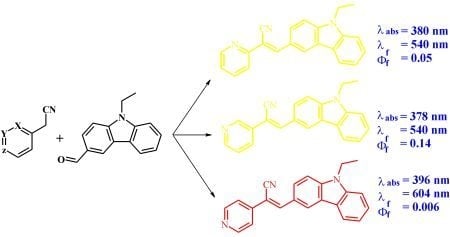
Synthesis, Characterization and Photophysical Properties of Pyridine-Carbazole Acrylonitrile Derivatives
Abstract
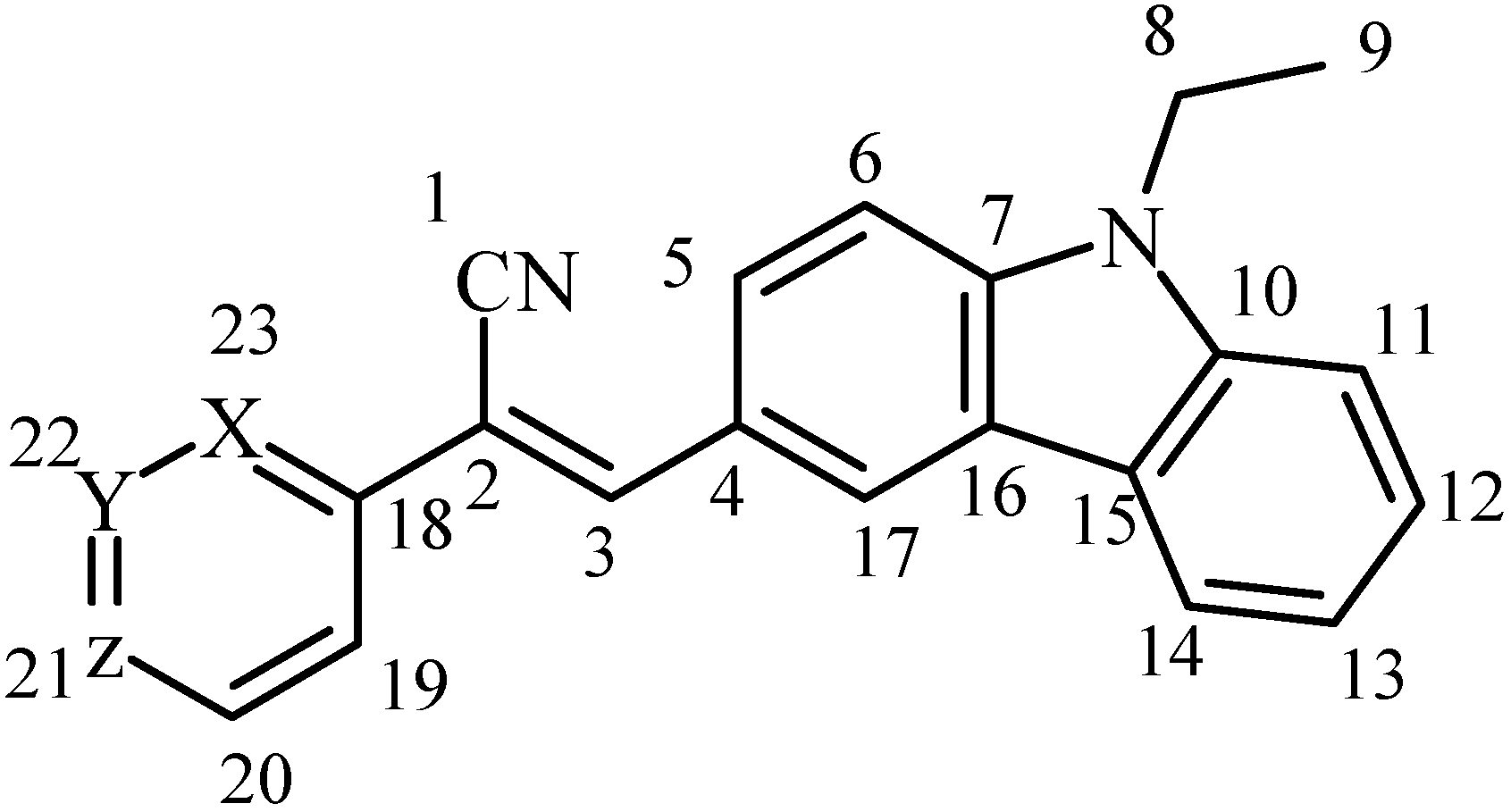
:1. Introduction

2. Results and Discussion
2.1. FT-IR and NMR Characteristics
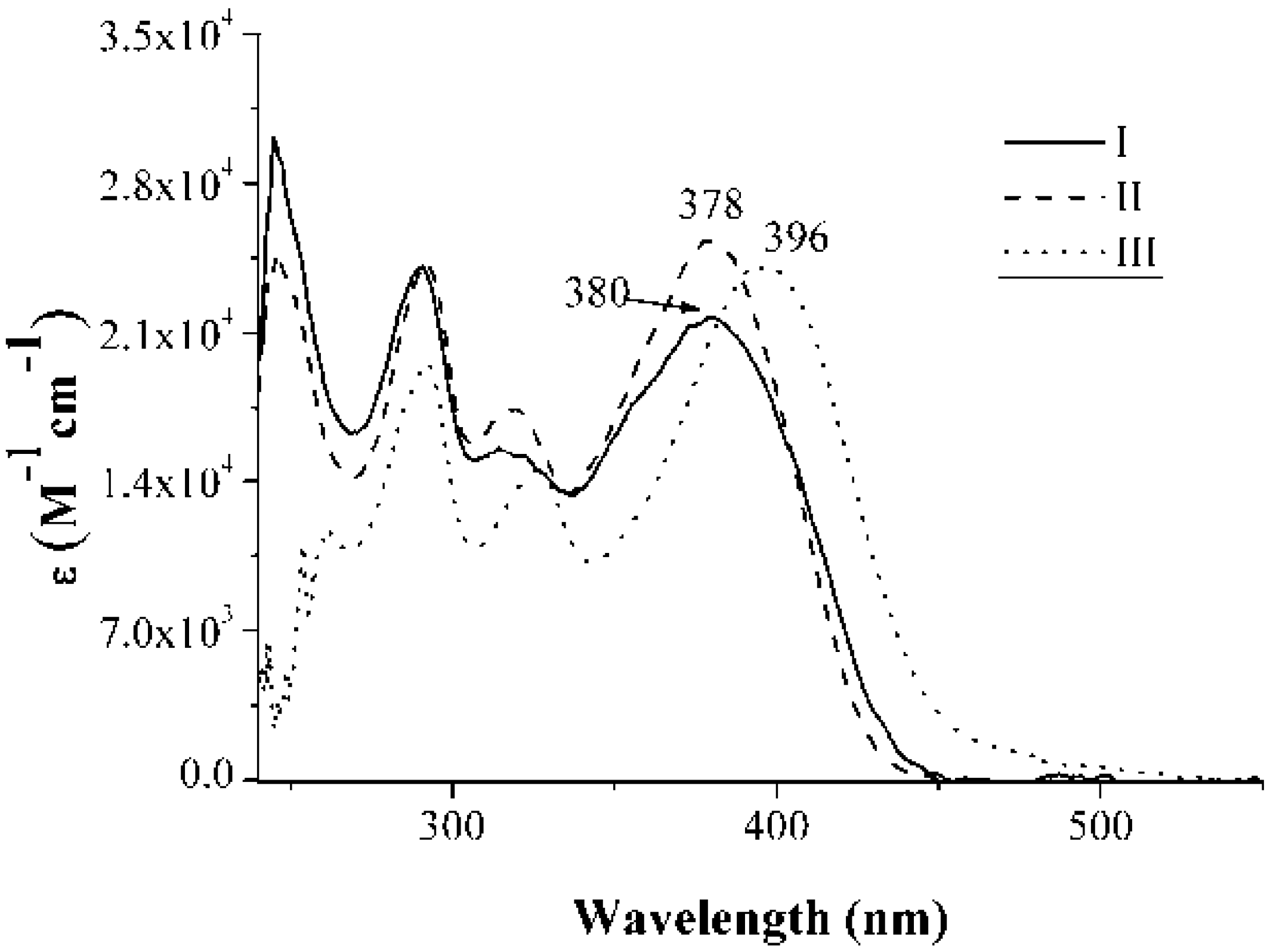
2.2. Absorption

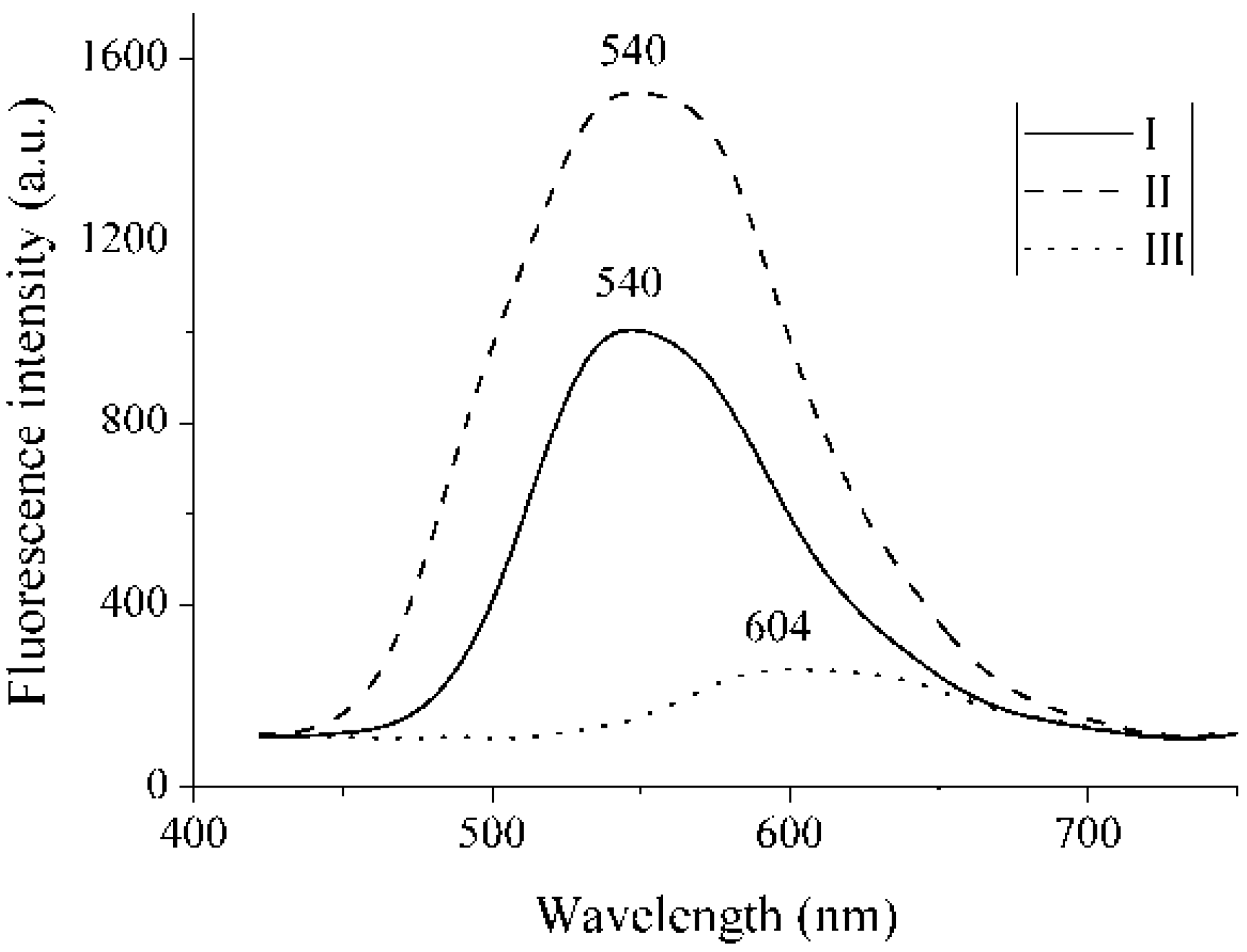
2.3. Fluorescence
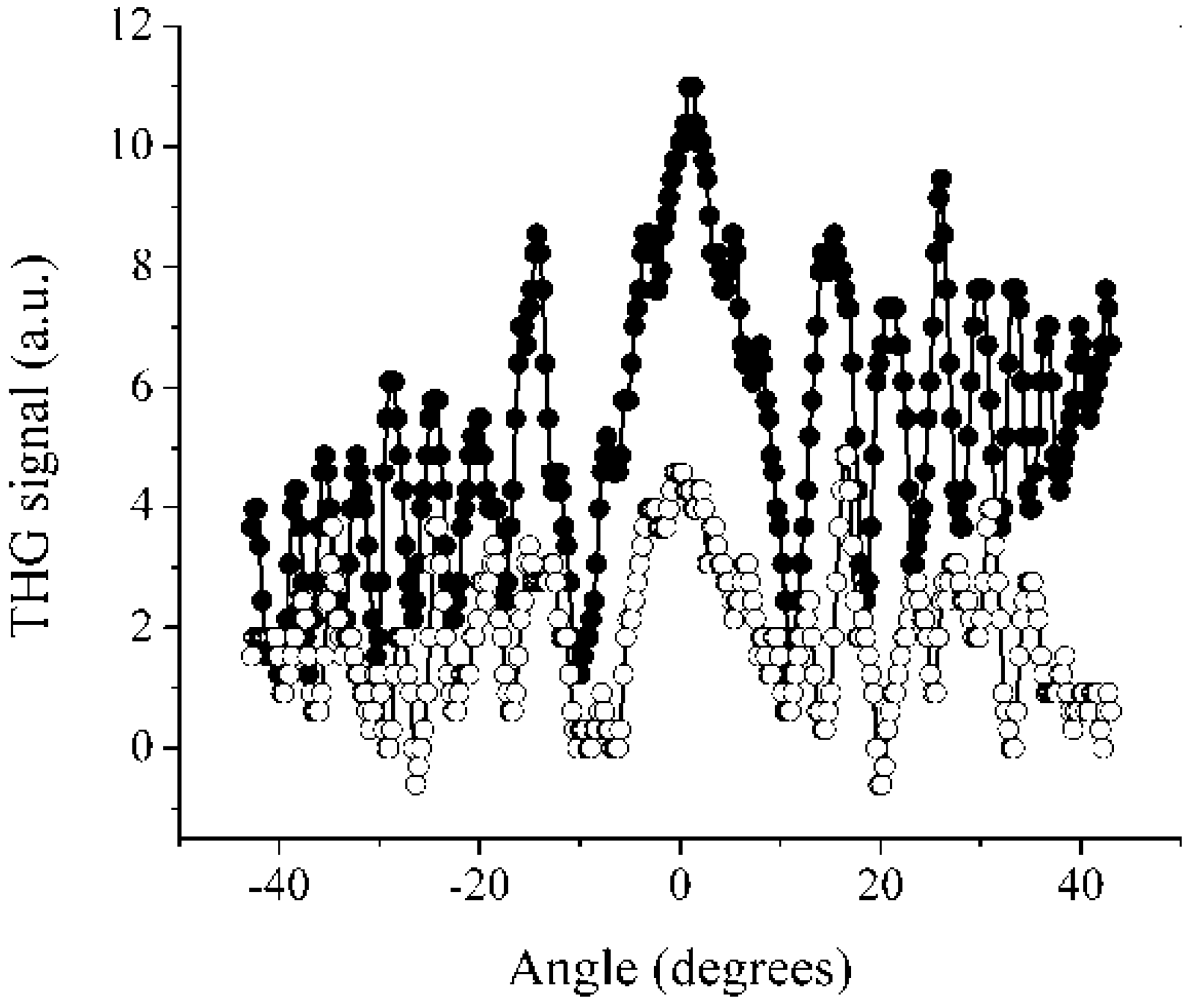
3. Experimental Section
3.1. Materials and Measurements
3.2. Synthesis and Characterization of Novel Acrylonitrile Compounds
4. Conclusions
Acknowledgements
References
- Gondek, E.; Danel, A.; Kityk, I.V. Single-layered light-emitting diodes possessing methoxy-modified pyrazoloquinoline dyes in poly-N-vinylcarbazole matrix. J. Lumin. 2008, 128, 348–354. [Google Scholar] [CrossRef]
- Patra, A.; Pan, M.; Friend, C.S.; Lin, T.-Ch.; Cartwright, A.N.; Prasad, P.N. Electroluminescence properties of systematically derivatized organic chromophores containing electron donor and acceptor groups. Chem. Mater. 2002, 14, 4044–4048. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar]
- Shaheen, E.S.; Christoph, J.B.; Sariciftci, N.S.; Padinger, F.; Fromherz, T.; Hummelen, J.C. 2.5% efficient organic plastic solar cells. Appl. Phys. Lett. 2001, 78, 841–843. [Google Scholar] [CrossRef]
- Tessler, N.; Denton, G.J.; Friend, R.H. Lasing from conjugated-polymer microcavities. Nature 1996, 382, 695–697. [Google Scholar] [CrossRef]
- Samuel, I.D.W.; Turnbull, G.A. Organic semiconductor lasers. Chem. Rev. 2007, 107, 1272–1295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiao, Y.; Chi, S.; Qian, X. Isomeric boron-fluorine complexes with donor-acceptor architecture: Strong solid/liquid fluorescence and large stokes shift. Org. Lett. 2008, 10, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Adès, D.; Boucard, V.; Cloutet, E.; Siove, A.; Olivero, C.; Castex, M.C.; Pichler, G. Photoluminescence of donor-acceptor carbazole-based molecules in amorphous and powder forms. J. Appl. Phys. 2000, 87, 7290–7293. [Google Scholar] [CrossRef]
- Molecular Association Including Molecular Complexes; Foster, R. (Ed.) Academic Press: New York, NY, USA, 1979; Volume 2.
- Adhikari, R.M.; Mondal, R.; Shah, B.K.; Neckers, D.C. Synthesis and photophysical properties of carbazole-based blue light-emitting dendrimers. J. Org. Chem. 2007, 72, 4727–4732. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, K.; Bernéde, J.C.; Marsillac, S.; Godoy, A.; Diaz, F.R. Carbazole based electroluminescent devices obtained by vacuum evaporation. Synth. Met. 2001, 122, 127–129. [Google Scholar] [CrossRef]
- Liu, Y.; Nishiura, M.; Wang, Y.; Hou, Z. π-Conjugated aromatic enynes as a single-emitting component for white electroluminescence. J. Am. Chem. Soc. 2006, 128, 5592–5593. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.R.J.; Lin, J.T.; Tao, Y.-T.; Ko, C.-W. Light-emitting carbazole derivatives: Potential electroluminescent materials. J. Am. Chem. Soc. 2001, 123, 9404–9411. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Chen, Z.; Bian, Z.; Liu, Z.; Gong, Z.; Baik, W.; Lee, H.; Huang, C. The host materials containing carbazole and oxadiazole fragment for red triplet emitter in organic light-emitting diodes. Org. Electron. 2006, 7, 330–336. [Google Scholar] [CrossRef]
- Strohriegl, P.; Grazulevicius, J.V. Handbook of Organic Conductive Molecules and Polymers; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Blouin, N.; Leclerc, M. Poly(2,7-carbazole)s: Structure-property relationships. Accounts Chem. Res. 2008, 41, 1110–1119. [Google Scholar] [CrossRef]
- Murphy, A.R.; Fréchet, J.M.J. Organic semiconducting oligomers for use in thin film transistors. Chem. Rev. 2007, 107, 1066–1096. [Google Scholar] [CrossRef] [PubMed]
- Drolet, N.; Morin, J.-F.; Leclerc, N.; Wakim, S.; Tao, Y.; Leclerc, M. 2,7-Carbazolenevinylene-based oligomer thin-film transistors: high mobility through structural ordering. Adv. Funct. Mater. 2005, 15, 1671–1682. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Leclerc, M. A low-bandgap poly(2,7-carbazole) derivative for use in high-performance solar cells. Adv. Mater. 2007, 19, 2295–2300. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Gendron, D.; Wakim, S.; Blair, E.; Neagu-Plesu, R.; Belletete, M.; Durocher, G.; Tao, Y.; Leclerc, M. Toward a rational design of poly(2,7-carbazole) derivatives for solar cells. J. Am. Chem. Soc. 2008, 130, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.S.; Li, Z.H.; Shek, M.F.; Samoc, M.; Samoc, A.; Luther-Davies, B. Synthesis and third-order nonlinear optical properties of end-functionalized oligo-phenylenevinylenes. Chem. Mater. 2002, 14, 2999–3004. [Google Scholar] [CrossRef]
- Samoc, M.; Samoc, A.; Luther-Davies, B.; Humphrey, M.G.; Wong, M.-S. Third-order optical nonlinearities of oligomers, dendrimers and polymers derived from solution Z-scan studies. Opt. Mater. 2003, 21, 485–488. [Google Scholar] [CrossRef]
- Meier, H.; Ickenroth, D.; Stalmach, U.; Koynov, K.; Bahtiar, A.; Bubeck, C. Preparation and nonlinear optics of monodisperse oligo(1,4-phenyleneethynylene)s. Eur. J. Org. Chem. 2001, 23, 4431–4435. [Google Scholar] [CrossRef]
- Yoon, C.B.; Lee, J.I.; Shim, H.K. Synthesis and third-order nonlinearity of dye-containing poly(1,4-phenylenevinylene) derivative. Synthetic Met. 1997, 84, 273–274. [Google Scholar] [CrossRef]
- Koynov, K.; Bahtiar, A.; Bubeck, C.; Muhling, B.; Meier, H. Effect of donor-acceptor substitution on the nonlinear optical properties of oligo(1,4-phenyleneethynylene)s studied by third harmonic generation spectroscopy. J. Phys. Chem. B 2005, 109, 10184–10188. [Google Scholar] [CrossRef] [PubMed]
- Mathy, A.; Ueberhofen, K.; Schenk, R.; Gregorius, H.; Garay, R.; Müllen, K.; Bubeck, C. Third-harmonic-generation spectroscopy of poly(p-phenylenevinylene): A comparison with oligomers and scaling laws for conjugated polymers. Phys. Rev. B 1996, 53, 4367–4376. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, F.; Gao, Y.; Song, Y.; Liu, H.; Gan, H.; Jiu, T.; Li, Y.; Li, Y.; Wang, S.; Zhu, D. Nonlinear optical properties of an ultrathin film containing porphyrin and poly (phenylenevinylene) units. Thin Solid Films 2006, 496, 311–316. [Google Scholar] [CrossRef]
- Bader, M.A.; Marowsky, G.; Bahtiar, A.; Koynov, K.; Bubeck, C.; Tillmann, H.; Hörhold, H.H.; Pereira, S. Poly(p-phenylenevinylene) derivatives: New promising materials for nonlinear all-optical waveguide switching. J. Opt. Soc. Am. B 2002, 19, 2250–2262. [Google Scholar] [CrossRef]
- Samoc, A.; Samoc, M.; Woodruff, M.; Luther-Davies, B. Tuning the properties of poly(p-phenylenevinylene) for use in all-optical switching. Opt. Lett. 1995, 20, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Samoc, A.; Samoc, M.; Woodruff, M.; Luther-Davies, B. Photonic Polymer Systems, Fundamentals, Methods and Applications; Ise, D.L., Wnek, G.E., Trantolo, D.J., Cooper, T.M., Gresser, J.D., Eds.; Marcel Dekker, Inc.: Basel, Switzerland, 1998. [Google Scholar]
- Dailey, S.; Halim, M.; Rebourt, E.; Hoursburgh, L.E.; Samuel, I.D.W.; Monkman, A.P. An efficient electron-transporting polymer for light-emitting diodes. J. Phys. Condens. Matter. 1998, 10, 5171–5178. [Google Scholar] [CrossRef]
- Epstein, A.J.; Blatchford, J.W.; Wang, Y.Z.; Jessen, S.W.; Gebler, D.D.; Lin, L.B.; Gustafson, T.L.; Wang, H.L.; Park, Y.W.; Swager, T.M.; MacDiarmind, A.G. Poly(p-pyridine)—and poly (p-pyridyl vinylene)-based polymers: Their photophysics and application to SCALE devices. Synt. Met. 1996, 78, 253–261. [Google Scholar] [CrossRef]
- Su, S.-J.; Sasabe, H.; Takeda, T.; Kido, J. Pyridine-containing bipolar host materials for highly efficient blue phosphorescent OLEDs. Chem. Mater. 2008, 20, 1691–1693. [Google Scholar] [CrossRef]
- Percino, M.J.; Chapela, V.M.; Urzúa, O.; Montiel, L.-F.; Rodríguez de Barbarín, C. 1-(p-Fluorophenyl)-2-(2’-pyridyl)ethanol and 1-(p-fluorophenyl)-2-(2’-pyridyl)ethene obtained from the condensation reaction of 2-picoline and p-fluorophenylaldehyde under catalyst-and solvent-free conditions. Res. Chem. Intermediat. 2007, 33, 623–629. [Google Scholar] [CrossRef]
- Percino, M.J.; Chapela, V.M.; Montiel, L.-F.; Rodríguez-Babarín, C. X-Ray crystal structures of a 1-(p-fluorophenyl)-2-(α-pyridyl)ethanol intermediate and the 1-(p-fluorophenyl)-2-(α-pyridyl)ethene dehydration compound obtained from the condensation reaction of 2-methylpyridine and p-fluorobenzaldehyde. Open Crystallogr. J. 2008, 1, 37–41. [Google Scholar] [CrossRef]
- Percino, M.J.; Chapela, V.M.; Sanchez, A.; Maldonado-Rivera, J.L. Condensation reactions of methylpyridines and aromatic aldehydes under catalyst and solvent free Conditions. Chem. Indian J. 2006, 3, 262–267. [Google Scholar]
- Percino, M.J.; Chapela, V.M.; Montiel, L.-F.; Pérez-Gutiérrez, E.; Maldonado-Rivera, J.L. Spectroscopic characterization of halogen- and cyano-substituted pyridinevinylenes synthesized without catalyst or solvent. Chem. Pap. 2010, 64, 360–367. [Google Scholar] [CrossRef]
- Williams, D.H.; Fleming, I. Spectroscopic Methods in Organic Chemistry, 3rd ed.; McGraw-Hill: Maidenhead, UK, 1980. [Google Scholar]
- Gatial, A.; Milata, V.; Biskupič, S.; Pigosšová, J.; Herzog, K.; Salzer, R. The vibrational and NMR spectra and conformations of methoxymethylene- and 1-methoxyethylidenepropanedinitrile including solvent effect calculations. Asian Chem. Letter. 2004, 8, 169–186. [Google Scholar]
- Gróf, M.; Polovková, J.; Gatial, A.; Milata, V.; Černuchová, P.; Prónayová, N.; Matějka, P. Isomers and conformers of two push-pull hydrazines studied by NMR and vibrational spectroscopy and by ab initio calculations. J. Mol. Struc. 2007, 834–836, 284–293. [Google Scholar] [CrossRef]
- Vida, M.; Gatial, A.; Polovková, J.; Zalibera, L.; Milata, V.; Salzer, R. The vibrational and NMR spectra, conformations and ab initio calculations of 1-(cyclopropylamino)-ethylidene propanedinitrile. Asian Chem. Letter. 2007, 11, 11–24. [Google Scholar]
- Kobayashi, T. J-Aggregates; World Scientific Publishing: Hackensack, NJ, USA, 1996; pp. 111–160. [Google Scholar]
- Oelkrug, D.; Tompert, A.; Gierschner, J.; Egelhaaf, H.-J.; Hanack, M.; Hohloch, M.; Steinhuber, E. Tuning of fluorescence in films and nanoparticles of oligophenylenevinylenes. J. Phys. Chem. B 1998, 102, 1902–1907. [Google Scholar] [CrossRef]
- Davis, R.; Kumar, N.S.S.; Abraham, S.; Suresh, C.H.; Rath, N.P.; Tamaoki, N.; Das, S. Molecular packing and solid-state fluorescence of alkoxy-cyano substituted diphenylbutadienes: Structure of the luminescent aggregates. J. Phys. Chem. C 2008, 112, 2137–2146. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, X.; Wang, F.; Shi, J.; Yu, W.; Ren, Y.; Zou, D.; Jiang, M. Intermolecular hydrogen bonds induce highly emissive excimers: Enhancement of solid-state luminescence. J. Phys. Chem. C 2007, 111, 6544–6549. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B.Z. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- De Mello, J.C.; Wittmann, H.F.; Friend, R.H. An improved experimental determination of external photoluminescence quantum efficiency. Adv. Mater. 1997, 9, 230–232. [Google Scholar] [CrossRef]
- Kumar, N.S.S.; Varghese, S.; Rath, N.P.; Das, S. Solid state optical properties of 4-alkoxy-pyridine butadiene derivatives: Reversible thermal switching of luminescence. J. Phys. Chem. C 2008, 112, 8429–8437. [Google Scholar] [CrossRef]
- Kawamura, Y.; Sasabe, H.; Adachi, C. Simple accurate system for measuring absolute photoluminescence quantum efficiency in organic solid-state thin films. Jpn. J. Appl. Phys. 2004, 43, 7729–7730. [Google Scholar] [CrossRef]
- Mattoussi, H.; Murata, H.; Merritt, C.D.; Lizumi, Y.; Kido, J.; Kafafi, Z.H. Photoluminescence quantum yield of pure and molecularly doped organic solid films. J. Appl. Phys. 1999, 86, 2642–2650. [Google Scholar] [CrossRef]
- Palsson, L.-O.; Monkman, A.P. Measurements of solid-state photoluminescence quantum yields of films using a fluorimeter. Adv. Mater. 2002, 14, 757–758. [Google Scholar] [CrossRef]
- Ramos-Ortiz, G.; Maldonado, J.L.; Meneses-Nava, M.A.; Barbosa-García, O.; Olmos, M.; Cha, M. Third-harmonic generation performance of organic polymer films doped with triphenylmethane derivative dyes. Opt. Mater. 2007, 29, 636–641. [Google Scholar] [CrossRef]
- Gordon, A.J.; Ford, R.A. The Chemist’s Companion—A Handbook of Practical Data, Techniques, and References; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1972. [Google Scholar]
- Klimova, E.; Klimova, T.; Martínez-Mendoza, J.M.; Ortiz-Frade, L.; Maldonado, J.-L.; Ramos-Ortiz, G.; Flores-Alamo, M.; Martínez-García, M. 5-Aryl-1-ferrocenylpenta-1,4-dien-3-ones: Synthesis, structures, electrochemistry and third-order nonlinear optical properties. Inorg. Chim. Acta 2009, 362, 2820–2827. [Google Scholar] [CrossRef]
© 2011 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pérez-Gutiérrez, E.; Percino, M.J.; Chapela, V.M.; Cerón, M.; Maldonado, J.L.; Ramos-Ortiz, G. Synthesis, Characterization and Photophysical Properties of Pyridine-Carbazole Acrylonitrile Derivatives. Materials 2011, 4, 562-574. https://doi.org/10.3390/ma4030562
Pérez-Gutiérrez E, Percino MJ, Chapela VM, Cerón M, Maldonado JL, Ramos-Ortiz G. Synthesis, Characterization and Photophysical Properties of Pyridine-Carbazole Acrylonitrile Derivatives. Materials. 2011; 4(3):562-574. https://doi.org/10.3390/ma4030562
Chicago/Turabian StylePérez-Gutiérrez, Enrique, M. Judith Percino, Víctor M. Chapela, Margarita Cerón, José Luis Maldonado, and Gabriel Ramos-Ortiz. 2011. "Synthesis, Characterization and Photophysical Properties of Pyridine-Carbazole Acrylonitrile Derivatives" Materials 4, no. 3: 562-574. https://doi.org/10.3390/ma4030562