A Review on the Synthesis and Applications of Mesostructured Transition Metal Phosphates
Abstract
:1. Introduction
2. General Synthesis Strategies
2.1. Structure-Directing Synthesis Routes
2.1.1. Soft-Templating Route

2.1.2. Hard-Templating Route
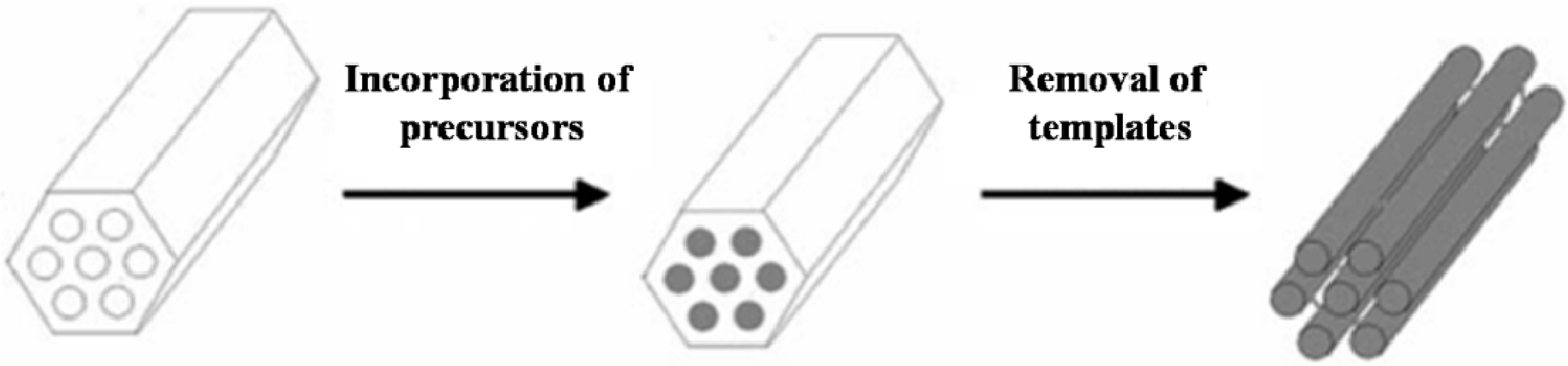
2.2. Template-Free Synthesis Routes
3. Synthesis and Application of Mesostructured Transition Metal Phosphates
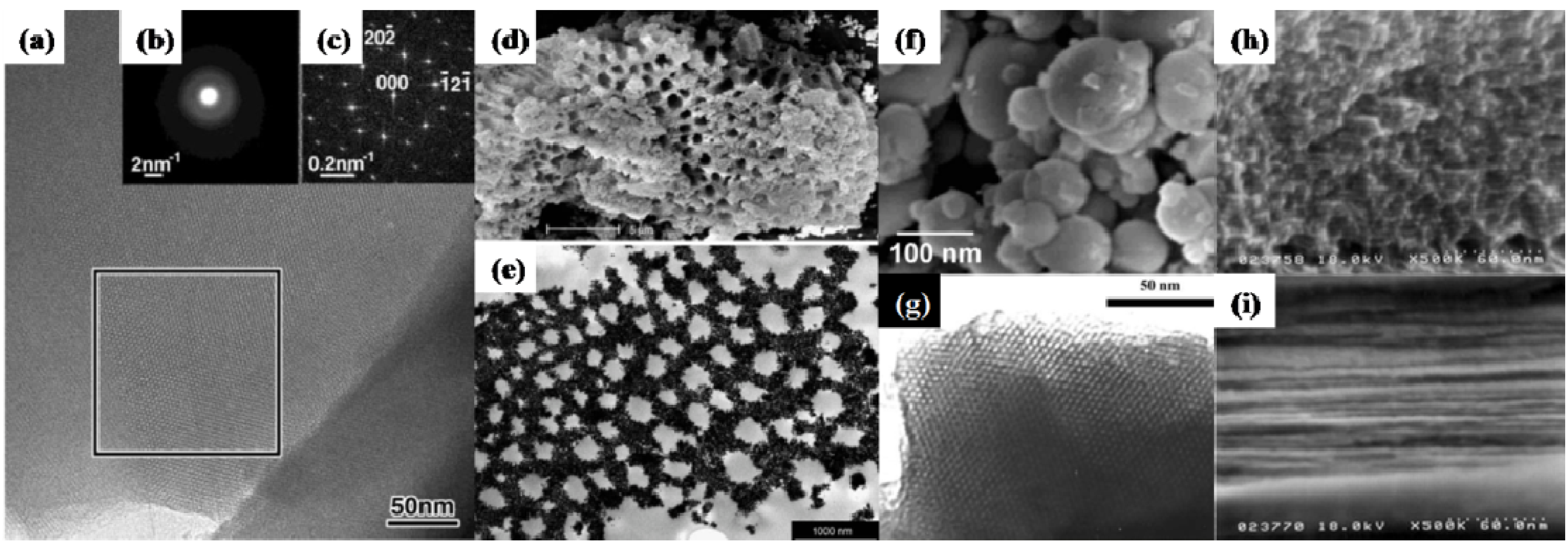
3.1. Zirconium Phosphates and Titanium Phosphates
3.1.1. Zirconium Phosphates (ZrP)
| Materials | Synthesis conditions | Physical properties | Applications | Reference |
|---|---|---|---|---|
| ZrP | Yeast as biotemplate, assembly, ambient conditions | Wormhole-like mesoporous structure, SBET ca. 217 m2/g, narrow pore size of 2.7 nm | Electrode for oxygen reduction reaction | [6] |
| Spin coating, P123, zirconium isopropoxide, triethyl phosphate | Ordered mesoporous films, hexagonal structure | Proton conducting devices | [7] | |
| Coassembly, pluronic-F127, zirconium butoxide, phosphorous trichloride | Randomly ordered mesostructures, SBET 84 m2/g, average pore size of 17 nm | Nafion-zirconium phosphate composite membranes | [39,69] | |
| Thermal decomposing a mesoporous zirconium phosphite diphosphonate | Globular particles of 10~20 nm diameter, SBET 215 m2/g, average pore size ca. 4.0 nm | Potential uses as an acid catalyst at high temperature | [60] | |
| Post-treating surfactant-assisted zirconium oxide mesophase with phosphoric acid | Ordered hexagonal pore, SBET 456~547 m2/g, pore size of 1.30~1.66 nm | Untested | [61] | |
| Precipitation of zirconium sulfate, followed by hydrothermal treating | Ordered mesostructures, SBET 230~390 m2/g | Untested | [62] | |
| Supramolecular, self-assembly (C18BDAC), aging at 363 K, 3 day | Cubic Ia3d structure | Potential solid acid catalyst (Brønsted and Lewis acid sites) | [63] | |
| Precipitation of zirconium sulfate with gemini cationic surfactants, followed by hydrothermal treating | Highly ordered mesostructures | Untested | [64] | |
| Sol-gel, CTMA template, aging at room temperature 2~3 day | Less ordered mesoporous structure, SBET 250~320 m2/g, average pore size 2.5~2.7 nm | Proton conducting devices, potential solid acid catalyst | [65,66] | |
| Surfactant-assisted precipitation (CTAC, HDA, SDS), aging 24 h | Porous, SBET 400~500 m2/g | Untested | [67] | |
| Precipitation of (Zr(OC3H7)4 with Brij 56, followed by hydrothermal treating | Hierarchical structure with supermicroporous walls, uniform diameters ranging from 300 to 800 nm | Potential applications in catalysis | [68] | |
| “Surfactant-assisted” approach (AOT) | Porous, SBET 83 m2/g, pore size of 2~30 nm | Support for protein adsorption (myoglobin) | [70] | |
| Precipitation of a zirconium carbonate complex, pH 8.0 | Spherical, SBET 299 m2/g, narrow pore size of 3.91 nm | Ethyl acetate hydrolysis | [72] | |
| Evaporation-induced self-assembly, F127, strongly acidic conditions | Wormhole-like disordered mesostructure, SBET 260~312 m2/g, narrow pore size of 4.5~5.5 nm | Conversion of the long chain fatty acids to their respective methyl esters | [73] |
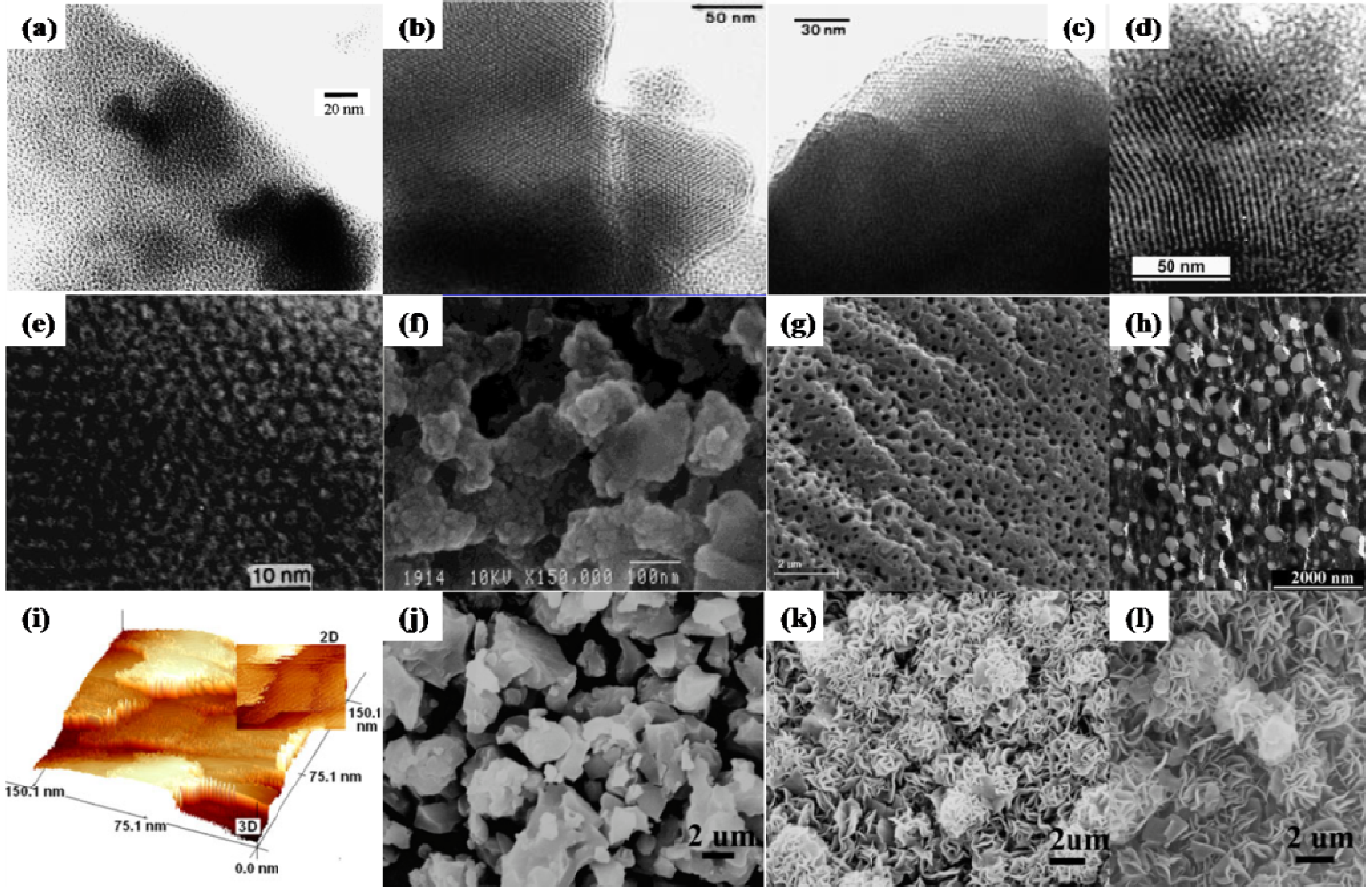
3.1.2. Titanium Phosphates (TiP)
| Materials | Synthesis conditions | Physical properties | Applications | Reference |
|---|---|---|---|---|
| TiP | Template-directing assembly (P123, Tergitol 15-S-9, CTAC), pH 4 | SBET 107~340 m2/g, pore size of 4.0~4.5 nm | Radionuclide sorbent materials Np(V) | [9] |
| Template-directing assembly and hydrothermal, SDS, DBSA, ODTMABr/Cl | Uniform hexagonal mesopore, SBET 407~701 m2/g, average pore size of 2.18~3.13 nm | Ion-exchange | [37] | |
| Precipitation of titanium propoxide or titanium chloride with H3PO4 in the presence of trimethylammonium | SBET 207~740 m2/g, average pore size of 2.3~5.1 nm | Potential acid catalyst | [74] | |
| Non-ionic template route, [Dodecanol +5 EO] | Disordered hexagonal pore structure, SBET 350 m2/g, average pore size ca. 4.5 nm | Untested | [76] | |
| Precipitation of titanium isopropoxide with surfactant CTAB | Hexagonally packed porous structure or lamellar structure, | Untested | [77,78] | |
| Self-formation process, hydrothermal 353 K 24 h, with/without Brij 56 | Disordered framework with wormhole-like channels, SBET 165~312 m2/g | Potential optical material and acid catalyst | [79] | |
| Yeast cells induced self-assembly | Lamellas with mesopores, pore size 3~12 nm | Untested | [80] | |
| Neutral templating route, hydrothermal aging at 363 K 48 h, long-chain n-alkyl amine | Wormlike mesopore, SBET 359~497 m2/g, average pore size of 1.7~3.3 nm | Liquid-phase partial oxidation of cyclohexene with H2O2 | [81] | |
| Hydrothermal combined with evaporation-induced self-assembly, Brij 56 | Ordered hexagonal pore structure, SBET 230~1021 m2/g, average pore size of 2.6~3.4 nm | Photocatalysts for organic dye degradation, adsorbents for heavy metal ions | [82] |
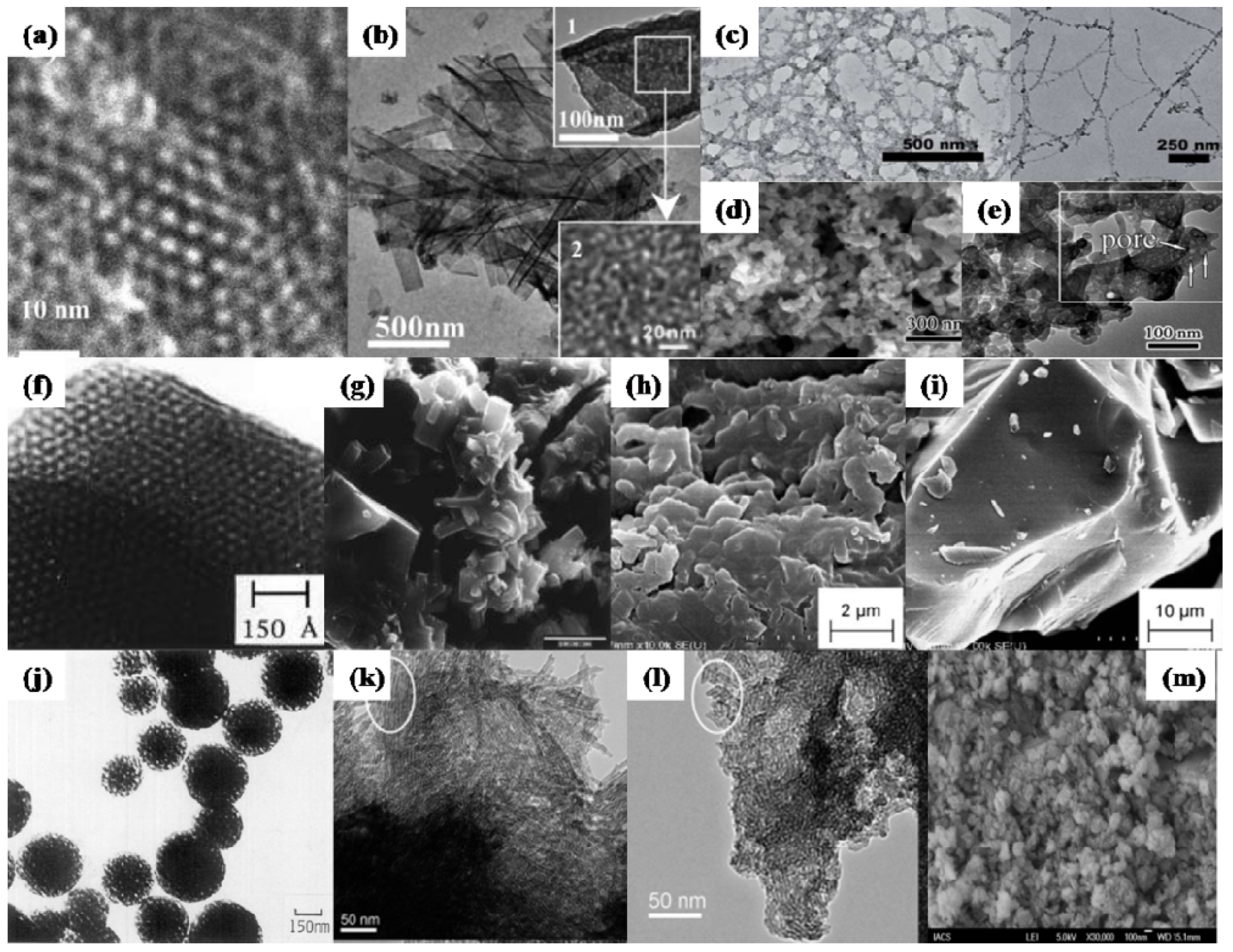
3.2. Iron, Vanadium and Nickel Phosphates
| Materials | Synthesis conditions | Physical properties | Applications | Reference |
|---|---|---|---|---|
| FeP | Electrochemical | Nanoparticals, 20–80 nm, SBET ca. 65 m2/g, dominant pore size 23.6 nm | LiFePO4/C, cathode materials | [55] |
| Solution precipitation, HF, sodium dodecyl sulfate (SDS) | Ordered mesopores, SBET ca. 254 m2/g, average pore size 2.6 nm | Prins condensation of β-pinene and paraformaldehyde | [84,85] | |
| Solvothermal, SDS-templating assembly | Mesoporous nanotubes, 50~400 nm (diameter), lengths of several microns; SBET 232 m2/g | Direct hydroxylation of benzene | [86] | |
| G4-NH2-terminated PAMAM dendrimer, single template assembly | Hexagonal ordering structures | Untested | [87] | |
| VP | Thermal treatment of vanadyl n-butylphosphate, 525–705 K | Highly porous, SBET ca. 225 m2/g, mesopore diameter ca. 4.4 nm | Precursors of the (VO)2P2O7 catalyst | [59] |
| CTAB-templating and/or hydrothermal post-treatment | Hexagonal structures | The same as above | [90] | |
| Hydrothermal, C16TMA(OH,Cl), 473 K, 48 h | Hexagonal-, cubic-, and lamellar-mesostructures | The same as above | [91] | |
| Surfactant templating (CTAB) of exfoliated VOPO4 sheets | Hexagonal- and lamellar-mesostructures | The same as above | [92] | |
| NiP | Hydrothermal, template-free, HDTMP, 443 K, 36 h | Crystalline porous organic–inorganic hybrid, SBET 241 m2/g | Adsorption of heavy metal cations like Cr3+, Pb2+, Hg2+ and Cd2+; Nitrobenzenes reduction to the respective anilines | [10] |
| Hydrothermal, NiSO4 + SDS + NaH2PO4, 353 K | Spherical particles (ca. 245 nm) with high porosity, SBET 130 m2/g | Selective adsorption of H2O | [95] | |
| Sol-gel, CTAB, aging at 373 K, 24 h | Nanotubular structures, 200~400 nm (length) × 4~5 nm (diameter); SBET 205–292 m2/g | Epoxidation of cyclododecene with H2O2 as an oxidant | [96] |
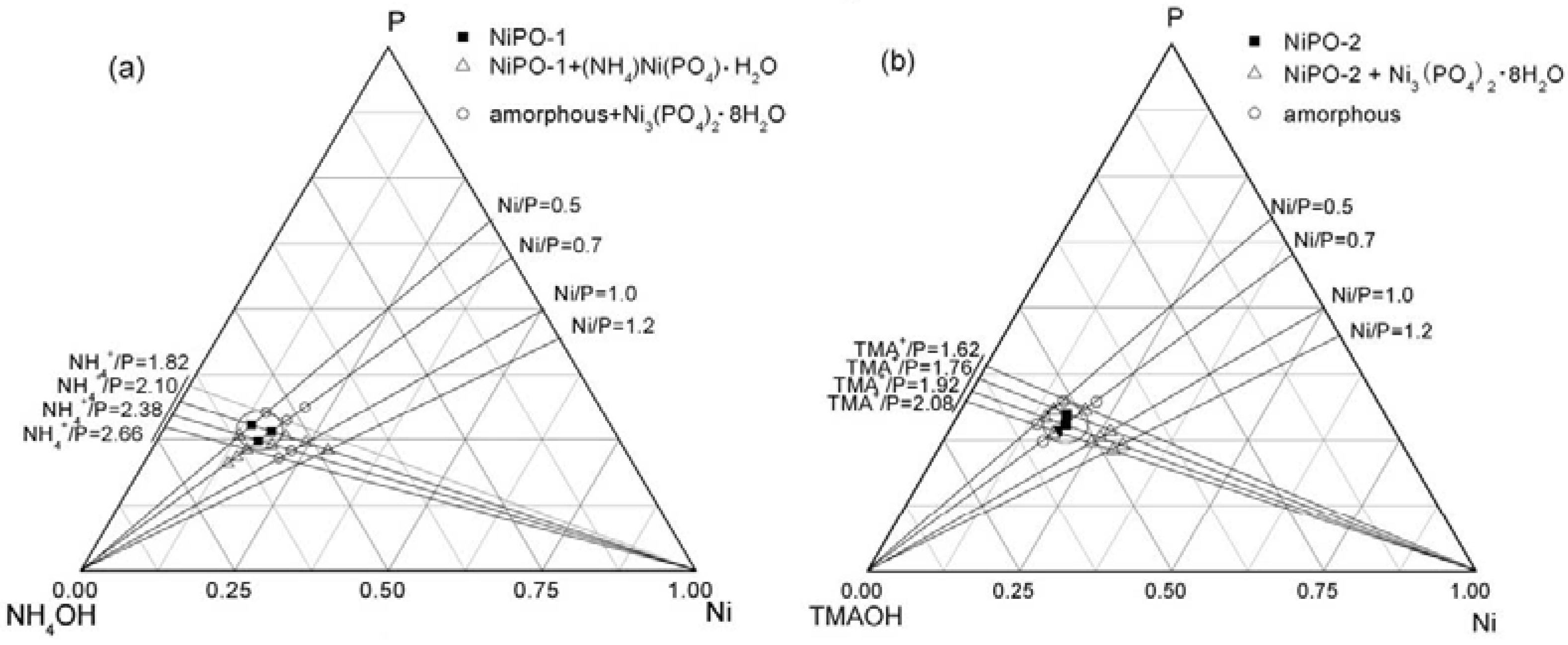
3.3. Other Mesostructured Transition Metal Phosphates
| Materials | Synthesis conditions | Physical properties | Applications | Reference |
|---|---|---|---|---|
| CrP | Sol-gel combined with programmed hydrothermal treating, TTBr | Ink-bottle pores, SBET 384 m2/g, average pore size 3.6 nm | Untested | [98] |
| Solid-state reaction at 373 K, CTAB | Banger-like pores, SBET 250 m2/g, average pore size 3.48 nm | Isopropanol dehydration to propene | [99] | |
| NbP | Hydrothermal, TTBr as template, aging at 403 K, 24 h | Wormhole-like structure, SBET 427 m2/g, average pore size 3.35 nm | Potential solid acid catalyst | [100] |
| Precipitation combined with hydrothermal treating, CTAB | Wormhole-like structure, SBET 210~290 m2/g, average pore size 3.5 nm | Isomerization of xylose to xylulose and subsequent dehydration; dehydration of fructose to 5-hydroxymethylfurfural | [101,102] | |
| ZnP | Chemical precipitation with yeast cells as biotemplates, pH 8~10 | Agglomerates of isolated nanoparticles of 10 nm, SBET 146 m2/g, average pore size 10 nm | Untested | [103] |
| TaP | Precipitation combined with hydrothermal treating, TTBr | Wormhole-like structure, SBET 324~359 m2/g, average pore size 2.7~3.5 nm | Untested | [104] |
| YP | Microwave-assisted precipitation | Lenticular nanoparticles with internal porosity and a pore diameter of ca. 4.0 nm, SBET 145 m2/g | Photoluminescence material | [11] |
| Nanocasting route, KIT-6 as hard template | Cubic ordered mesopores, SBET 114 m2/g, average pore size 4.3 nm | The same as above | [12] |
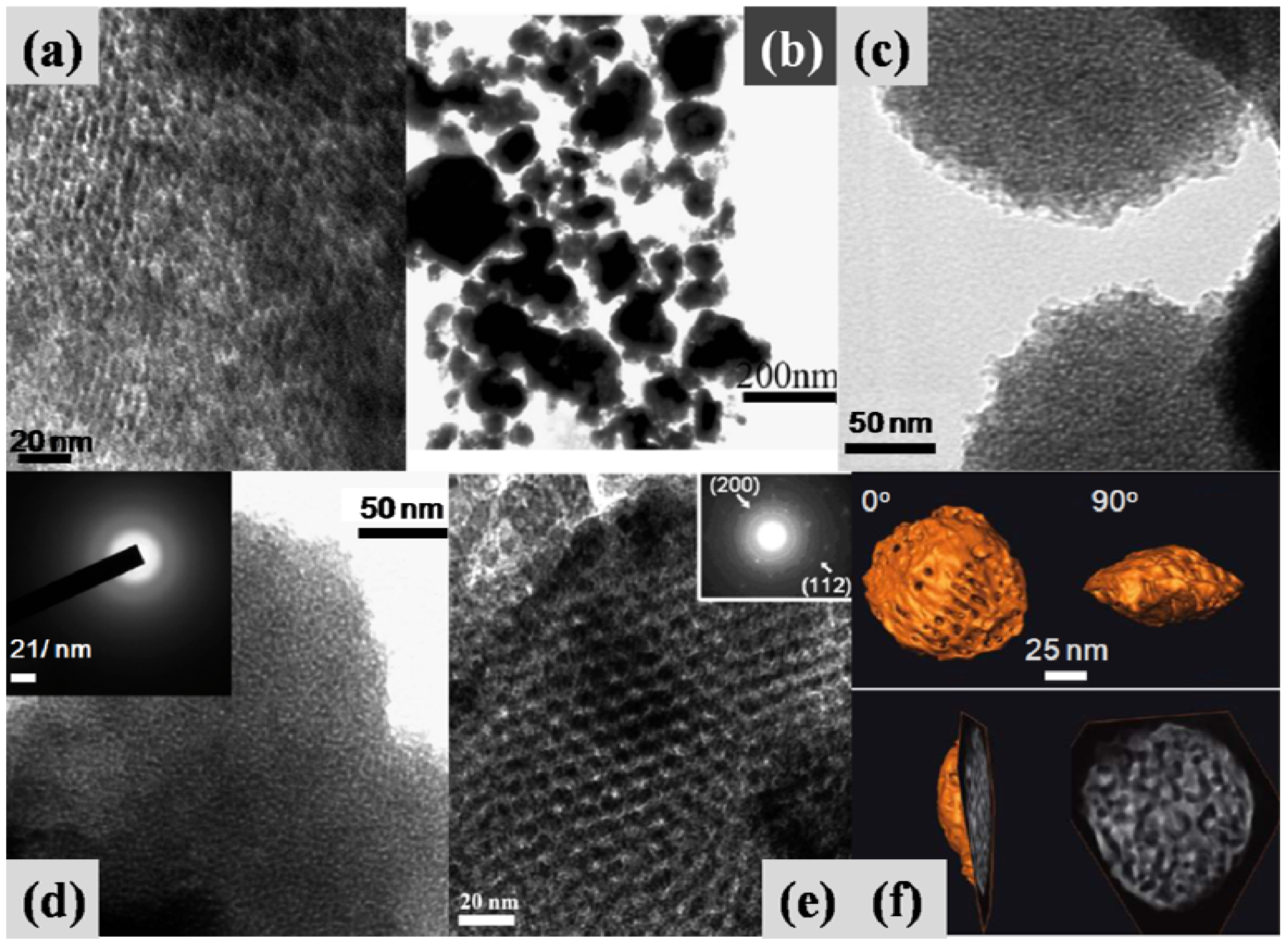
4. Conclusions and Future Perspectives
Acknowledgments
References
- Hutchings, G.J. Vanadium phosphate: A new look at the active components of catalysts for the oxidation of butane to maleic anhydride. J. Mater. Chem. 2004, 14, 3385–3395. [Google Scholar] [CrossRef]
- Muneyama, E.; Kunishige, A.; Ohdan, K.; Ai, M. Reduction and reoxidation of iron phosphate and its catalytic activity for oxidative dehydrogenation of isobutyric acid. J. Catal. 1996, 158, 378–384. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.X.; Su, Z.; Guo, Q.; Tang, Q.H.; Zhang, Q.H.; Wan, H.L. SBA-15-supported iron phosphate catalyst for partial oxidation of methane to formaldehyde. Catal. Today 2004, 93–95, 155–161. [Google Scholar]
- Lin, R.H.; Ding, Y.J.; Gong, L.F.; Dong, W.D.; Wang, J.H.; Zhang, T. Efficient and stable silica-supported iron phosphate catalysts for oxidative bromination of methane. J. Catal. 2010, 272, 65–73. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Masquelier, C.; Okada, S.; Goodenough, J.B. Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates. J. Electrochem. Soc. 1997, 144, 1609–1613. [Google Scholar] [CrossRef]
- Tian, X.Y.; He, W.; Cui, J.J.; Zhang, X.D.; Zhou, W.J.; Yan, S.P.; Sun, X.N.; Han, X.X.; Han, S.S.; Yue, Y.Z. Mesoporous zirconium phosphate from yeast biotemplate. J. Colloid Interface Sci. 2010, 343, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Tanaka, S.; Hillhouse, H.W.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Synthesis of ordered mesoporous zirconium phosphate films by spin coating and vapor treatments. Langmuir 2006, 22, 9469–9472. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Zhang, X.J.; Yuan, Z.Y. Hierarchically meso-/macroporous titanium tetraphosphonate materials: Synthesis, photocatalytic activity and heavy metal ion adsorption. Microporous Mesoporous Mater. 2009, 123, 234–242. [Google Scholar] [CrossRef]
- Li, X.S.; Courtney, A.R.; Yantasee, W.; Mattigod, S.V.; Fryxell, G.E. Templated synthesis of mesoporous titanium phosphates for the sequestration of radionuclides. Inorg. Chem. Commun. 2006, 9, 293–295. [Google Scholar] [CrossRef]
- Dutta, A.; Patra, A.K.; Bhaumik, A. Porous organic-inorganic hybrid nickel phosphonate: Adsorption and catalytic applications. Microporous Mesoporous Mater. 2012, 155, 208–214. [Google Scholar] [CrossRef]
- Rodriguez-Liviano, S.; Aparicio, F.J.; Rojas, T.C.; Hungria, A.B.; Chinchilla, L.E.; Ocana, M. Microwave-assisted synthesis and luminescence of mesoporous RE-doped YPO4 (RE = Eu, Ce, Tb, and Ce plus Tb) nanophosphors with lenticular shape. Cryst. Growth Des. 2012, 12, 635–645. [Google Scholar] [CrossRef]
- Luo, Q.L.; Shen, S.D.; Lu, G.Z.; Xiao, X.Z.; Mao, D.S.; Wang, Y.Q. Synthesis of cubic ordered mesoporous YPO4:Ln3+ and their photoluminescence properties. J. Mater. Chem. 2009, 19, 8079–8085. [Google Scholar] [CrossRef]
- Kim, T.W.; Chung, P.W.; Slowing, I.I.; Tsunoda, M.; Yeung, E.S.; Lin, V.S.Y. Structurally ordered mesoporous carbon nanoparticles as transmembrane delivery vehicle in human cancer cells. Nano Lett. 2008, 8, 3724–3727. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Stace-Naughton, A.; Jiang, X.M.; Brinker, C.J. Porous nanoparticle supported lipid bilayers (protocells) as delivery vehicles. J. Am. Chem. Soc. 2009, 131, 1354–1355. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; Mccullen, S.B. A new family of mesoporous molecular-sieves prepared with liquid-crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Jackowska, K.; Biegunski, A.T.; Tagowska, M. Hard template synthesis of conducting polymers: A route to achieve nanostructures. J. Solid State Electr. 2008, 12, 437–443. [Google Scholar] [CrossRef]
- Huang, Z.; Luan, D.Y.; Shen, S.C.; Hidajat, K.; Kawi, S. Supercritical fluid extraction of the organic template from synthesized porous materials: effect of pore size. J. Supercrit. Fluid 2005, 35, 40–48. [Google Scholar] [CrossRef]
- Jiao, F.; Bruce, P.G. Two- and three-dimensional mesoporous iron oxides with microporous walls. Angew. Chem. Int. Ed. 2004, 43, 5958–5961. [Google Scholar] [CrossRef]
- Yang, L.M.; Wang, Y.J.; Luo, G.S.; Dai, Y.Y. Simultaneous removal of copolymer template from SBA-15 in the crystallization process. Microporous Mesoporous Mater. 2005, 81, 107–114. [Google Scholar] [CrossRef]
- Lu, A.H.; Spliethoff, B.; Schuth, F. Aqueous synthesis of ordered mesoporous carbon via self-assembly catalyzed by amino acid. Chem. Mater. 2008, 20, 5314–5319. [Google Scholar] [CrossRef]
- Wang, D.S.; Xie, T.; Peng, Q.; Li, Y.D. Ag, Ag2S, and Ag2Se nanocrystals: Synthesis, assembly, and construction of mesoporous structures. J. Am. Chem. Soc. 2008, 130, 4016–4022. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Takai, A.; Komatsu, M.; Sawada, M.; Ohsuna, T.; Kuroda, K. Vapor infiltration of a reducing agent for facile synthesis of mesoporous Pt and Pt-based alloys and its application for the preparation of mesoporous Pt microrods in anodic porous membranes. Chem. Mater. 2008, 20, 1004–1011. [Google Scholar] [CrossRef]
- Hill, A.H.; Jiao, F.; Bruce, P.G.; Harrison, A.; Kockelmann, W.; Ritter, C. Neutron diffraction study of mesoporous and bulk hematite, alpha-Fe2O3. Chem. Mater. 2008, 20, 4891–4899. [Google Scholar] [CrossRef]
- Nistor, L.C.; Mateescu, C.D.; Birjega, R.; Nistor, S.V. Synthesis and characterization of mesoporous ZnS with narrow size distribution of small pores. Appl. Phys. A Mater. 2008, 92, 295–301. [Google Scholar] [CrossRef]
- Ma, T.Y.; Yuan, Z.Y. Metal phosphonate hybrid mesostructures: Environmentally friendly multifunctional materials for clean energy and other applications. ChemSusChem 2011, 4, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.F.; Wan, Y.; Zhang, R.Y.; Zhao, D.Y. Synthesis of self-supported ordered mesoporous cobalt and chromium nitrides. Adv. Funct. Mater. 2008, 18, 2436–2443. [Google Scholar] [CrossRef]
- Wan, Y.; Shi, Y.F.; Zhao, D.Y. Supramolecular aggregates as templates: Ordered mesoporous polymers and carbons. Chem. Mater. 2008, 20, 932–945. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Kuroda, K. Rational design of mesoporous metals and related nanomaterials by a soft-template approach. Chem. Asian J. 2008, 3, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ma, Z.; Bruce, P.G. Ordered mesoporous metal oxides: synthesis and applications. Chem. Soc. Rev. 2012, 41, 4909–4927. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K. Silica-based mesoporous molecular sieves derived from a layered polysilicate kanemite-a review. J. Porous Mater. 1996, 3, 107–114. [Google Scholar] [CrossRef]
- Sayari, A.; Hamoudi, S. Periodic mesoporous silica-based organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3151–3168. [Google Scholar] [CrossRef]
- Lin, H.P.; Mou, C.Y. Structural and morphological control of cationic surfactant-templated mesoporous silica. Acc. Chem. Res. 2002, 35, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Vinu, A.; Hossain, K.Z.; Ariga, K. Recent advances in functionalization of mesoporous silica. J. Nanosci. Nanotechnol. 2005, 5, 347–371. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular-sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Tanev, P.T.; Pinnavaia, T.J. A neutral templating route to mesoporous molecular-sieves. Science 1995, 267, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.A.; Prouzet, E.; Pinnavaia, T.J. Templating of mesoporous molecular-sieves by nonionic polyethylene oxide surfactants. Science 1995, 269, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, A.; Inagaki, S. Mesoporous titanium phosphate molecular sieves with ion-exchange capacity. J. Am. Chem. Soc. 2001, 123, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.D.; Zhao, D.Y.; Margolese, D.I.; Chmelka, B.F.; Stucky, G.D. Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks. Nature 1998, 396, 152–155. [Google Scholar] [CrossRef]
- Tian, B.Z.; Liu, X.Y.; Tu, B.; Yu, C.Z.; Fan, J.; Wang, L.M.; Xie, S.H.; Stucky, G.D.; Zhao, D.Y. Self-adjusted synthesis of ordered stable mesoporous minerals by acid-base pairs. Nat. Mater. 2003, 2, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R. Nanomaterials-a membrane-based synthetic approach. Science 1994, 266, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Schuth, F. Non-siliceous mesostructured and mesoporous materials. Chem. Mater. 2001, 13, 3184–3195. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Kim, T.W.; Ryoo, R.; Terasaki, O. Three-dimensional structure of large-pore mesoporous cubic Ia3 over-bard silica with complementary pores and its carbon replica by electron crystallography. Angew. Chem. Int. Ed. 2004, 43, 5231–5234. [Google Scholar] [CrossRef]
- Liu, X.Y.; Tian, B.Z.; Yu, C.Z.; Gao, F.; Xie, S.H.; Tu, B.; Che, R.C.; Peng, L.M.; Zhao, D.Y. Room-temperature synthesis in acidic media of large-pore three-dimensional bicontinuous mesoporous silica with Ia3d symmetry. Angew. Chem. Int. Ed. 2002, 41, 3876–3878. [Google Scholar] [CrossRef]
- Yang, H.F.; Zhao, D.Y. Synthesis of replica mesostructures by the nanocasting strategy. J. Mater. Chem. 2005, 15, 1217–1231. [Google Scholar]
- Dickinson, C.; Zhou, W.Z.; Hodgkins, R.P.; Shi, Y.F.; Zhao, D.Y.; He, H.Y. Formation mechanism of porous single-crystal Cr2O3 and Co3O4 templated by mesoporous silica. Chem. Mater. 2006, 18, 3088–3095. [Google Scholar] [CrossRef]
- Yue, W.B.; Hill, A.H.; Harrison, A.; Zhou, W.Z. Mesoporous single-crystal Co3O4 templated by cage-containing mesoporous silica. Chem. Commun. 2007, 2518–2520. [Google Scholar] [CrossRef]
- Jiao, K.; Zhang, B.; Yue, B.; Ren, Y.; Liu, S.X.; Yan, S.R.; Dickinson, C.; Zhou, W.Z.; He, H.Y. Growth of porous single-crystal Cr2O3 in a 3-D mesopore system. Chem. Commun. 2005, 5618–5620. [Google Scholar] [CrossRef]
- Scott, B.J.; Wirnsberger, G.; Stucky, G.D. Mesoporous and mesostructured materials for optical applications. Chem. Mater. 2001, 13, 3140–3150. [Google Scholar] [CrossRef]
- Shon, J.K.; Kong, S.S.; Kim, J.M.; Ko, C.H.; Jin, M.; Lee, Y.Y.; Hwang, S.H.; Yoona, J.A.; Kim, J.N. Facile synthesis of highly ordered mesoporous silver using cubic mesoporous silica template with controlled surface hydrophobicity. Chem. Commun. 2009, 650–652. [Google Scholar] [CrossRef]
- Lu, A.H.; Schuth, F. Nanocasting: A versatile strategy for creating nanostructured porous materials. Adv. Mater. 2006, 18, 1793–1805. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Krawiec, P.; Kockrick, E.; Borchardt, L.; Geiger, D.; Corma, A.; Kaskel, S. Ordered mesoporous carbide derived carbons: Novel materials for catalysis and adsorption. J. Phys. Chem. C 2009, 113, 7755–7761. [Google Scholar] [CrossRef]
- Chang, S.C.; Huang, M.H. Formation of indium nitride nanorods within mesoporous silica SBA-15. Inorg. Chem. 2008, 47, 3135–3139. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.C.; Xia, Y.; Zhang, W.K.; Huang, H.; Gan, Y.P.; Zeng, H.J.; Tao, X.Y. Electrochemical synthesis of mesoporous FePO4 nanoparticles for fabricating high performance LiFePO4/C cathode materials. Microporous Mesoporous Mater. 2012, 152, 128–133. [Google Scholar] [CrossRef]
- Clearfield, A. Metal phosphonate chemistry. Prog. Inorg. Chem. 1998, 47, 371–510. [Google Scholar]
- Clearfield, A. Organically pillared micro- and mesoporous materials. Chem. Mater. 1998, 10, 2801–2810. [Google Scholar] [CrossRef]
- Alberti, G.; Costantino, U.; Marmottini, F.; Vivani, R.; Zappelli, P. Zirconium phosphite (3,3',5,5'-tetramethylbiphenyl)diphosphonate, a microporous, layered, inorganic-organic polymer. Angew. Chem. Int. Ed. 1993, 32, 1357–1359. [Google Scholar] [CrossRef]
- Kamiya, Y.; Nishikawa, E.; Satsuma, A.; Yoshimune, M.; Okuhara, T. Highly porous vanadium phosphorus oxides derived from vanadyl n-butylphosphate. Microporous Mesoporous Mater. 2002, 54, 277–283. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Marmottini, F.; Vivani, R. Preparation of mesoporous zirconium phosphate-pyrophosphate with a large amount of thermally stable acid groups on the pore surface. J. Porous Mater. 1999, 6, 299–305. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Y.M.; He, M.Y.; Iwamoto, M. Postsynthesis of hexagonally packed porous zirconium phosphate through a novel anion exchange between zirconium oxide mesophase and phosphoric acid. Chem. Mater. 2005, 17, 3921–3928. [Google Scholar] [CrossRef]
- Ciesla, U.; Schacht, S.; Stucky, G.D.; Unger, K.K.; Schuth, F. Formation of a porous zirconium oxo phosphate with a high surface area by a surfactant-assisted synthesis. Angew. Chem. Int. Ed. 1996, 35, 541–543. [Google Scholar] [CrossRef]
- Kleitz, F.; Thomson, S.J.; Liu, Z.; Terasaki, O.; Schuth, F. Porous mesostructured zirconium oxophosphate with cubic (Ia3d) symmetry. Chem. Mater. 2002, 14, 4134–4144. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, Y.S.; Sakamoto, Y.; Terasaki, O.; Che, S. Structure and thermal stability of mesostructured zirconium oxophosphates. Microporous Mesoporous Mater. 2007, 100, 295–301. [Google Scholar] [CrossRef]
- Jimenez-Jimenez, J.; Maireles-Torres, P.; Olivera-Pastor, P.; Rodriguez-Castellon, E.; Jimenez-Lopez, A.; Jones, D.J.; Roziere, J. Surfactant-assisted synthesis of a mesoporous form of zirconium phosphate with acidic properties. Adv. Mater. 1998, 10, 812–815. [Google Scholar] [CrossRef]
- Rodriguez-Castellon, E.; Jimenez-Jimenez, J.; Jimenez-Lopez, A.; Maireles-Torres, P.; Ramos-Barrado, J.R.; Jones, D.J.; Roziere, J. Proton conductivity of mesoporous MCM type of zirconium and titanium phosphates. Solid State Ionics 1999, 125, 407–410. [Google Scholar] [CrossRef]
- Sun, Y.; Afanasiev, P.; Vrinat, M.; Coudurier, G. Porous zirconium phosphates prepared by surfactant-assisted precipitation. J. Mater. Chem. 2000, 10, 2320–2324. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Ren, T.Z.; Azioune, A.; Pireaux, J.J.; Su, B.L. Marvelous self-assembly of hierarchically nanostructured porous zirconium phosphate solid acids with high thermal stability. Catal. Today 2005, 105, 647–654. [Google Scholar] [CrossRef]
- Sahu, A.K.; Pitchumani, S.; Sridhar, P.; Shukla, A.K. Co-assembly of a nafion-mesoporous zirconium phosphate composite membrane for PEM fuel cells. Fuel Cells 2009, 9, 139–147. [Google Scholar] [CrossRef]
- Bellezza, F.; Cipiciani, A.; Costantino, U.; Marmottini, F. Adsorption of myoglobin onto porous zirconium phosphate and zirconium benzenephosphonate obtained with template synthesis. Langmuir 2006, 22, 5064–5069. [Google Scholar] [CrossRef] [PubMed]
- Um, W.; Mattigod, S.; Serne, R.J.; Fryxell, G.E.; Kim, D.H.; Troyer, L.D. Synthesis of nanoporous zirconium oxophosphate and application for removal of U(VI). Water Res. 2007, 41, 3217–3226. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Panda, A.B.; Pradhan, N.C.; Pramanik, P. Synthesis of spherical mesostructured zirconium phosphate with acidic properties. Microporous Mesoporous Mater. 2006, 95, 360–365. [Google Scholar] [CrossRef]
- Das, S.K.; Bhunia, M.K.; Sinha, A.K.; Bhaumik, A. Synthesis, characterization, and biofuel application of mesoporous zirconium oxophosphates. ACS Catal. 2011, 1, 493–501. [Google Scholar] [CrossRef]
- Jones, D.J.; Aptel, G.; Brandhorst, M.; Jacquin, M.; Jimenez-Jimenez, J.; Jimenez-Lopez, A.; Maireles-Torres, P.; Piwonski, I.; Rodriguez-Castellon, E.; Zajac, J. High surface area mesoporous titanium phosphate: synthesis and surface acidity determination. J. Mater. Chem. 2000, 10, 1957–1963. [Google Scholar] [CrossRef]
- Feng, J.; Huo, Q.; Petroff, P.M.; Stucky, G.D. Morphology definition by disclinations and dislocations in a mesostructured silicate crystal. Appl. Phys. Lett. 1997, 71, 620–622. [Google Scholar] [CrossRef]
- Thieme, M.; Schuth, F. Preparation of a mesoporous high surface area titanium oxo phosphate via a non-ionic surfactant route. Microporous Mesoporous Mater. 1999, 27, 193–200. [Google Scholar] [CrossRef]
- Blanchard, J.; Schuth, F.; Trens, P.; Hudson, M. Synthesis of hexagonally packed porous titanium oxo-phosphate. Microporous Mesoporous Mater. 2000, 39, 163–170. [Google Scholar] [CrossRef]
- Wang, L.; Yan, Z.; Qiao, S.Z.; Lu, G.Q.M.; Huang, Y. Structural and morphological transformations of mesostructured titanium phosphate through hydrothermal treatment. J. Colloid Interface Sci. 2007, 316, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.Z.; Yuan, Z.Y.; Azioune, A.; Pireaux, J.J.; Su, B.L. Tailoring the porous hierarchy of titanium phosphates. Langmuir 2006, 22, 3886–3894. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, M.M.; Zhang, X.D.; Cui, J.J.; Yue, Y.Z. Large-scale synthesis of hierarchically mesoporous phosphate nanocomposites using yeast cells as the template reactor. Res. Chem. Intermed. 2011, 37, 309–318. [Google Scholar] [CrossRef]
- Pan, C.H.; Yuan, S.; Zhang, W.X. A neutral templating route to mesoporous titanium phosphate molecular sieves with enhanced thermal stability. Appl. Catal. A 2006, 312, 186–193. [Google Scholar] [CrossRef]
- Ma, T.Y.; Lin, X.Z.; Yuan, Z.Y. Periodic mesoporous titanium phosphonate hybrid materials. J. Mater. Chem. 2010, 20, 7406–7415. [Google Scholar] [CrossRef]
- Nagaraju, P.; Srilakshmi, C.; Pasha, N.; Lingaiah, N.; Suryanarayana, I.; Prasad, P.S.S. Effect of method of preparation on the activity and selectivity of iron phosphate catalyst in the ammoxidation of 2-methyl pyrazine. Catal. Today 2008, 131, 393–401. [Google Scholar] [CrossRef]
- Guo, X.F.; Ding, W.P.; Wang, X.G.; Yan, Q.J. Synthesis of a novel mesoporous iron phosphate. Chem. Commun. 2001, 709–710. [Google Scholar] [CrossRef]
- Pillai, U.R.; Sahle-Demessie, E. Mesoporous iron phosphate as an active, selective and recyclable catalyst for the synthesis of nopol by Prins condensation. Chem. Commun. 2004, 826–827. [Google Scholar] [CrossRef]
- Yu, D.H.; Qian, J.S.; Xue, N.H.; Zhang, D.Y.; Wang, C.Y.; Guo, X.F.; Ding, W.P.; Chen, Y. Mesoporous nanotubes of iron phosphate: Synthesis, characterization, and catalytic property. Langmuir 2007, 23, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Z.; Imae, T. Synthesis of mesoporous iron phosphate using PAMAM dendrimer as a single molecular template. Chem. Lett. 2005, 34, 1132–1133. [Google Scholar] [CrossRef]
- Shi, Z.C.; Attia, A.; Ye, W.L.; Wang, Q.; Li, Y.X.; Yang, Y. Synthesis, characterization and electrochemical performance of mesoporous FePO4 as cathode material for rechargeable lithium batteries. Electrochim. Acta 2008, 53, 2665–2673. [Google Scholar] [CrossRef]
- Lee, Y.J.; Belcher, A.M. Nanostructure design of amorphous FePO4 facilitated by a virus for 3 V lithium ion battery cathodes. J. Mater. Chem. 2011, 21, 1033–1039. [Google Scholar] [CrossRef]
- El Haskouri, J.; Roca, M.; Cabrera, S.; Alamo, J.; Beltran-Porter, A.; Beltran-Porter, D.; Marcos, M.D.; Amoros, P. Interface charge density matching as driving force for new mesostructured oxovanadium phosphates with hexagonal structure, [CTA]xVOPO4·zH2O. Chem. Mater. 1999, 11, 1446–1454. [Google Scholar]
- Mizuno, N.; Hatayama, H.; Uchida, S.; Taguchi, A. Tunable one-pot syntheses of hexagonal-, cubic-, and lamellar-mesostructured vanadium-phosphorus oxides. Chem. Mater. 2001, 13, 179–184. [Google Scholar] [CrossRef]
- Kamiya, Y.; Yamamoto, N.; Imai, H.; Komai, S.; Okuhara, T. Mesostructured vanadium phosphorus oxides assembled with exfoliated VOPO4 nanosheets. Microporous Mesoporous Mater. 2005, 81, 49–57. [Google Scholar] [CrossRef]
- Sanz, F.; Parada, C.; Rojo, J.M.; Ruiz-Valero, C. Crystal structure, magnetic properties, and ionic conductivity of a new mixed-anion phosphate Na4Ni5(PO4)2(P2O7)2. Chem. Mater. 1999, 11, 2673–2679. [Google Scholar] [CrossRef]
- Guillou, N.; Gao, Q.; Forster, P.M.; Chang, J.S.; Nogues, M.; Park, S.E.; Ferey, G.; Cheetham, A.K. Nickel(II) phosphate VSB-5: A magnetic nanoporous hydrogenation catalyst with 24-ring tunnels. Angew. Chem. Int. Ed. 2001, 40, 2831–2834. [Google Scholar] [CrossRef]
- Kandori, K.; Nakashima, H.; Ishikawa, T. Inner structure of uniform spherical metal phosphate particles : 2. Nickel phosphate. J. Colloid Interface Sci. 1993, 160, 499–501. [Google Scholar] [CrossRef]
- Yu, J.; Wang, A.; Tan, J.; Li, X.; van Bokhoven, J.A.; Hu, Y.K. Synthesis of novel nanotubular mesoporous nickel phosphates with high performance in epoxidation. J. Mater. Chem. 2008, 18, 3601–3607. [Google Scholar] [CrossRef]
- Yu, D.H.; Wu, C.; Kong, Y.; Xue, N.H.; Guo, X.F.; Ding, W.P. Structural and catalytic investigation of mesoporous iron phosphate. J. Phys. Chem. C 2007, 111, 14394–14399. [Google Scholar] [CrossRef]
- Tarafdar, A.; Biswas, S.; Pramanik, N.K.; Pramanik, P. Synthesis of mesoporous chromium phosphate through an unconventional sol-gel route. Microporous Mesoporous Mater. 2006, 89, 204–208. [Google Scholar] [CrossRef]
- Liu, Q.; He, H.; Chao, Z.S.; Xie, J.; Ruchenstein, E. Synthesis of mesoporous chromium phosphates via solid-state reaction at low temperature. New J. Chem. 2012, 36, 139–147. [Google Scholar] [CrossRef]
- Sarkar, A.; Pramanik, P. Synthesis of mesoporous niobium oxophosphate using niobium tartrate precursor by soft templating method. Microporous Mesoporous Mater. 2009, 117, 580–585. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.J.; Ren, J.W.; Liu, X.H.; Li, X.C.; Xia, Y.J.; Lu, G.Z.; Wang, Y.Q. Mesoporous niobium phosphate: An excellent silid acid for the dehydration of fructose to 5-hydroxymethylfurfural in water. Catal. Sci. Technol. 2012, 2, 2485–2491. [Google Scholar] [CrossRef]
- Li, X.C.; Zhang, Y.; Xia, Y.J.; Hu, B.C.; Zhong, L.; Wang, Y.Q.; Lu, G.Z. One-pot catalytic conversion of xylose to furfural on mesoporous niobium phosphate. Acta. Phys. Chim. Sin. 2012, 28, 2349–2354. [Google Scholar]
- He, W.; Yan, S.P.; Wang, Y.J.; Zhang, X.D.; Zhub, W.J.; Tian, X.Y.; Sun, X.A.; Han, X.X. Biomimetic synthesis of mesoporous zinc phosphate nanoparticles. J. Alloy Compd. 2009, 477, 657–660. [Google Scholar] [CrossRef]
- Sarkar, A.; Pramanik, P. A new and facile route to prepare mesoporous tantalum phosphate with high surface area using tantalum tartrate precursor. J. Non-Cryst. Solids 2010, 356, 2709–2713. [Google Scholar] [CrossRef]
- Huo, Q.S.; Feng, J.L.; Schuth, F.; Stucky, G.D. Preparation of hard mesoporous silica spheres. Chem. Mater. 1997, 9, 14–17. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, R.; Ding, Y. A Review on the Synthesis and Applications of Mesostructured Transition Metal Phosphates. Materials 2013, 6, 217-243. https://doi.org/10.3390/ma6010217
Lin R, Ding Y. A Review on the Synthesis and Applications of Mesostructured Transition Metal Phosphates. Materials. 2013; 6(1):217-243. https://doi.org/10.3390/ma6010217
Chicago/Turabian StyleLin, Ronghe, and Yunjie Ding. 2013. "A Review on the Synthesis and Applications of Mesostructured Transition Metal Phosphates" Materials 6, no. 1: 217-243. https://doi.org/10.3390/ma6010217




