Synthesis of Uniform Polyaniline Nanofibers through Interfacial Polymerization
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
2.3. Characterization
3. Results and Discussion
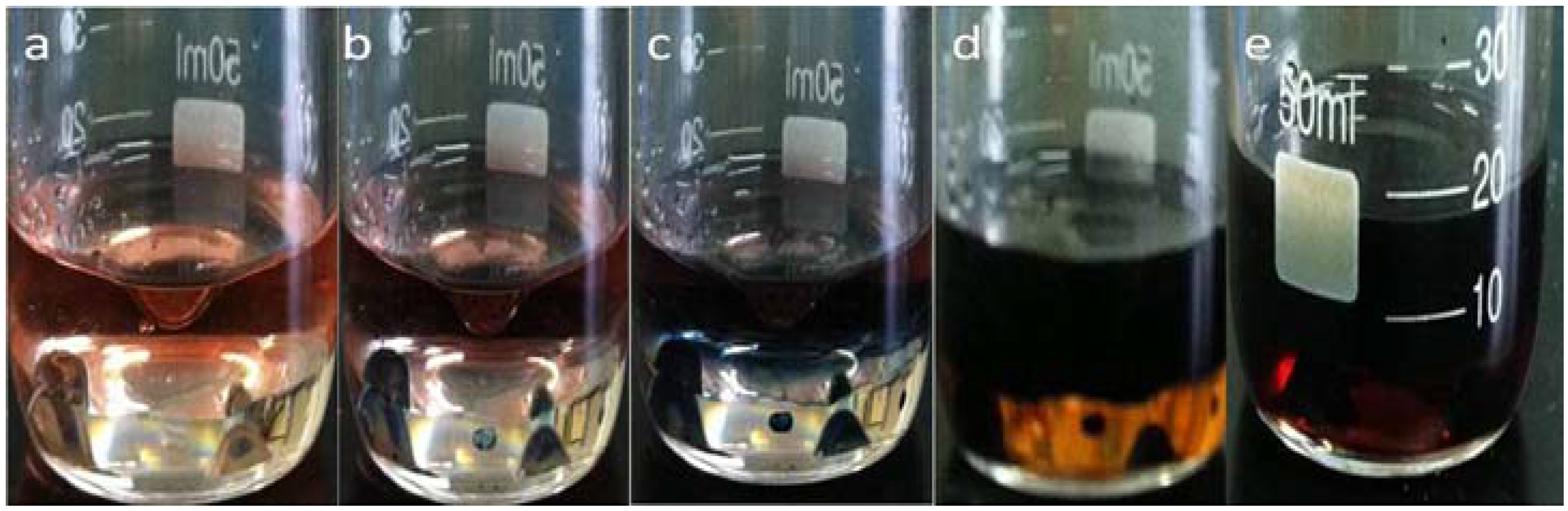
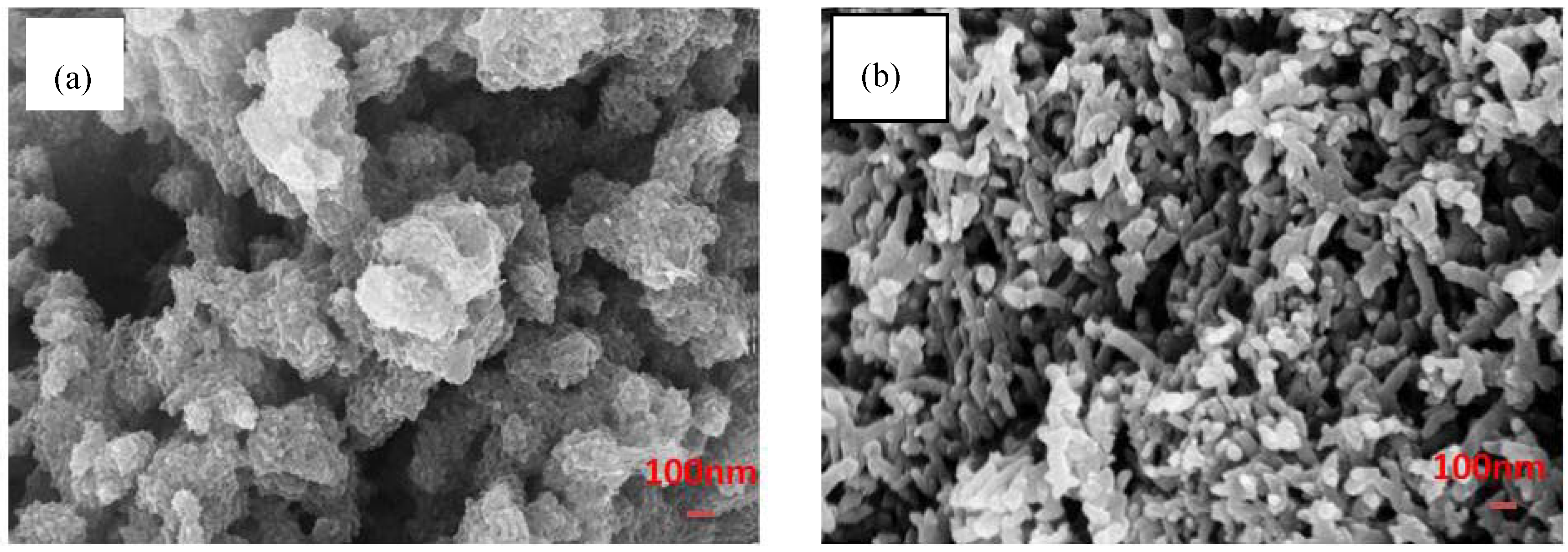
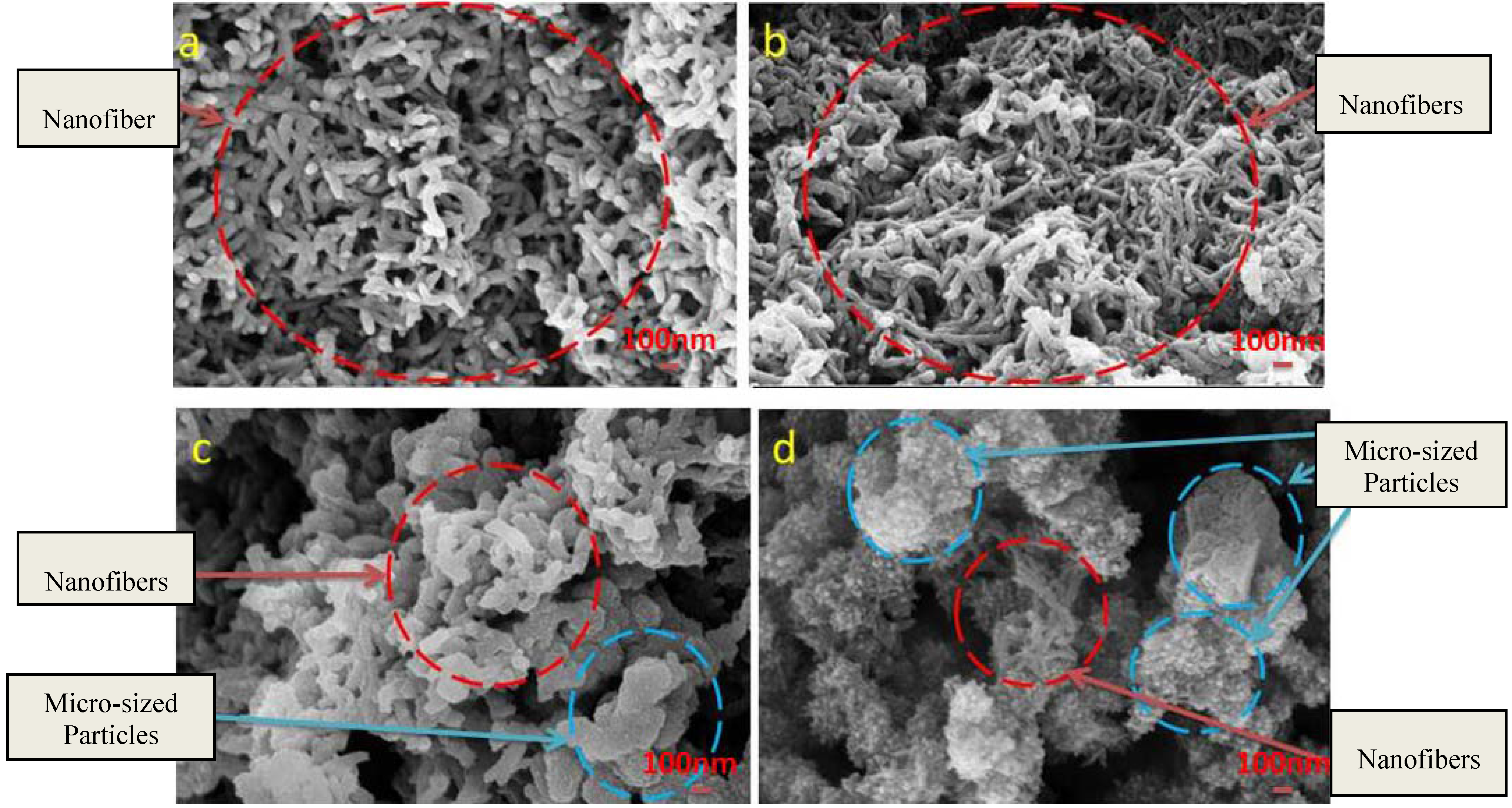
3.1. Reaction and Mechanism
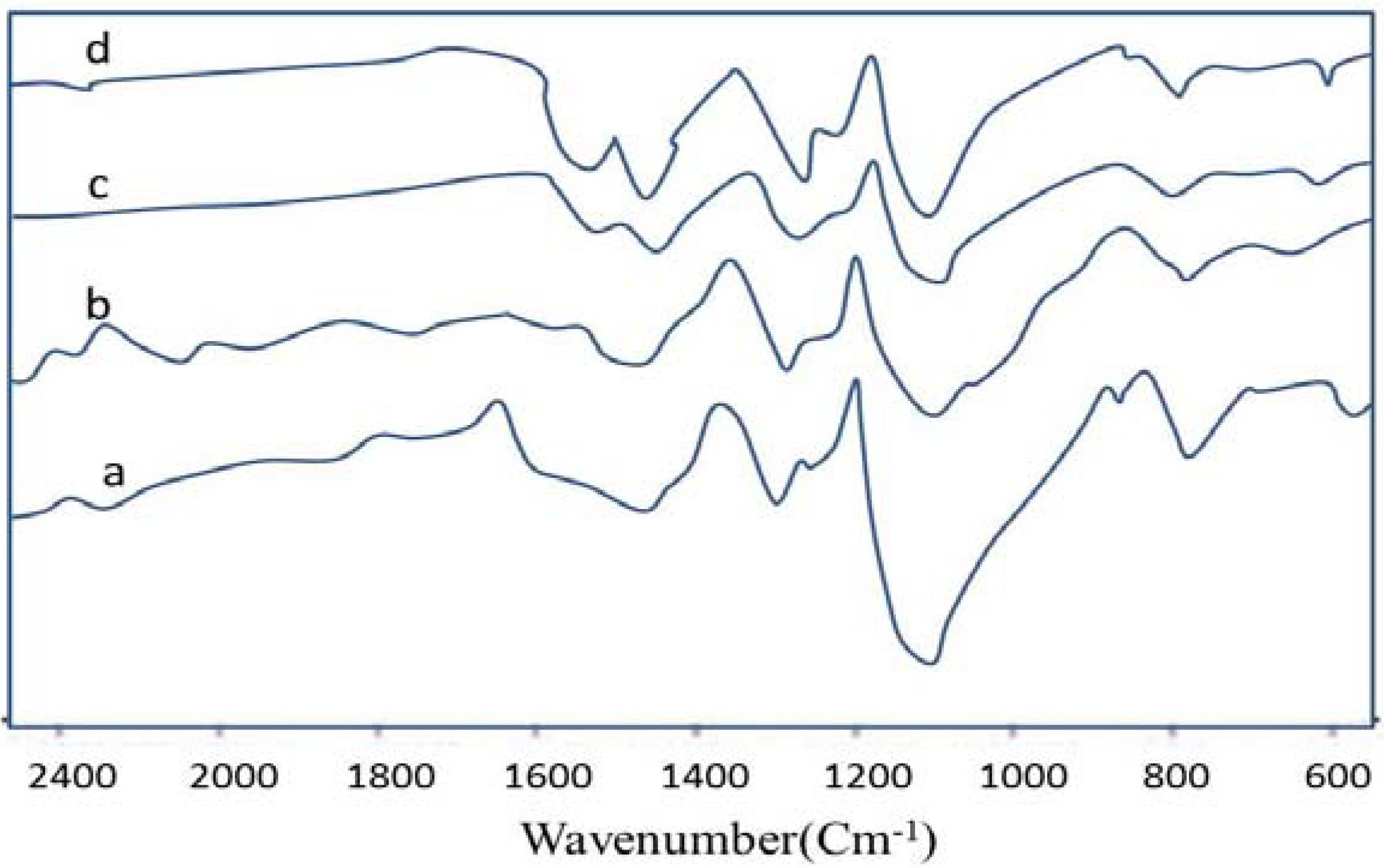
3.2. FTIR Spectroscopy
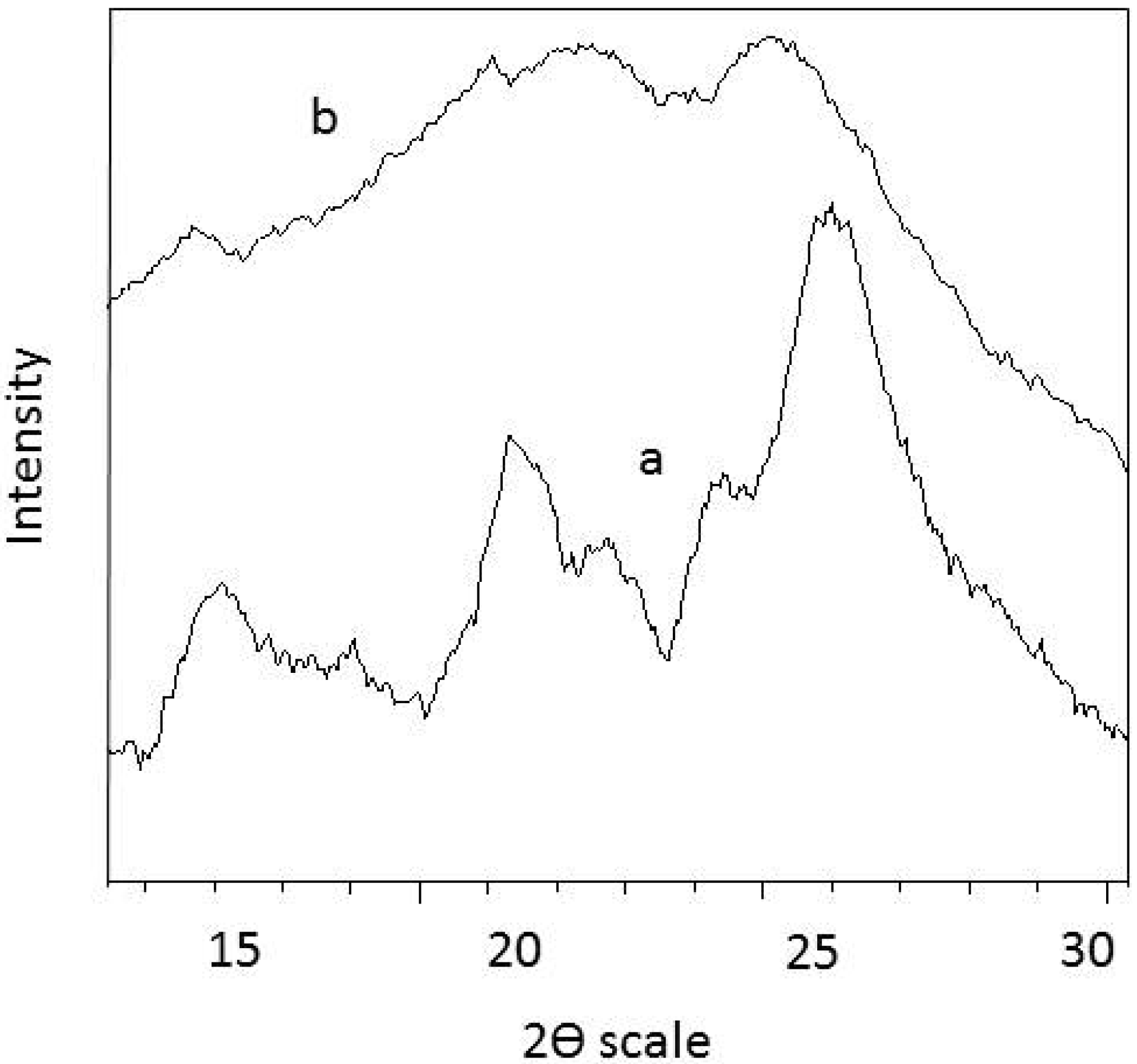
3.3. XRD Analysis
4. Conclusions
References
- Patil, A.O.; Heeger, A.J.; Wudl, F. Optical properties of conducting polymers. Chem. Rev. 1988, 88, 183–200. [Google Scholar] [CrossRef]
- Keiichi, K.; Katsumi, Y.; Yoshio, I. Electrical properties of conducting polymer, poly-thiophene, prepared by electrochemical polymerization. Jpn. J. Appl. Phys. 1982, 21, 567–568. [Google Scholar] [CrossRef]
- Niemann, M.U.; Srinivasan, S.S.; Phani, A.R.; Kumar, A.; Goswami, D.Y.; Stefanakos, E.K. Room temperature hydrogen storage in polyaniline nanofibers. J. Nanosci. Nanotechnol. 2009, 9, 4561–4565. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.S.; Niemann, M.U.; Ratnadurai, R.; Phani, A.R.; Goswami, D.Y.; Stefanakos, E.K. Reversible hydrogen storage in electrospunpolyaniline fibers. Int. J. Hydrog. Energy 2010, 35, 225–230. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Nanostructured polyaniline sensors. Chemistry 2004, 10, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, J.; Xue, H.; Shen, L.; Zang, H.; Chen, W. Polyaniline/TiO2 solar cells. Synthet. Metal. 2006, 156, 721–723. [Google Scholar] [CrossRef]
- Sotooviedo, M.A.; Araujo, O.A.; Faez, R.; Rezende, M.C.; Depaoli, M.A. Antistatic coating and electromagnetic shielding properties of a hybrid material based on polyaniline/organoclaynanocomposite and EPDM rubber. Synthet. Metal. 2006, 156, 1249–1255. [Google Scholar] [CrossRef]
- Yang, Y. Polyaniline as a transparent electrode for polymer light-emitting diodes: Lower operating voltage and higher efficiency. Appl. Phys. Lett. 1994, 64, 1245–1247. [Google Scholar] [CrossRef]
- Armelina, E.; Martia, M.; Liesab, F.; Iribarrena, J.I.; Alemana, C. Partial replacement of metallic zinc dust in heavy duty protective coatings by conducting polymer. Prog. Org. Coating. 2010, 69, 26–30. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 211–324. [Google Scholar] [CrossRef]
- Bhadra, S.; Singha, N.K.; Khastgir, D. Electrochemical synthesis of polyaniline and its comparison with chemically synthesized polyaniline. J. Appl. Polym. Sci. 2007, 104, 1900–1904. [Google Scholar] [CrossRef]
- Hanyand, P.; Geniis, E.M. Polyanilines with covalently bonded alkyl sulfonates as doping agent. Synthesis and Properties. Synthet. Metal. 1989, 31, 369–378. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Kaner, R.B. Polyaniline nanofibers: A unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2009, 42, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Lia, M.; Guob, Y.; Weib, Y.; Macdiarmidc, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wang, Y.; Wu, D.; She, L.; Guo, Y. Polyaniline nanofibers prepared with ultrasonic irradiation. J. Polym. Sci. A Polym. Chem. 2006, 44, 1014–1019. [Google Scholar] [CrossRef]
- Wu, C.G.; Bein, T. Conducting polyaniline filaments in a mesoporous channel host. Science 1994, 264, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Deng, J.; Yang, Y.; Yang, W. Synthesis of large-area three-dimensional polyaniline nanowire networks using a soft template. Macromol. Rapid Commun. 2005, 26, 395–400. [Google Scholar] [CrossRef]
- Huang, J.; Kaner, R.B. A general chemical route to polyaniline nanofibers. J. Am. Chem. Soc. 2004, 126, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goux, W.J.; Manohar, S.K. Synthesis of polyaniline nanofibers by nanofiber seeding. J. Am. Chem. Soc 2004, 126, 4502–4503. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Tong, Y.; Bai, J.; Lei, Z.; Wang, K.; Mu, H.; Dong, N. Acid doped polyaniline nanofibers synthesised by interfacial polymerization. Indian J. Chem. 2007, 46, 595–599. [Google Scholar]
- Ge, C.Y.; Yang, X.G.; Hou, B.R. Synthesis of polyaniline nanofiber and anticorrosion property of polyaniline—Epoxy composite coating for Q235 steel. J. Coat. Technol. Res. 2012, 9, 56–59. [Google Scholar] [CrossRef]
- Zhang, X.; Kolla, H.S.; Wang, X.; Raja, K.; Manohar, S.K. Fibrillar growth in polyaniline. Adv. Funct. Mater. 2006, 16, 1145–1152. [Google Scholar] [CrossRef]
- Ma, S.; Song, G.; Feng, N.; Zhao, P. Corrosion protection of mild steel with nanofibrous polyaniline-based coatings. J. Appl. Polym. Sci. 2012, 125, 1601–1605. [Google Scholar] [CrossRef]
- Huang, J. Syntheses and applications of conducting polymer polyaniline nanofibers. Pure Appl. Chem. 2006, 78, 15–27. [Google Scholar] [CrossRef]
- Rahy, A.; Yang, D.J. Synthesis of highly conductive polyaniline nanofibers. Mater. Lett. 2008, 62, 4311–4314. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Synthesis of Uniform Polyaniline Nanofibers through Interfacial Polymerization. Materials 2012, 5, 1487-1494. https://doi.org/10.3390/ma5081487
Abdolahi A, Hamzah E, Ibrahim Z, Hashim S. Synthesis of Uniform Polyaniline Nanofibers through Interfacial Polymerization. Materials. 2012; 5(8):1487-1494. https://doi.org/10.3390/ma5081487
Chicago/Turabian StyleAbdolahi, Ahmad, Esah Hamzah, Zaharah Ibrahim, and Shahrir Hashim. 2012. "Synthesis of Uniform Polyaniline Nanofibers through Interfacial Polymerization" Materials 5, no. 8: 1487-1494. https://doi.org/10.3390/ma5081487



