A Preliminary Study on the Effect of Macro Cavities Formation on Properties of Carbon Nanotube Bucky-Paper Composites
Abstract
:1. Introduction
2. Experimental Section
2.1. Processing of the CNTs Based Composite Materials
2.2. Characterization Techniques
2.3. Gas Permeability
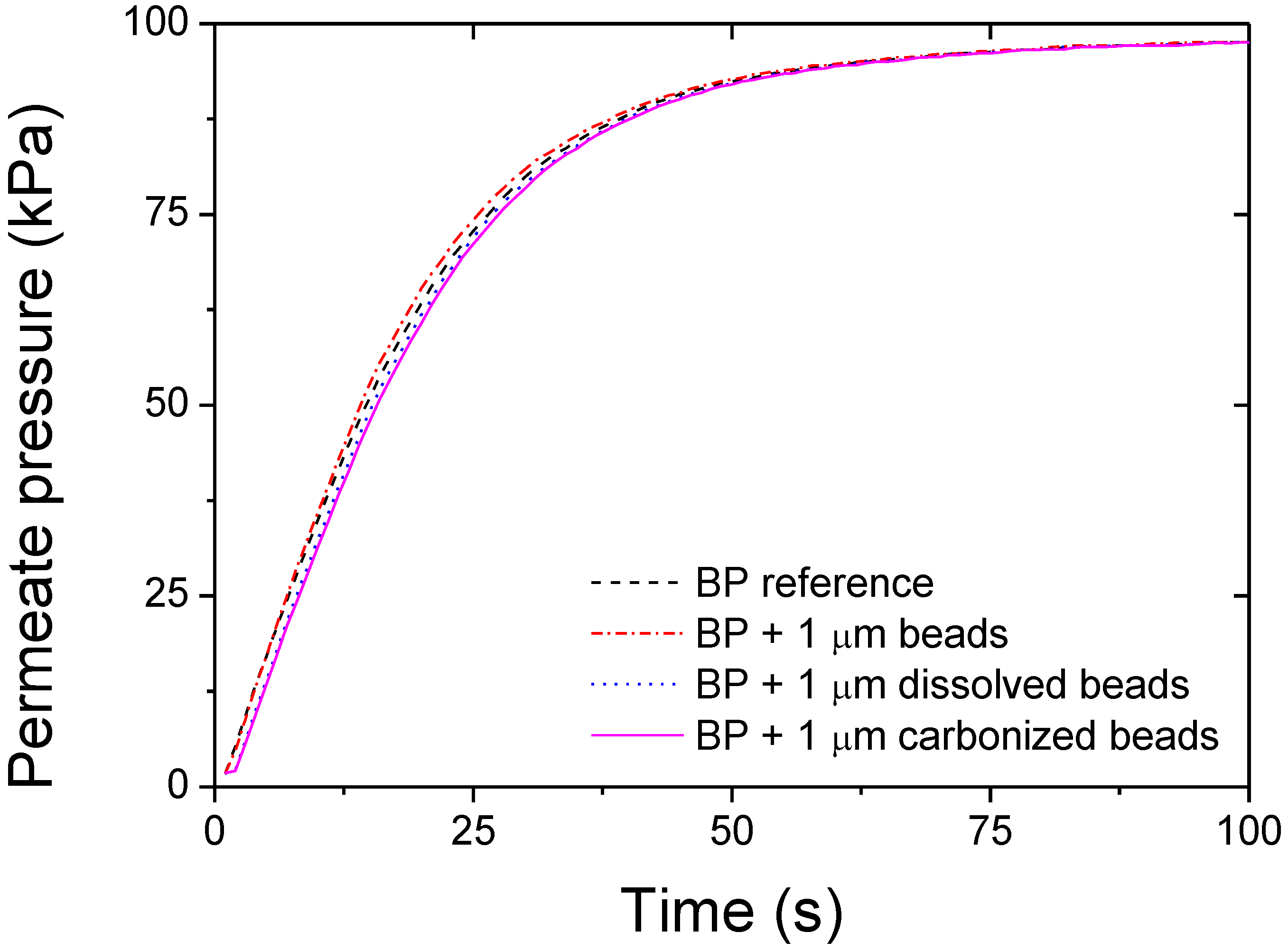
3. Results and Discussion
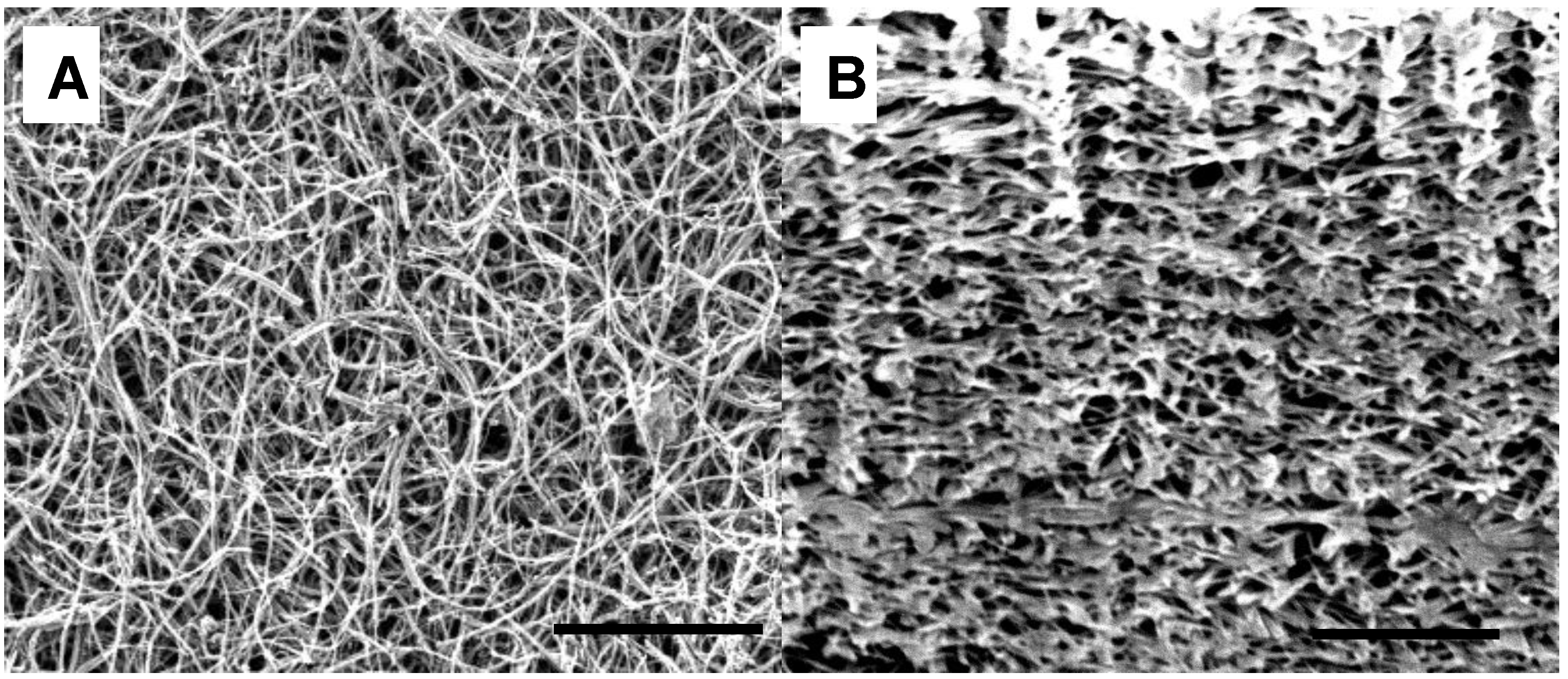
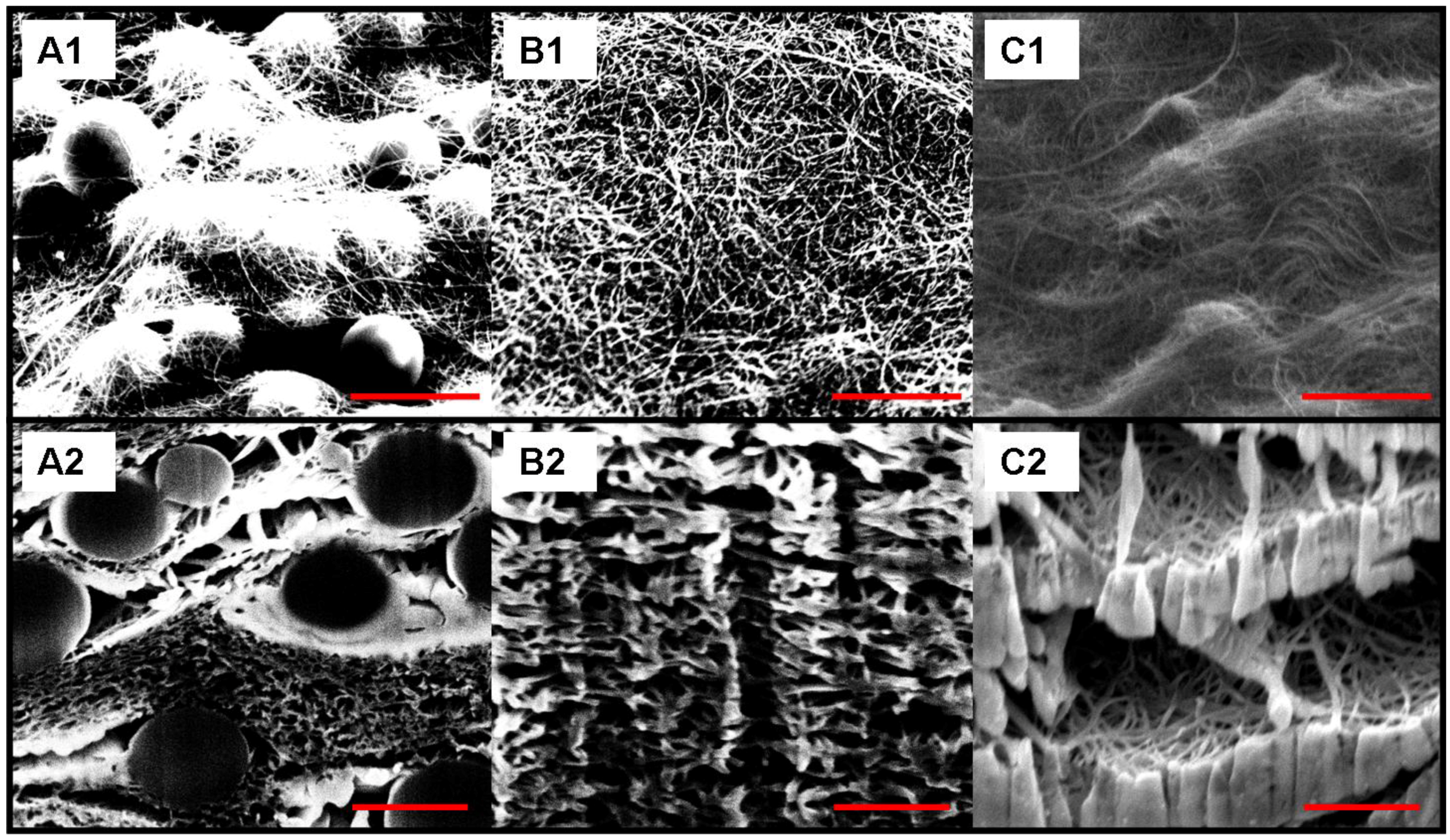
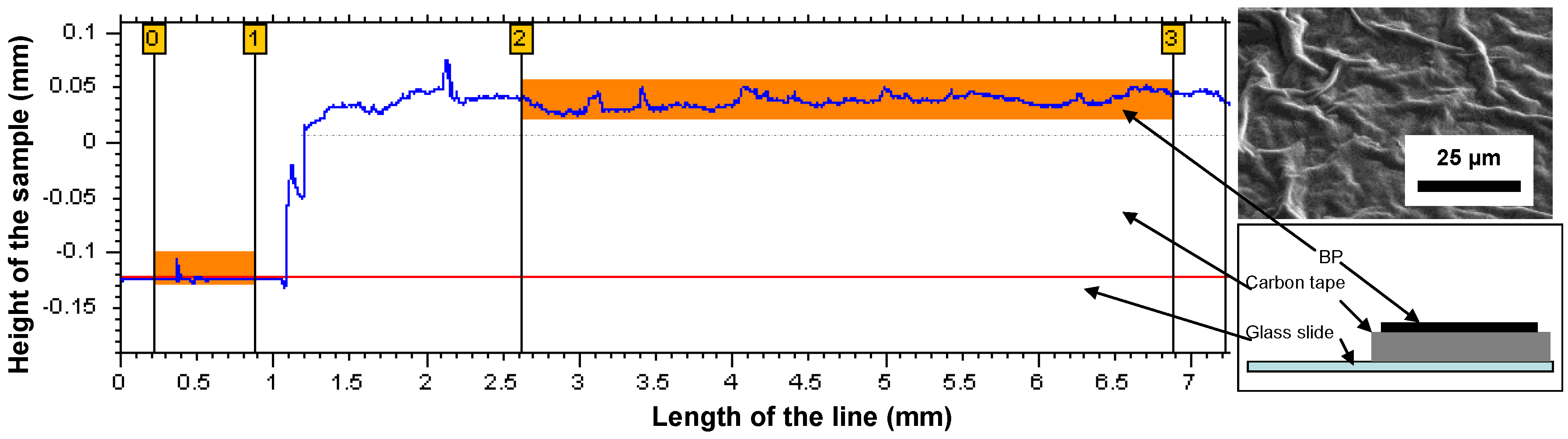
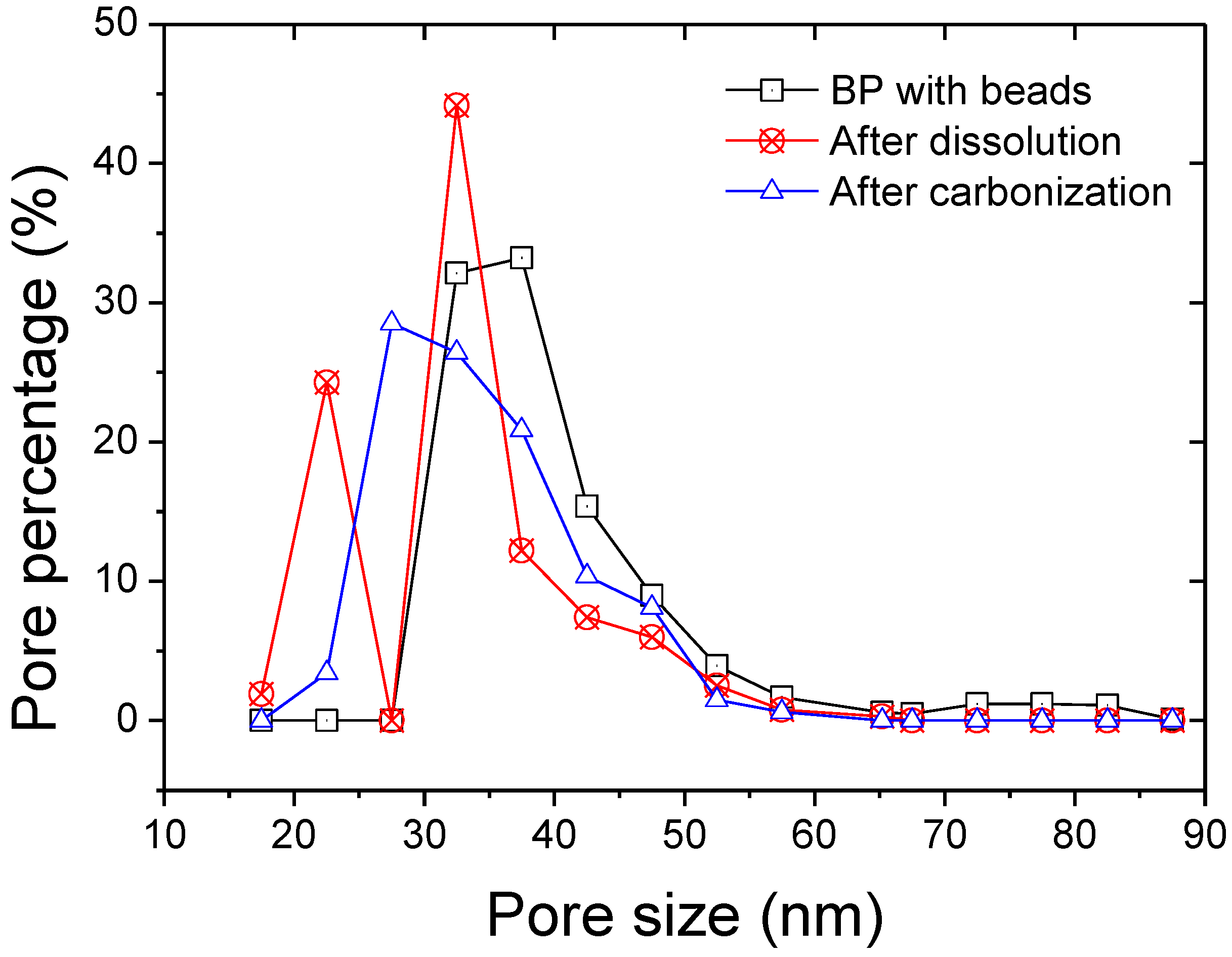

| Thickness | Tensile Modulus | Average pore size | Bubble point | BET | Permeability | |
|---|---|---|---|---|---|---|
| μm | GPa | nm | kPa | m2/g | x10 −11 kg.m−1.h−1.Pa−1 | |
| BP reference | 10 (+/−2) | 0.95 | 32 | 783 | 197 | 2.61 |
| BP + beads | 8 (+/−3) | 0.35 | 37 | 532 | 90.7 | 2.55 |
| BP + beads dissolved | 7 (+/−2) | 0.21 | 32 | 735 | 135.6 | 2.23 |
| BP + beads carbonized | 6 (+/−3) | 0.99 | 33 | 760 | 97.5 | 1.91 |
4. Conclusions
Acknowledgements
References
- Bernholc, J.; Brenner, D.; Buongiorno, M.; Nardelli, V.; Meunier, C. Roland, mechanical and electrical properties of nanotubes. Ann. Rev. Mater. Res. 2002, 32, 347–375. [Google Scholar] [CrossRef]
- Gonnet, P.; Liang, Z.; Choi, E.S.; Kadambala, R.S.; Zhang, C.; Brooks, J.S.; Wang, B.; Kramer, L. Thermal conductivity of magnetically aligned carbon nanotube buckypapers and nanocomposites. Curr. Appl. Phys. 2006, 6, 119–122. [Google Scholar] [CrossRef]
- Gou, J.; Liang, Z.Y.; Wang, B. Experimental design and optimization of dispersion process for single-walled carbon nanotube bucky paper. Int. J. Nanosci. 2004, 3, 293–307. [Google Scholar] [CrossRef]
- Smajda, R.; Kukovecz, A.; Konya, Z.; Kiricsi, I. Structure and gas permeability of multi-wall carbon nanotube buckypapers. Carbon 2007, 45, 1176–1184. [Google Scholar] [CrossRef]
- Nuriel, S.; Liu, L.; Barber, A.H.; Wagner, H.D. Direct measurement of multiwall nanotube surface tension. Chem. Phys. Lett. 2005, 404, 263–266. [Google Scholar] [CrossRef]
- Dumée, L.F.; Sears, K.; Schütz, J.; Finn, N.; Duke, M.; Gray, S. Characterization and evaluation of carbon nanotube bucky-paper membranes for direct contact membrane distillation. J. Membr. Sci. 2010, 351, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Bajpai, V.; Ji, T.; Dai, L.M. Chemistry of carbon nanotubes. Chem. Inform. 2003, 34, 635–651. [Google Scholar]
- Whitby, R.L.D.; Fukuda, T.; Maekawa, T.; James, S.L.; Mikhalovsky, S.V. Geometric control and tuneable pore size distribution of buckypaper and buckydiscs. Carbon 2008, 46, 949–956. [Google Scholar] [CrossRef]
- Whitby, R.L.D.; Mikhalovsky, S.V.; Gun'ko, V.M. Mechanical performance of highly compressible multi-walled carbon nanotube columns with hyperboloid geometries. Carbon 2010, 48, 145–152. [Google Scholar] [CrossRef]
- Whitby, R.L.D.; Gun'ko, V.M.; Fukuda, T.; Maekawa, T. Relating bulk resistivity to nanoscale mechanical responses of carbon nanotubes randomly orientated in monoliths under compression. Carbon 2010, 48, 3635–3637. [Google Scholar] [CrossRef]
- Das, R.K.; Liu, B.; Reynolds, J.R.; Rinzler, A.G. Engineered macroporosity in single-wall carbon nanotube films. Nano Lett. 2009, 9, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Kukovecz, Á.; Smajda, R.; Kónya, Z.; Kiricsi, I. Controlling the pore diameter distribution of multi-wall carbon nanotube buckypapers. Carbon 2007, 45, 1696–1716. [Google Scholar]
- Sears, K.; Dumée, L.; Schütz, J.; She, M.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Recent developments in carbon nanotube membranes for water purification and gas separation. Materials 2009, 3, 127–149. [Google Scholar] [CrossRef] [Green Version]
- Smajda, R.; Kukovecz, A.; Hopp, B.; Mohl, M.; Kónya, Z.; Kiricsi, I. Morphology and N2 permeability of multi-wall carbon nanotube-teflon membranes. J. Nanosci. Nanotechnol. 2007, 7, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Dumée, L.; Sears, K.; Schutz, J.; Finn, N.; Duke, M.; Gray, S. Carbon nanotube based composite membranes for water desalination by membrane distillation, desalination and water treatment. Desalination Water Treat. 2010, 17, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Dumee, L.; Velleman, L.; Sears, K.; Hill, M.; Schutz, J.; Finn, N.; Duke, M.; Gray, S. Control of porosity and pore size of metal reinforced carbon nanotube membranes. Membranes 2010, 1, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, C.P.; Hawkins, S.C. Understanding the synthesis of directly spinnable carbon nanotube forests. Carbon 2010, 48, 1105–1115. [Google Scholar] [CrossRef]
- Cooper, S.M.; Chuang, H.F.; Cinke, M.; Cruden, B.A.; Meyyappan, M. Gas permeability of a buckypaper membrane. Nano Lett. 2003, 3, 189–192. [Google Scholar] [CrossRef]
- Franken, A.C.M.; Nolten, J.A.M.; Mulder, M.H.V.; Bargeman, D.; Smolders, C.A. Wetting criteria for the applicability of membrane distillation. J. Membr. Sci. 1987, 33, 315–328. [Google Scholar] [CrossRef]
- Hernández-Pérez, A.; Avilés, F.; May-Pat, A.; Valadez-González, A.; Herrera-Franco, P.J.; Bartolo-Pérez, P. Effective properties of multiwalled carbon nanotube/epoxy composites using two different tubes. Compos. Sci. Technol. 2008, 68, 1422–1431. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dumée, L.; Sears, K.; Schütz, J.; Finn, N.; Duke, M.; Gray, S. A Preliminary Study on the Effect of Macro Cavities Formation on Properties of Carbon Nanotube Bucky-Paper Composites. Materials 2011, 4, 553-561. https://doi.org/10.3390/ma4030553
Dumée L, Sears K, Schütz J, Finn N, Duke M, Gray S. A Preliminary Study on the Effect of Macro Cavities Formation on Properties of Carbon Nanotube Bucky-Paper Composites. Materials. 2011; 4(3):553-561. https://doi.org/10.3390/ma4030553
Chicago/Turabian StyleDumée, Ludovic, Kallista Sears, Jürg Schütz, Niall Finn, Mikel Duke, and Stephen Gray. 2011. "A Preliminary Study on the Effect of Macro Cavities Formation on Properties of Carbon Nanotube Bucky-Paper Composites" Materials 4, no. 3: 553-561. https://doi.org/10.3390/ma4030553






