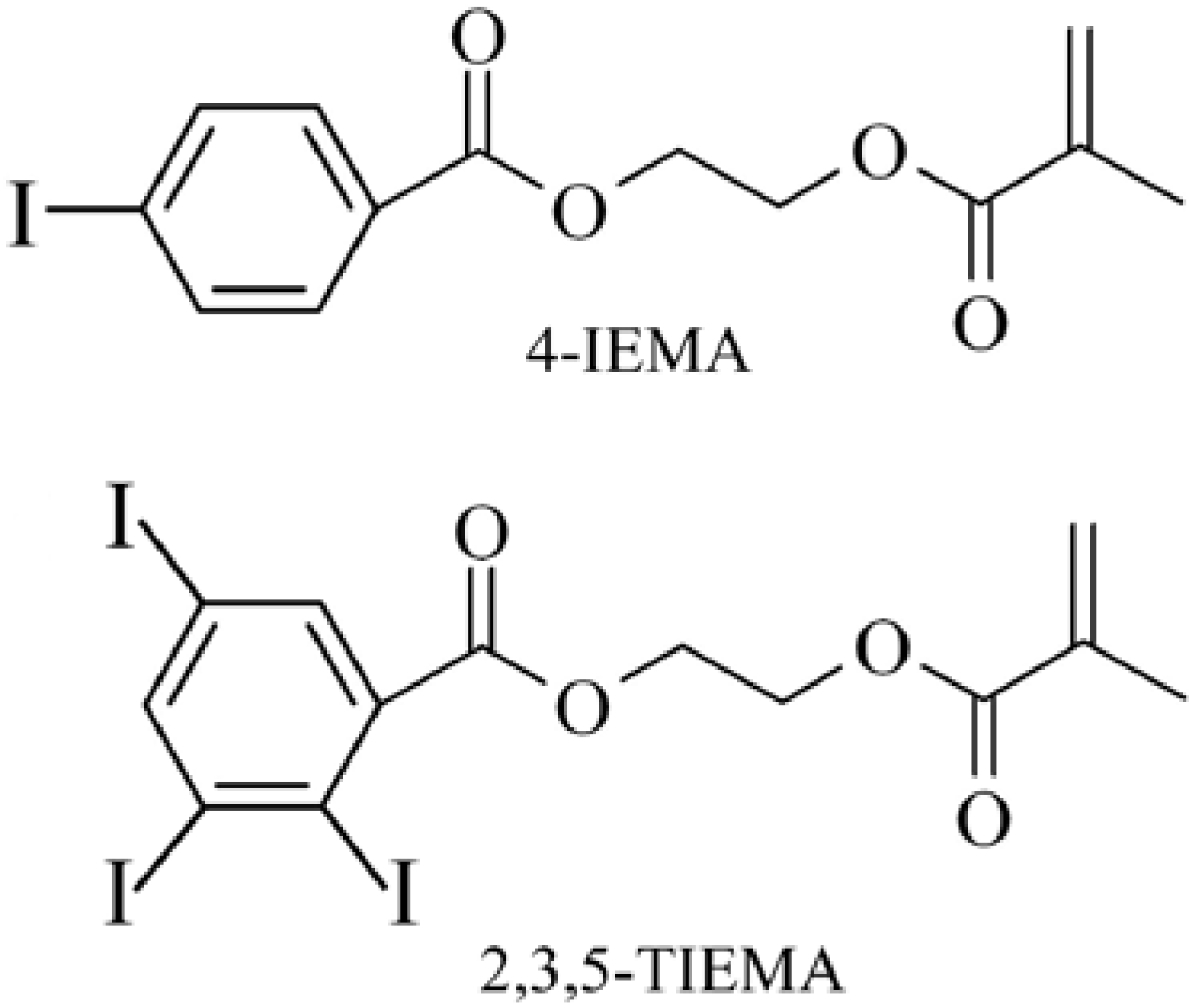
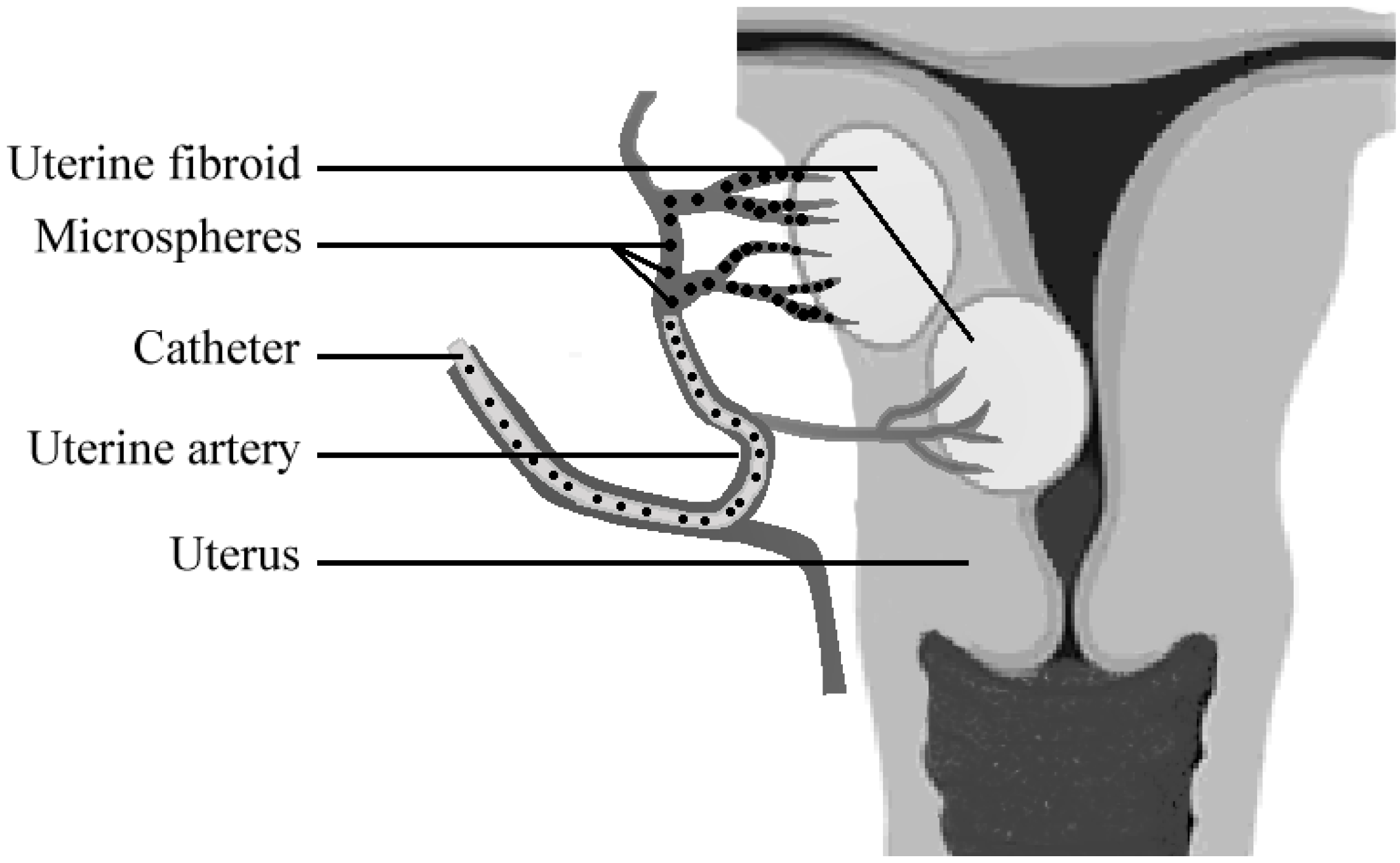
3.2. Microspheres for embolization therapy
3.2.1. Embolization of tumors
4. Improving Microsphere-based Therapies by Controlled Local Drug Delivery
5. Concluding Remarks and Outlook
References and Notes
- Eppley, B.L.; Dadvand, B. Injectable soft-tissue fillers: clinical overview. Plast. Reconstr. Surg. 2006, 118, e98–e106. [Google Scholar] [CrossRef]
- Laeschke, K. Biocompatibility of microparticles into soft tissue fillers. Semin. Cutan. Med. Surg. 2004, 23, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Amrute, K.V.; Badlani, G.H. The science behind biomaterials in female stress urinary incontinence surgery. Sci. World J. 2009, 9, 23–31. [Google Scholar] [CrossRef]
- Broder, K.W.; Cohen, S.R. An overview of permanent and semipermanent fillers. Plast. Reconstr. Surg. 2006, 118 (suppl. 3), S7–S14. [Google Scholar] [CrossRef]
- Garvin, K.; Feschuk, C. Polylactide-polyglycide antibiotic implants. Clin. Orthop. Relat. Res. 2005, 437, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Starkman, J.S.; Scarpero, H.; Dmochowski, R.R. Emerging periurethral bulking agents for female stress urinary incontinence: is new necessarily better? Curr. Urol. Rep. 2006, 7, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Kotb, A.F.; Campeau, L.; Corcos, J. Urethal bulking agents: techniques and outcomes. Curr. Urol. Rep. 2009, 10, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, R.R.; Appell, R.A. Injectable agents in the treatment of stress urinary incontinence in women: where are we now? Urology 2000, 56 (suppl. 1), 32–40. [Google Scholar] [CrossRef]
- Laurent, A. Microspheres and nonspherical particles for embolization. Tech. Vasc. Interv. Radiol. 2007, 10, 248–256. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Ebert, A.D. Treatment of uterine fibroids by embolization–advantages, disadvantages, and pitfalls. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 123, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kettenbach, J.; Stadler, A.; Katzler, I.; Schernthaner, R.; Blum, M.; Lammer, J.; Rand, T. Drug-loaded microspheres for the treatment of liver cancer: review of current results. Cardiovasc. Intervent. Radiol. 2008, 31, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chem. Soc. Rev. 2008, 37, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Chitkara, D.; Shikanov, A.; Kumar, N.; Domb, A.J. Biodegradable injectable in situ depot-forming drug delivery systems. Macromol. Biosci. 2006, 6, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Packhäuser, C.B.; Schnieders, J.; Oster, C.G.; Kissel, T. In situ forming parenteral drug delivery systems: an overview. Eur. J. Pharm. Biopharm. 2004, 58, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Wischle, C.; Schwendeman, S.P. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Varde, N.K.; Pack, D.W. Microspheres for controlled release drug delivery. Exp. Opin. Biol. Ther. 2004, 4, 35–51. [Google Scholar] [CrossRef]
- Nicolini, A.; Martinetti, L.; Crespi, S.; Maggioni, M.; Sangiovani, A. Transarterial chemoembolization with epirubicin-eluting beads versus trransarterial embolization before liver transplantation for hepatocellular carcinoma. J. Vasc. Interven. Radiol. 2010, 21, 327–332. [Google Scholar] [CrossRef]
- Cavalieri, F.; Chiessi, E.; Villa, R.; Vigano, L.; Zaffaroni, N.; Telling, M.F.; Paradossi, G. Novel PVA-based hydrogel microparticles for doxorubicin delivery. Biomacromolecules 2008, 9, 1967–1973. [Google Scholar] [CrossRef] [PubMed]
- Saralidze, K.; Knetsch, M.L.W.; van Hooy-Corstjens, C.S.J.; Koole, L.H. Radio-opaque and surface-functionalized polymer microparticles: potentially safer biomaterials for different injection therapies. Biomacromolecules 2006, 7, 2991–2996. [Google Scholar] [CrossRef] [PubMed]
- Arshady, R. Preparation of polymer nano- and microspheres by vinyl polymerization techniques. J. Microencapsul. 1988, 5, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tian, W.; Gao, S.; Yu, Y.; Yang, W.; Bai, G. Immobilization staphylococcal protein a on magnetic cellulose microspheres for IgG affinity purification. Artif. Cells Blood Substit. Immobil. Biotechnol. 2007, 35, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Chilian, W.M.; Eastham, C.L.; Layne, S.M.; Marcus, M.L. Small vessel phenomena in the coronary microcirculation: phasic intramyocardial perfusion and coronary microvascular dynamics. Progr. Cardiovasc. Dis. 1988, 31, 17–38. [Google Scholar] [CrossRef]
- Quinones, A.; Cheirif, B.J. New perspectives for perfusion imaging echocardiography. Circulation 1991, 83 (suppl. 5), III104–III110. [Google Scholar]
- Hong, S.J.; Yu, H.S.; Kim, H.W. Preparation of porous bioactive ceramic microspheres and in vitro osteoblastic culturing for tissue engineering application. Acta Biomater. 2009, 5, 1725–1731. [Google Scholar] [CrossRef] [PubMed]
- Yaparpalvi, R.; Loyalka, S.K.; Tompson, R.V., Jr. Production of spherical ZrO2-Y2O3 and ZnO particles. J. Biomed. Mater. Res. 1994, 28, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ε-caprolactone microspheres and nanospheres: an overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by solvent evaporation: state of the art for process engineering approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Saralidze, K.; Aldenhoff, Y.B.J.; Knetsch, M.L.W.; Koole, L.H. Injectable polymeric microspheres with X-ray visibility. Preparation, properties, and potential utility as new traceable bulking agents. Biomacromolecules 2003, 4, 793–798. [Google Scholar] [CrossRef]
- Gorodetsky, S.N.; Marx, G.; Gal, D.; Rivkin, R.; Ben-Ari, A.; Landsman, A.; Haviv, Y.S. Fibrin microbeads (FMB) as a 3D platform for kidney gene and cell therapy. Kidney Int. 2006, 69, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, R.; Clark, R.A.; An, J.; Gailit, J.; Levdansky, L.; Vexler, A.; Berman, E.; Marx, G. Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. J. Invest. Dermatol. 1999, 112, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Sengel, C.T.; Hascicek, C.; Gönül, N. Development and in vitro evaluation of modified release tablets including ethylcellulose microspheres loaded with diltiazem hydrochloride. J. Microencapsul. 2006, 23, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J. The promise of chitosan microspheres in drug delivery systems. Expert Opin. Drug Deliv. 2007, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Changerath, R.; Nair, P.D.; Mathew, S.; Nair, C.P.R. Poly(ethyl methacrylate)-grafted chitosan microspheres for controlled release of ampicillin. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2008, 89B, 65–76. [Google Scholar]
- Gupta, P.K.; Hung, C.T. Albumin microspheres I: physico-chemical characteristics. J. Microencapsul. 1989, 6, 427–462. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, H.; Sharma, R.K.; Mishra, A.K.; Chuttani, K.; Murhty, R.R. Albumin microspheres as carriers for the antiarthritic drug celecoxib. AAPS Pharm. Sci. Tech. 2005, 6, E65–E73. [Google Scholar] [CrossRef]
- Bitz, C.; Doelker, E. Influence of the preparation method on residual solvents in biodegradable microspheres. Int. J. Pharm. 1996, 131, 171–181. [Google Scholar] [CrossRef]
- Witschi, C.; Doelker, E. Influence of the microencapsulation method and peptide loading on poly(lactic acid) and poly(lactic-co-glycolic acid) degradation during in vitro testing. J. Control. Release 1998, 51, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Giunchedi, P.; Alpar, H.O.; Conte, U. PDLLA microspheres containing steroids: spray-drying, o/w and w/o/w emulsifications as preparation methods. J. Microencapsul. 1998, 15, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Panduranga Rao, K. Synthesis, characterization, and in vitro release of ibuprofen from poly(MMA-HEMA) copolymeric core-shell hydrogel microspheres for biomedical applications. J. Appl. Polym. Sci. 2002, 83, 3045–3054. [Google Scholar] [CrossRef]
- Jayakrishnan, A.; Thanoo, B.C. suspension polymerization of 2-hydroxyethyl methacrylate in the presence of polymeric diluents: a novel route to spherical highly porous beads for biomedical applications. J. Biomed. Mater. Res. 1990, 24, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Watts, P.J.; Davies, M.C.; Melia, C.D. Microencapsulation using emulsification/solvent evaporation: an overview of techniques and applications. Crit. Rev. Ther. Drug Carrier Syst. 1990, 7, 235–259. [Google Scholar] [PubMed]
- Ahmad, H.; Upin, D.; Armes, S.P.; Lewis, A.L. Synthesis of biocompatible sterically-stabilized poly(2-(methacryloyloxy)ethyl posphorylcholine) latexes via dispersion polymerization in alcohol/water mixtures. Langmuir 2009, 25, 11442–11449. [Google Scholar] [CrossRef] [PubMed]
- Piskin, E.; Tuncel, A.; Denizli, A.; Ayhan, H. Monosize microbeads based on polystyrene and their modified forms for some selected medical and biological applications. J. Biomater. Sci. Polym. Ed. 1994, 5, 451–471. [Google Scholar] [CrossRef] [PubMed]
- Ruckenstein, E.; Sun, Y. Preparation and characteristics of polymer-based large adsorbent particles. J. Appl. Polym. Sci. 1996, 61, 1949–1956. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hong, L. Sedimentation polymerization. Polymer 1995, 36, 2857–2860. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E.; Passirani, C.; Montero-Menei, C.N.; Benoit, J.P. Biocompatibility of implantable synthetic polymerc drug crriers: focus on brain biocompatibility. Biomaterials 2003, 24, 331–3331. [Google Scholar] [CrossRef]
- Christensen, L.H. Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatol. Surg. 2009, 35 (Suppl. 2), 1612–1619. [Google Scholar] [CrossRef]
- Kusin, S.; Lippitz, J. Skin fillers. Dis. Month 2009, 55, 236–256. [Google Scholar] [CrossRef]
- Segall, L.; Ellis, D.A.F. Therapeutic options for lip augmentation. Facial Plast. Surg. Clin. N. Am. 2007, 15, 485–490. [Google Scholar] [CrossRef]
- Keegan, P.E.; Atiemo, K.; Cody, J.D.; McClinton, S.; Pickard, R. Periurethral injection therapy for urinary incontinence in women. Cochrane Database Syst. Rev. 2007, 3. No. CD003881. [Google Scholar]
- Appell, R.A.; Dmochowski, R.R.; Herschorn, S. Urethral injections for female stress incontinence. BJU Int. 2006, 98 (Suppl. 1), 27–30. [Google Scholar] [CrossRef]
- Herschorn, S. Current role of injectable agents for female stress urinary incontinence. Can. J. Urol. 2006, 13 (suppl. 1), 5–12. [Google Scholar]
- Saralidze, K.; van Hooy-Corstjens, C.S.J.; Koole, L.H.; Knetsch, M.L.W. New acrylic microspheres for arterial embolization: combining radiopacity for precise localization with immobilized thrombin to trigger local blood coagulation. Biomaterials 2007, 28, 2457–2464. [Google Scholar] [CrossRef]
- van Hooy-Corstjens, C.S.J.; Saralidze, K.; Knetsch, M.L.W.; Emans, P.J.; de Haan, M.W.; Magusin, P.C.; Mezari, B.; Koole, L.H. New intrinsically radiopaque hydrophilic microspheres for embolization: synthesis and characterization. Biomacromolecules 2008, 9, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Abrams, P.; Cardozo, L.; Fall, M.; Griffiths, D.; Rosier, P.; Ulmsten, U.; Van Kerrebroeck, P.; Victor, A.; Wein, A. Standardization Sub-Committee of the International Continence Society. The standardization of terminology in lower urinary tract function: report from the standardization sub-committee of the International Continence Society. Urology 2003, 61, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Breasted, J.H. Edwin Smith surgical papyrus in facsimile and hieroglyphic transliteration with translation and commentary; University of Chicago Oriental Institute: Chicago, IL, USA, 1930. [Google Scholar]
- Ebbel, B. The papyrus Ebers: The greatest Egyptian medical document; Levin & Munksgaard: Copenhagen, Denmark, 1937. [Google Scholar]
- Kegel, A.H. Progressive resistance exercise in the functional restoration of the perineal muscles. Am. J. Obste. Gynecol. 1948, 56, 238–248. [Google Scholar]
- Cammu, H.; Van Nylen, M.; Amy, J. A ten-year follow-up after Kegel pelvic floor muscle exercises for genuine stress incontinence. BJU Int. 2000, 85, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Hay-Smith, E.J.; Dumoulin, C. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst. Rev. 2006, 25, CD005654. [Google Scholar]
- Bo, K. Pelvic floor muscle training is effective in treatment of female stress urinary incontinence, but how does it work? Int. Urogynecol. J. Pelvic Floor Dysfunct. 2004, 15, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Oelke, M.; Roovers, J.P.; Michel, M.C. Safety and tolerability of duloxetine in women with stress urinary incontinence. Br. J. Obstet. Gynaecol. 2006, 113 (Suppl. 1), 22–26. [Google Scholar] [CrossRef]
- Guay, D.R. Duloxetine for management of stress urinary incontinence. Am. J. Geriatr. Pharmacother. 2005, 3, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, P.; Ballantyne, Z.; N'Dow, J.M.; Alhasso, AA. Serotonin and noradrenaline reuptake inhibitors (SNRI) for stress urinary incontinence in adults. Cochrane Database Syst. Rev. 2005, 3, CD004742. [Google Scholar] [PubMed]
- Mariappan, P.; Alhasso, A.; Ballantyne, Z.; Grant, A.; N'Dow, J. Duloxetine, a serotonin and noradrenaline reuptake inhibitor (SNRI) for the treatment of stress urinary incontinence: a systematic review. Eur. Urol. 2007, 51, 67–74. [Google Scholar] [CrossRef] [PubMed]
- van Kerrebroeck, P.; Abrams, P.; Lange, R.; Slack, M.; Wyndaele, J.J.; Yalcin, I.; Bump, R.C. Duloxetine Urinary Incontinence Study Group. Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. Br. J. Obstet. Gynaecol. 2004, 111, 249–257. [Google Scholar] [CrossRef]
- Lenzer, J. FDA warns that antidepressants may increase suicidality in adults. BMJ 2005, 331, 70. [Google Scholar] [CrossRef] [PubMed]
- Ulmsten, U.; Henriksson, L.; Johnson, P.; Varhos, G. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 1996, 7, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Comiter, C.V. Surgery insight: management of failed sling surgery for female stress urinary incontinence. Nat. Clin. Pract. Urol. 2006, 3, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Flock, F.; Reich, A.; Muche, R.; Kreienberg, R.; Reister, F. Hemorrhagic complications associated with tension-free vaginal tape procedure. Obstet. Gynecol. 2004, 104, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Amundsen, C.L.; Flynn, B.J.; Webster, G.D. Urethral erosion after synthetic and nonsynthetic pubovaginal slings: differences in management and continence outcome. J. Urol. 2003, 170, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Madjar, S.; Tchetgen, M.B.; van Antwerp, A.; Abdelmalak, J.; Rackley, R.R. Urethral erosion of tension-free vaginal tape. Urology 2002, 59, 601. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, M.; Sivanesan, K.; Ramsay, I.; Pringle, S.; Bjornsson, S. How common are tape erosions? A comparison of two versions of the transobturator tension-free vaginal tape procedure. BJU Int. 2006, 98, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Yamada, B.S.; Govier, F.E.; Stefanovic, K.B.; Kobashi, K.C. High rate of vaginal erosions associated with the mentor ObTape. J. Urol. 2006, 176, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.L. Vaginal mesh extrusion associated with use of Mentor transobturator sling. Urology 2005, 66, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Comiter, C.V.; Colegrove, P.M. High rate of vaginal extrusion of silicone-coated polyester sling. Urology 2004, 63, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Govier, F.E.; Kobashi, K.C.; Kuznetsov, D.D.; Comiter, C.; Jones, P.; Dakil, S.E.; James, R., Jr. Complications of transvaginal silicone-coated polyester synthetic mesh sling. Urology 2005, 66, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Frederick, R.W.; Carey, J.M.; Leach, G.E. Osseous complications after transvaginal bone anchor fixation in female pelvic reconstructive surgery: report from single largest prospective series and literature review. Urology 2004, 64, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.P.; Tchetgen, M.B.; Sand, P.K.; Koduri, S.; Rackley, R.; Appell, R.; Culligan, P.J. Incidence of pubic osteomyelitis after bladder neck suspension using bone anchors. Urology 2004, 63, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.D.; Dmochowski, R.R.; Appell, R.A.; Sand, P.K.; Klimberg, I.W.; Jacoby, K.; Graham, C.W.; Snyder, J.A.; Nitti, V.W.; Winters, J.C. Multicenter prospective randomized 52-week trial of calcium hydroxylapatite versus bovine dermal collagen for treatment of stress urinary incontinence. Urology 2007, 69, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.R.; Haab, F.; Cervigni, M.; Dannecker, C.; Fianu-Jonasson, A.; Sultan, A.H. An open, multicentre study of NASHA/Dx Gel (Zuidex) for the treatment of stress urinary incontinence. Eur. Urol. 2005, 48, 488–494. [Google Scholar] [CrossRef] [PubMed]
- van Kerrebroeck, P.; ter Meulen, F.; Larsson, G.; Farrelly, E.; Edwall, L.; Fianu-Jonasson, A. Efficacy and safety of a novel system (NASHA/Dx copolymer using the Implacer device) for treatment of stress urinary incontinence. Urology 2004, 64, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, H.A.; Ghoniem, G.M. Obstructive suburethral mass after transurethral injection of dextranomer/hyaluronic acid copolymer. Int. Urogynecol. J. 2007, 18, 1379–1380. [Google Scholar] [CrossRef]
- Castillo-Vico, M.T.; Checa-Vizcaíno, M.A.; Payà-Panadés, A.; Rueda-García, C.; Carreras-Collado, R. Periurethral granuloma following injection with dextranomer/hyaluronic acid copolymer for stress urinary incontinence. Int. Urogynecol. J. 2007, 18, 95–97. [Google Scholar] [CrossRef]
- Lee, P.E.; Kung, R.C.; Drutz, H.P. Periurethral autologous fat injection as treatment for female stress urinary incontinence: a randomized double-blind controlled trial. J. Urol. 2001, 165, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.C. Long-term follow-up comparison of durasphere and contigen in the treatment of stress urinary incontinence. J. Low Genit. Tract. Dis. 2002, 6, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Chrouser, K.L.; Fick, F.; Goel, A.; Itano, N.B.; Sweat, S.D.; Lightner, D.J. Carbon coated zirconium beads in beta-glucan gel and bovine glutaraldehyde cross-linked collagen injections for intrinsic sphincter deficiency: continence and satisfaction after extended follow-up. J. Urol. 2004, 171, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, E.; McCrery, R.; Appell, R. The safety and efficacy of ethylene vinyl alcohol copolymer as an intra-urethral bulking agent in women with intrinsic urethral deficiency. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2007, 18, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Erekson, E.A.; Sung, V.W.; Rardin, C.R.; Myers, D.L. Ethylene vinyl alcohol copolymer erosions after use as a urethral bulking agent. Obstet. Gynecol. 2007, 109, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.F.; O'Reilly, B.A.; Dwyer, P.L.; Carey, M.P.; Cornish, A.; Schluter, P. Pubovaginal sling versus transurethral Macroplastique for stress urinary incontinence and intrinsic sphincter deficiency: a prospective randomised controlled trial. Br. J. Obstet. Gynaecol. 2005, 112, 797–801. [Google Scholar] [CrossRef]
- Radley, S.C.; Chapple, C.R.; Mitsogiannis, I.C.; Glass, K.S. Transurethral implantation of macroplastique for the treatment of female stress urinary incontinence secondary to urethral sphincter deficiency. Eur. Urol. 2001, 39, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Tamanini, J.T.; D'Ancona, C.A.; Netto, N.R. Treatment of intrinsic sphincter deficiency using the Macroplastique Implantation System: two-year follow-up. J. Endourol. 2004, 18, 906–911. [Google Scholar] [CrossRef]
- Kulkarni, S.; Davies, A.J.; Treurnicht, K.; Dudderidge, T.J.; Al-Akraa, M. Misplaced Macroplastique injection presenting as a vaginal nodule and a bladder mass. Int. J. Clin. Pract. Suppl. 2005, 147, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Peeker, R.; Edlund, C.; Wennberg, A.L.; Fall, M. The treatment of sphincter incontinence with periurethral silicone implants (macroplastique). Scand. J. Urol. Nephrol. 2002, 36, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Malizia, A.A.; Reiman, H.M.; Myers, R.P.; Sande, J.R.; Barham, S.S.; Benson, R.C.; Dewanjee, M.K.; Utz, W.J. Migration and granulomatous reaction after periurethral injection of polytef (Teflon). JAMA 1984, 251, 3277–3281. [Google Scholar] [PubMed]
- Kiilholma, P.J.; Chancellor, M.B.; Makinen, J.; Hirsch, I.H.; Klemi, P.J. Complications of Teflon injection for stress urinary incontinence. Neurourol. Urodyn. 1993, 12, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Dewan, P.A.; Owen, A.J.; Byard, R.W. Long-term histological response to subcutaneously injected Polytef and Bioplastique in a rat model. Br. J. Urol. 1995, 76, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Dewan, P.A. Is injected polytetrafluoroethylene (Polytef) carcinogenic? Br. J. Urol. 1992, 69, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Beavan, A. Material properties and applications of Pyrolite™ Carbon. Mater. Eng. 1990, 77, 39–41. [Google Scholar]
- Lightner, D.; Calvosa, C.; Andersen, R.; Klimberg, I.; Brito, C.G.; Snyder, J.; Gleason, D.; Killion, D.; Macdonald, J.; Khan, A.U.; Diokno, A.; Sirls, L.T.; Saltzstein, D. A new injectable bulking agent for treatment of stress urinary incontinence: results of a multicenter, randomized, controlled, double-blind study of Durasphere. Urology 2001, 58, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Lemperle, G.; Morhenn, V.B.; Pestonjamasp, V.; Gallo, R.L. Migration studies and histology of injectable microspheres of different sizes in mice. Plast. Reconstr. Surg. 2004, 113, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Pannek, J.; Brands, F.H.; Senge, T. Particle migration after transurethral injection of carbon coated beads for stress urinary incontinence. J. Urol. 2001, 166, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Hartanto, V.H.; Lightner, D.J.; Nitti, V.W. Endoscopic evacuation of Durasphere. Urology 2003, 62, 135–137. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration, Center for Devices and Radiological Health: Summary of safety and effectiveness data: Durasphere™ injectable bulking agent. 1999. Available at: www.fda.gov/cdrh/pdf/p980053.html (accessed April 2007).
- Madjar, S.; Sharma, A.K.; Waltzer, W.C.; Frischer, Z.; Secrest, L. Periurethral mass formations following bulking agent injection for the treatment of urinary incontinence. J. Urol. 2006, 175, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Ghoniem, G.M.; Khater, U. Urethral prolapse after durasphere injection. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006, 17, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Madjar, S.; Covington-Nichols, C.; Secrest, C.L. New periurethral bulking agent for stress urinary incontinence: modified technique and early results. J. Urol. 2003, 170, 2327–2329. [Google Scholar] [CrossRef] [PubMed]
- Chrouser, K.L.; Fick, F.; Goel, A.; Itano, N.B.; Sweat, S.D.; Lightner, D.J. Carbon coated zirconium beads in beta-glucan gel and bovine glutaraldehyde cross-linked collagen injections for intrinsic sphincter deficiency: continence and satisfaction after extended follow-up. J. Urol. 2004, 171, 1152–1155. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, Center for Devices and Radiological Health: Summary of safety and effectiveness data: Coaptite®, injectable implant for soft tissue augmentation. 10 November 2005. Available at: http://www.fda.gov/cdrh/PDF4/p040047b.pdf (accessed April 2007).
- Food and Drug Administration, Center for Devices and Radiological Health: Summary of safety and effectiveness data: Radiesse®, injectable dermal filler. 22 December 2006. Available at: http://www.fda.gov/cdrh/pdf5/p050037.html (accessed April 2007).
- Mayer, R.; Lightfoot, M.; Jung, I. Preliminary evaluation of calcium hydroxylapatite as a transurethral bulking agent for stress urinary incontinence. Urology 2001, 57, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Lemperle, G.; Morhenn, V.; Charrier, U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthet. Plast. Surg. 2003, 27, 354–366. [Google Scholar] [CrossRef]
- Onol, F.F.; Tarcan, T.; Tinay, I.; Kotiloglu, E.; Simsek, F. Kidney loss due to periureteral fibrosis and ureteral obstruction secondary to migration of subureterically injected calcium hydroxylapatite. J. Pediat. Urol. 2006, 2, 503–508. [Google Scholar] [CrossRef]
- Palma, P.C.; Riccetto, C.L.; Martins, M.H.; Herrmann, V.; de Fraga, R.; Billis, A.; Netto, N.R., Jr. Massive prolapse of the urethral mucosa following periurethral injection of calcium hydroxylapatite for stress urinary incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006, 17, 670–671. [Google Scholar] [CrossRef] [PubMed]
- Stedman, T. Stedman’s medical dictionary, 27th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2000. [Google Scholar]
- Coldwell, D.M.; Stokes, K.R.; Yakes, W.F. Embolotherapy: agents, clinical applications, and techniques. Radiographics 1994, 14, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, D.E.; Geschwind, J.F. New interventions for liver tumors. Semin. Roentgenol. 2002, 37, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Becattini, C. Venous thromboembolism and atherosclerosis: common denominators or different diseases? J. Thromb. Haemost. 2006, 4, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Eliasson, A.; Bergqvist, D.; Björck, M.; Acosta, S.; Sternby, N.H.; Ogren, M. Incidence and risk of venous thromboembolism in patients with verified arterial thrombosis: a population study based on 23,796 consecutive autopsies. J. Thromb. Haemost. 2006, 4, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Ghirarduzzi, A.; Prins, M.H.; Pengo, V.; Davidson, B.L.; Sørensen, H.; Pesavento, R.; Iotti, M.; Casiglia, E.; Iliceto, S.; Pagnan, A.; Lensing, A.W. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J. Thromb. Haemost. 2006, 4, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Turpie, A.G. Preventing thromboembolism in patients with prosthetic heart valves. Cardiol. Clin. 1994, 12, 487–493. [Google Scholar] [PubMed]
- Al-Mubarak, N.; Roubin, G.S.; Vitek, J.J.; Iyer, S.S. Microembolization during carotid stenting with the distal-balloon antiemboli system. Int. Angiol. 2002, 21, 344–348. [Google Scholar] [PubMed]
- Liapi, E.; Geschwind, J.F. Transcatheter and ablative therapeutic approaches for solid malignancies. J. Clin. Oncol. 2007, 25, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Lupattelli, T.; Basile, A.; Garaci, F.G.; Simonetti, G. Percutaneous uterine artery embolization for the treatment of symptomatic fibroids: current status. Eur. J. Radiol. 2005, 54, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Helmberger, T.K.; Jakobs, T.F.; Reiser, M.F. Embolization of uterine fibroids. Abdom. Imaging 2004, 29, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W. Embolic agents used for bronchial artery embolization in massive haemoptysis. Exp. Opin. Pharmacother. 2004, 5, 361–367. [Google Scholar] [CrossRef]
- Funaki, B. Superselective embolization of lower gastrointestinal hemorrhage: a new paradigm. Abdom. Imaging 2004, 29, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.A. Thoracic embolotherapy for life-threatening haemoptysis: a pulmonologist's perspective. Respirology 2005, 10, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Enting, D.; van der Werf, T.S.; Prins, T.R.; Zijlstra, J.G.; Ligtenberg, J.J.; Tulleken, J.E. Massive haemoptysis: primary care, diagnosis and treatment. Ned. Tijdschr. Geneeskd. 2004, 148, 1582–1586. [Google Scholar] [PubMed]
- Abe, Y.; Nakamura, M.; Suzuki, K.; Hashizume, T.; Tanigaki, T.; Saito, T.; Fujino, T.; Kikuchi, K. Massive hemoptysis due to Mycobacterium fortuitum infection controlled with bronchial artery embolization - a case report. Clin. Imag. 1999, 23, 361–363. [Google Scholar] [CrossRef]
- Parker, W.H. Etiology, symptomatology, and diagnosis of uterine myomas. Fert. Steril. 2007, 87, 725–736. [Google Scholar] [CrossRef]
- Munro, M.G.; Lukes, A.S. Abnormal Uterine Bleeding and Underlying Hemostatic Disorders Consensus Group. Abnormal uterine bleeding and underlying hemostatic disorders: report of a consensus process. Fert. Steril. 2005, 84, 1335–1337. [Google Scholar] [CrossRef]
- Wegienka, G.; Baird, D.D.; Hertz-Picciotto, I.; Harlow, S.D.; Steege, J.F.; Hill, M.C.; Schectman, J.M.; Hartmann, K.E. Self-reported heavy bleeding associated with uterine leiomyomata. Obstet. Gynecol. 2003, 101, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.A.; Warner, M.; Samuels, S.; Olive, D.; Vercellini, P.; Eskenazi, B. Uterine fibroids and gynecologic pain symptoms in a population-based study. Fert. Steril. 2003, 80, 1488–1494. [Google Scholar] [CrossRef]
- Dick, M.L. Chronic pelvic pain in women: assessment and management. Aust. Fam. Physician 2004, 33, 971–976. [Google Scholar] [PubMed]
- Hosokawa, Y.; Kishino, T.; Ono, T.; Oyama, N.; Momose, H. Two cases of female acute urinary retention caused by an impacted pelvic mass. Int. J. Urol. 2005, 12, 1069–1070. [Google Scholar] [CrossRef] [PubMed]
- Barnacle, S.; Muir, T. Intermittent urinary retention secondary to a uterine leiomyoma. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2007, 18, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Iavazzo, C.; Myriokefalitaki, E.; Vorgias, G.; Akrivos, T.; Lekka, I.; Katsoulis, M. Giant solid abdominal mass with cystic lesions: a case report and diaphorodiagnostic approach. Bratisl. Lek. Listy 2006, 107, 445–447. [Google Scholar] [PubMed]
- Vilos, G.A. Uterine fibroids: relationships to reproduction. Minerva Ginecol. 2003, 55, 417–423. [Google Scholar] [PubMed]
- Casini, M.L.; Rossi, F.; Agostini, R.; Unfer, V. Effects of the position of fibroids on fertility. Gynecol. Endocrinol. 2006, 22, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.S.; Jones, G.; Mauskopf, J.; Spalding, J.; DuChane, J. Uterine fibroids: a review of health-related quality of life assessment. J. Womens Health (Larchmt) 2006, 15, 818–829. [Google Scholar]
- Myers, E.R.; Barber, M.D.; Gustilo-Ashby, T.; Couchman, G.; Matchar, D.B.; McCrory, D.C. Management of uterine leiomyomata: what do we really know? Obstet. Gynecol. 2002, 100, 8–17. [Google Scholar] [PubMed]
- Kjerulff, K.H.; Langenberg, P.W.; Rhodes, J.C.; Harvey, L.A.; Guzinski, G.M.; Stolley, P.D. Effectiveness of hysterectomy. Obstet. Gynecol. 2000, 95, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.E.; Ma, C.; Lamvu, G.M.; Langenberg, P.W.; Steege, J.F.; Kjerulff, K.H. Quality of life and sexual function after hysterectomy in women with preoperative pain and depression. Obstet. Gynecol. 2004, 104, 701–709. [Google Scholar] [CrossRef] [PubMed]
- West, S.; Ruiz, R.; Parker, W.H. Abdominal myomectomy in women with very large uterine size. Fert. Steril. 2006, 85, 36–39. [Google Scholar] [CrossRef]
- Muñoz, J.L.; Jiménez, J.S.; Hernández, C.; Vaquero, G.; Pérez Sagaseta, C.; Noguero, R.; Miranda, P.; Hernández, J.M.; De la Fuente, P. Hysteroscopic myomectomy: our experience and review. JSLS 2003, 7, 39–48. [Google Scholar] [PubMed]
- Hurst, B.S.; Matthews, M.L.; Marshburn, P.B. Laparoscopic myomectomy for symptomatic uterine myomas. Fert. Steril. 2005, 83, 1–23. [Google Scholar] [CrossRef]
- Chwalisz, K.; Perez, M.C.; Demanno, D.; Winkel, C.; Schubert, G.; Elger, W. Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr. Rev. 2005, 26, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Chavez, N.F.; Stewart, E.A. Medical treatment of uterine fibroids. Clin. Obstet. Gynecol. 2001, 44, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W. Embolic agents used for bronchial artery embolization in massive haemoptysis. Expert Opin. Pharmacother. 2004, 5, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Weichert, W.; Denkert, C.; Gauruder-Burmester, A.; Kurzeja, R.; Hamm, B.; Dietel, M.; Kroencke, T.J. Uterine arterial embolization with tris-acryl gelatin microspheres. Am. J. Surg. Pathol. 2005, 29, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Gonzalez, M.V.; Leppard, S.W.; Brown, J.E.; Stratford, P.W.; Phillips, G.J.; Lloyd, A.W. Doxorubicin eluting beads–1: effects of drug loading on bead characteristics and drug distribution. J. Mater. Sci. Mater. Med. 2007, 18, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.V.; Tang, Y.; Phillips, G.J.; Lloyd, A.W.; Hall, B.; Stratford, P.W.; Lewis, A.L. Doxorubicin eluting beads–2: methods for evaluating drug elution and in vitro:in vivo correlation. J. Mater. Sci. Mater. Med. 2008, 19, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Bratby, M.J.; Belli, A.M. Radiological treatment of symptomatic uterine fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.K.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.L.; Gonzalez, M.V.; Lloyd, A.W.; Hall, B.; Tang, Y.; Willis, S.L.; Leppard, S.W.; Wolfenden, L.C.; Palmer, R.R.; Stratford, P.W. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J. Vasc. Interven. Radiol. 2006, 17, 335–342. [Google Scholar] [CrossRef]
- Nitta, N.; Ohta, S.; Tanaka, T.; Takazakura, R.; Toyama, T.; Sonoda, A.; Seko, A.; Furukawa, A.; Takahashi, M.; Murata, K.; Kurumi, Y.; Tani, T.; Sakamoto, T.; Tabata, Y. An initial clinical study on the fficacy of cisplatinpreleasing gelatin microspheres for metastatic liver tumors. Eur. J. Radiol. 2009, 71, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Gemeinhart, R.A. Cisplatin delivery from poly(acrylic acid-co-methyl methacrylate) microparticles. J. Control. Release 2005, 106, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Hovsepian, D.M.; Mandava, A.; Pilgram, T.K.; Holder, A.P.; Wong, V.; Chan, P.; Patel, T. Comparison of adjunctive use of rofecoxib versus ibuprofen in the management of postoperative pain after uterine artery embolization. J. Vasc. Interven. Radiol. 2006, 17, 665–670. [Google Scholar] [CrossRef]
- Borovac, T.; Pelage, J.P.; Kasselouri, A.; Prognon, P.; Guiffant, G.; Laurent, G. Release of ibuprofen from beads for embolization: in vitro and in vivo studies. J. Control. Release 2006, 115, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Wassef, M.; Pelage, J.P.; Velzenberger, E.; Namur, J.; Schwartz-Cornil, I.; Taylor, R.R.; Lewis, A.L.; Laurent, G. Anti-inflammatory effect of ibuprofen-loaded embolization beads in sheep uterus. J. Biomed. Mater. Res. Part B. Appl. Biomater. 2008, 86B, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Ping, Q.; Gao, Y. Effects of formulation factors on encapsulation efficiency and release behaviour in vitro of huperzine A-PLGA microspheres. J. Microencapsul. 2005, 22, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Hamishehkar, H.; Emami, J.; Najafabadi, A.R.; Gilani, K.; Minaiyan, M.; Mahdvi, H.; Nokodchi, A. The effect of formulation variables on the characteristics of insulin-loaded poly(lactic-co-glycolic acid) microspheres prepared by a single phase oil in oil solvent evaporation method. Colloids Surf. B 2009, 74, 340–349. [Google Scholar] [CrossRef]
- Raman, C.; Berkland, C.; Kim, K.; Pack, D.W. Modeling small-molecule release from PLG microspheres: effects of polymer degradation and non-uniform drug distribution. J. Control. Release 2005, 103, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Berchane, N.S.; Carson, K.H.; Rice-Ficht, A.C.; Andrews, M.J. Effect of mean diameter and polydispersity of PLG microspheres on drug release: experiment and theory. Int. J. Pharm. 2007, 337, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Berchane, N.; Jebrail, F.F.; Andrews, M.J. Optimization of PLG microspheres for tailored drug release. Int. J. Pharm. 2010, 383, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Berchane, N.S.; Jebrail, F.F.; Carson, K.H.; Rice-Ficht, A.C.; Andrews, M.J. About mean diameter and size distributions of poly(lactide-co-glycolide) [PLG] microspheres. J. Microencapsul. 2006, 23, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Dawes, G.J.S.; Fratila-Apachitie, L.E.; Mulia, K.; Apachitie, I.; Witkamp, G.J.; Duszczyk, J. Size effect of PLGA spheres on drug loading efficiency and release profiles. J. Mater. Sci. Mater. Med. 2009, 20, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Pandurangarao, K. In vitro release of ibuprofen and gentamicin from PMMA functional microspheres. J. Biomater. Sci. Polym. Ed. 2002, 13, 111–126. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saralidze, K.; Koole, L.H.; Knetsch, M.L.W. Polymeric Microspheres for Medical Applications. Materials 2010, 3, 3537-3564. https://doi.org/10.3390/ma3063537
Saralidze K, Koole LH, Knetsch MLW. Polymeric Microspheres for Medical Applications. Materials. 2010; 3(6):3537-3564. https://doi.org/10.3390/ma3063537
Chicago/Turabian StyleSaralidze, Ketie, Leo H. Koole, and Menno L.W. Knetsch. 2010. "Polymeric Microspheres for Medical Applications" Materials 3, no. 6: 3537-3564. https://doi.org/10.3390/ma3063537




