Preparation and Characterization of Guar-Montmorillonite Nanocomposites
Abstract
:1. Introduction
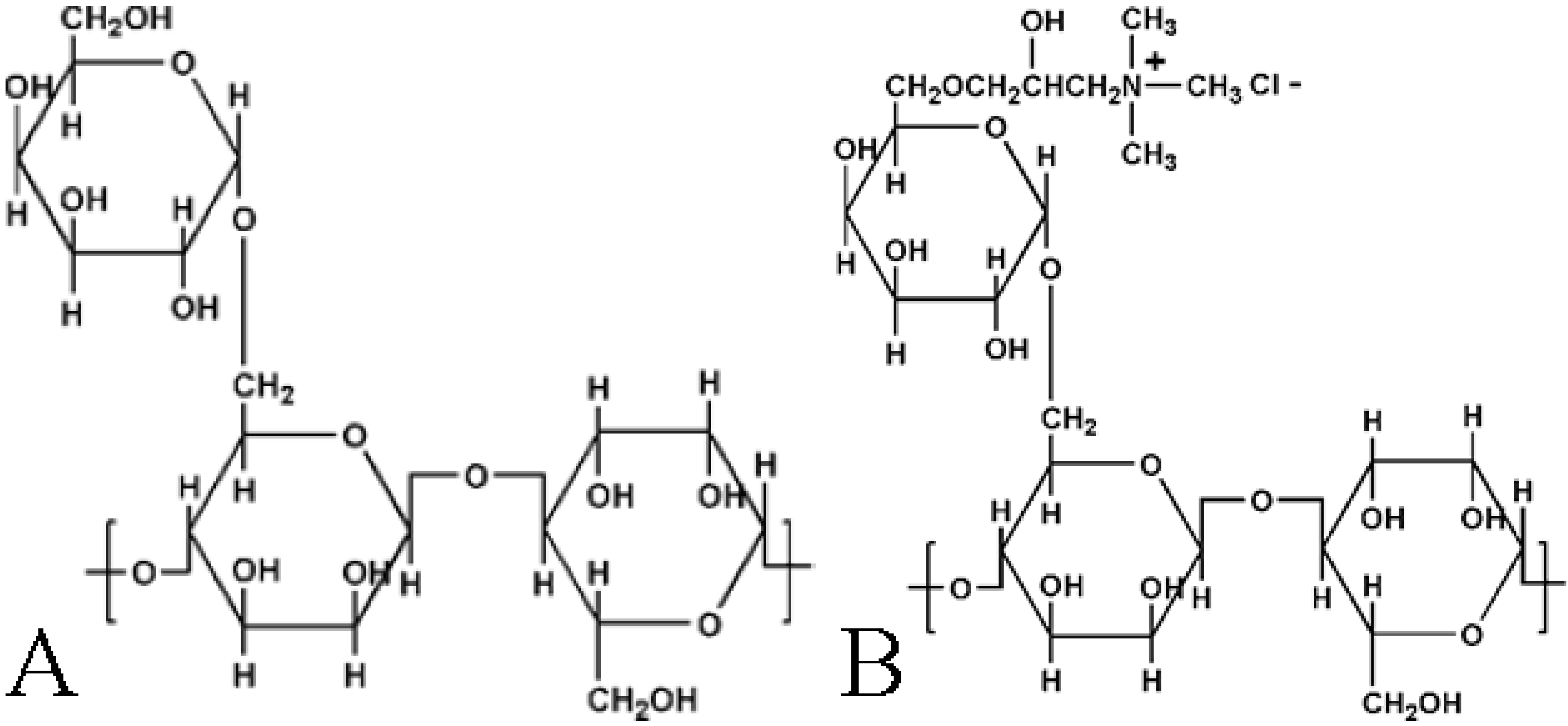
2. Results and Discussion
2.1. Interlayer Structure of the Modified Montmorillonite
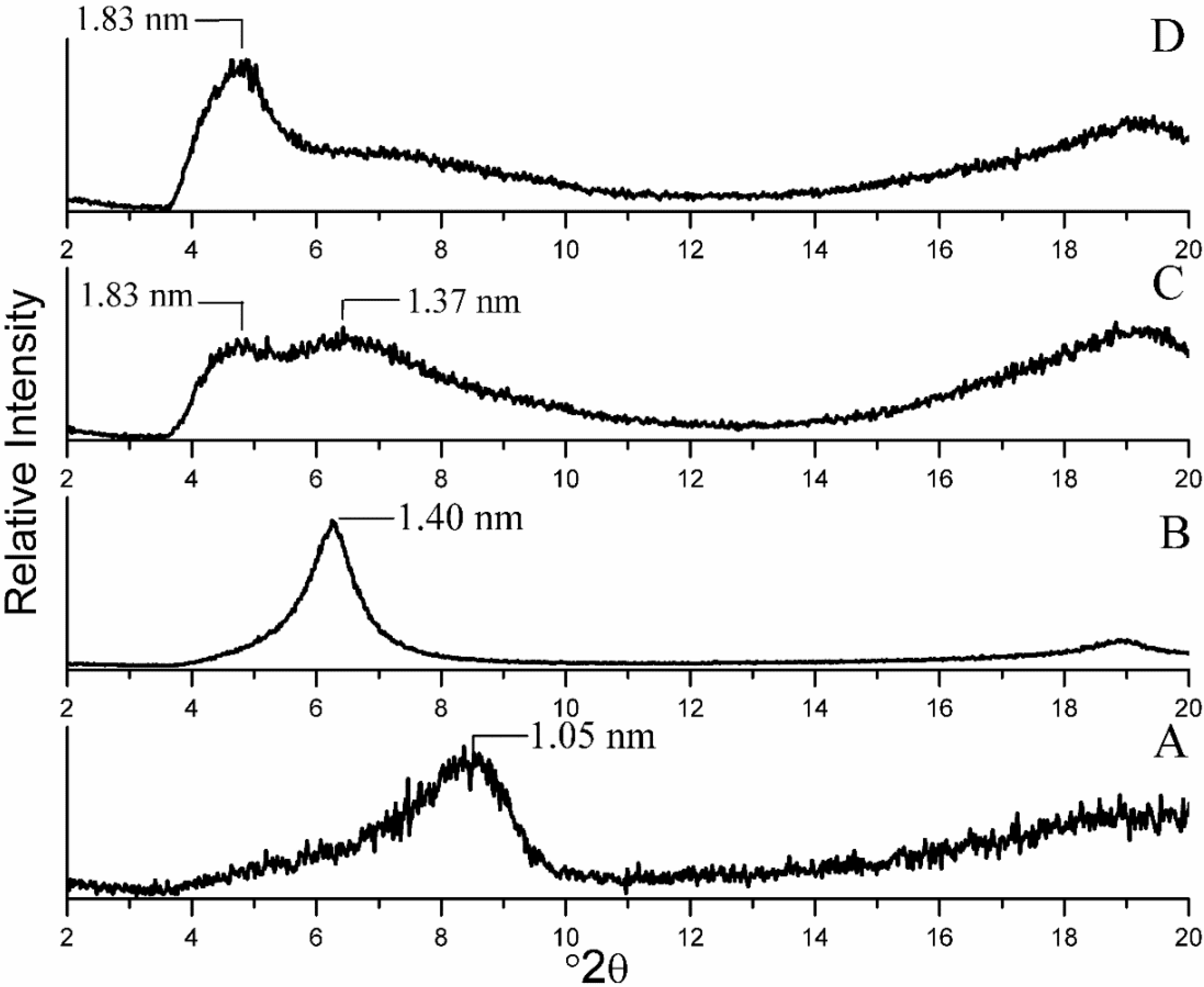
2.2. Morphology of the Nanocomposites by TEM Imaging

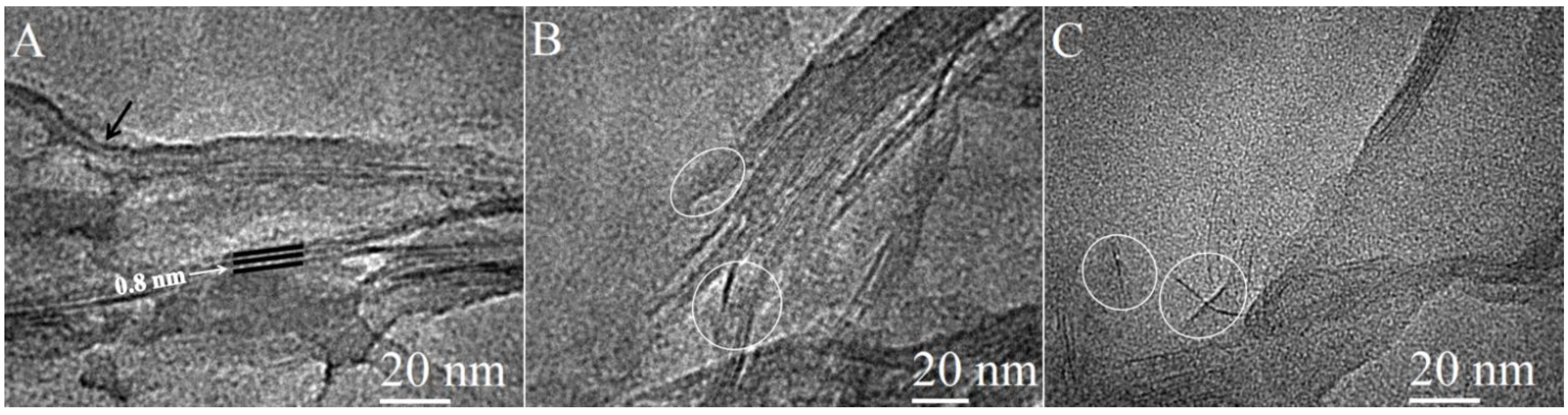
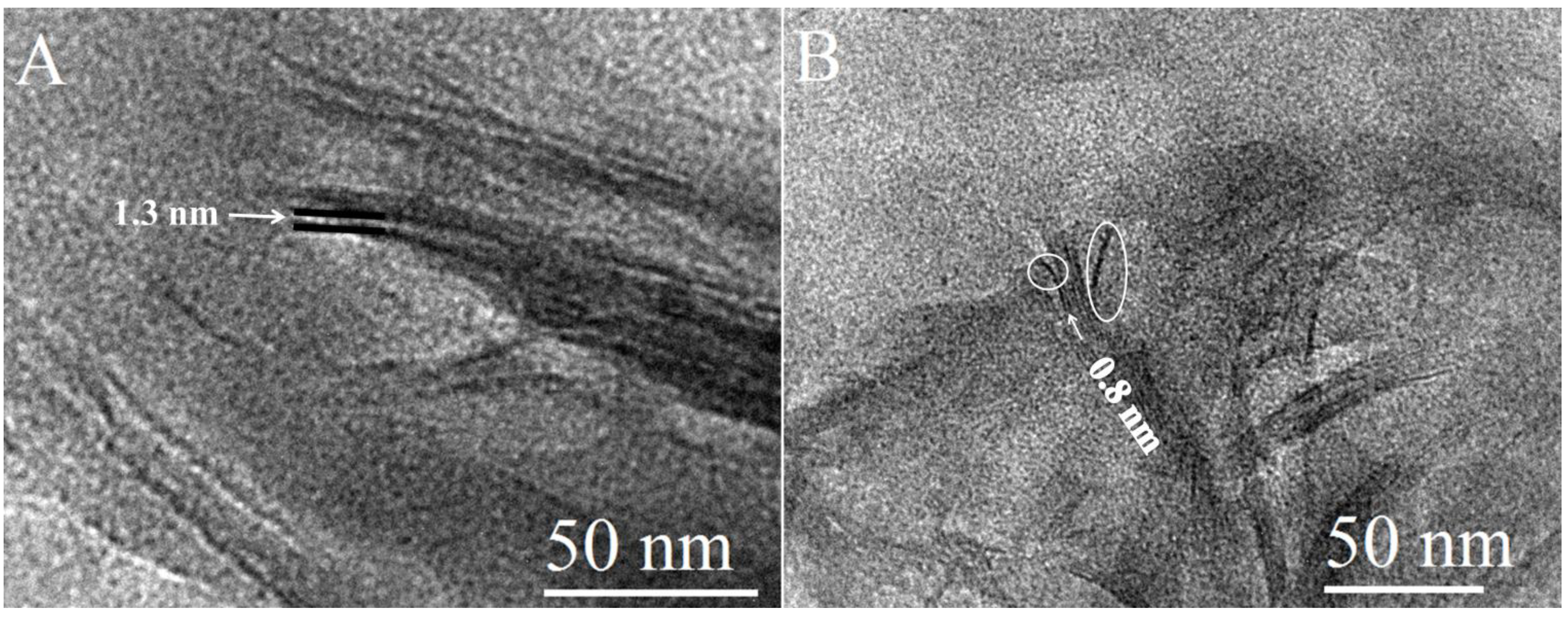

2.3. Thermal Analysis
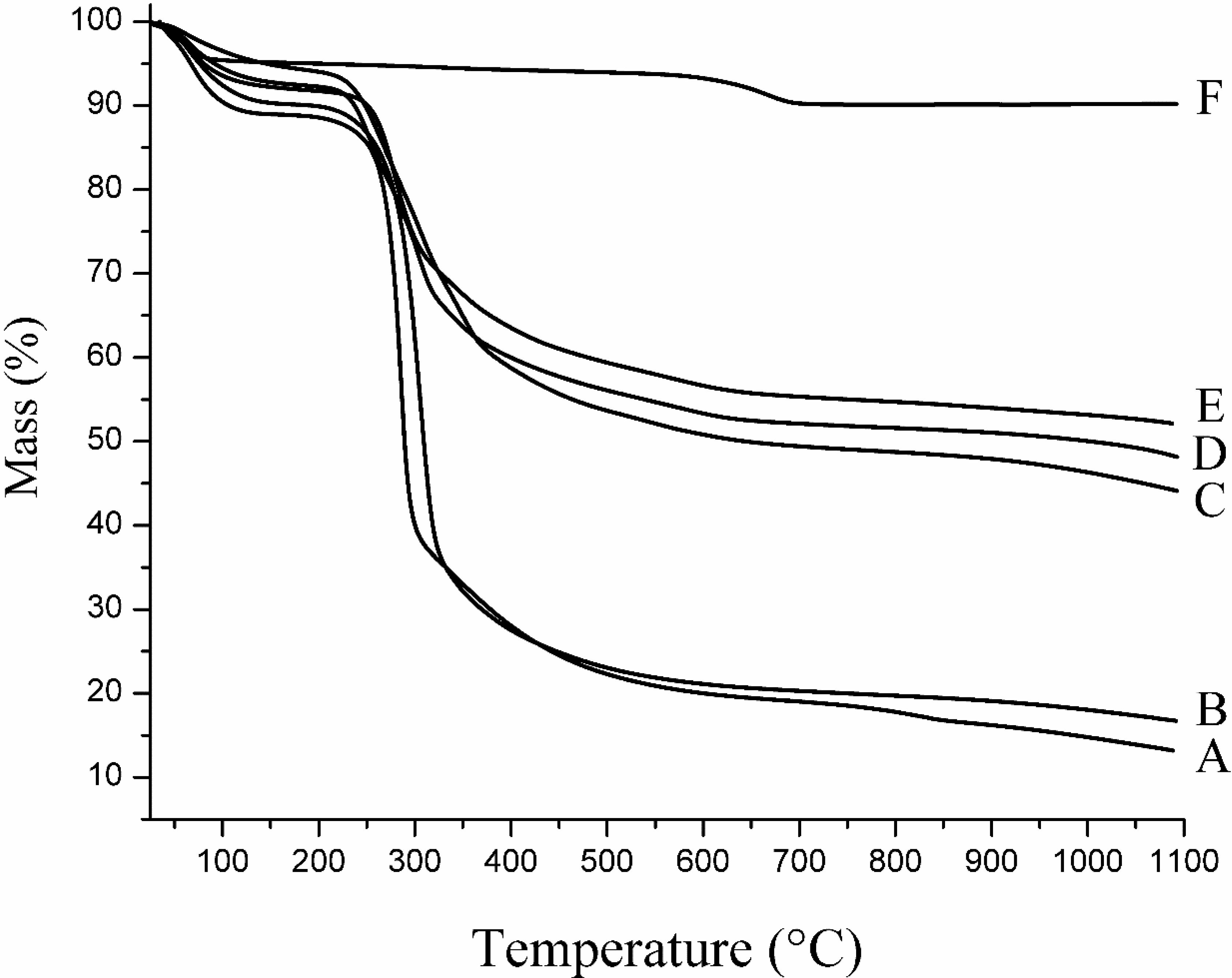
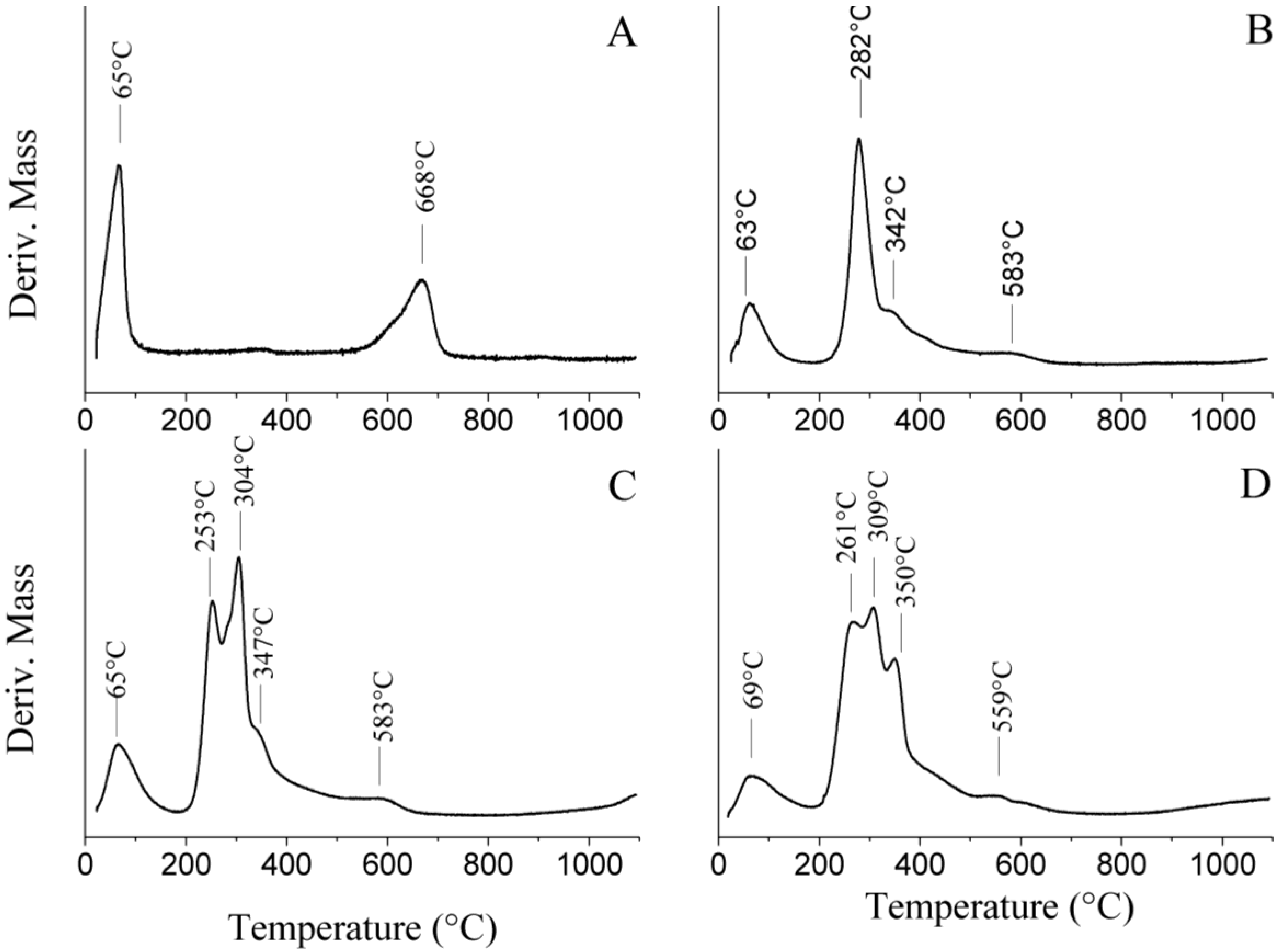
2.4. Structural Characterization
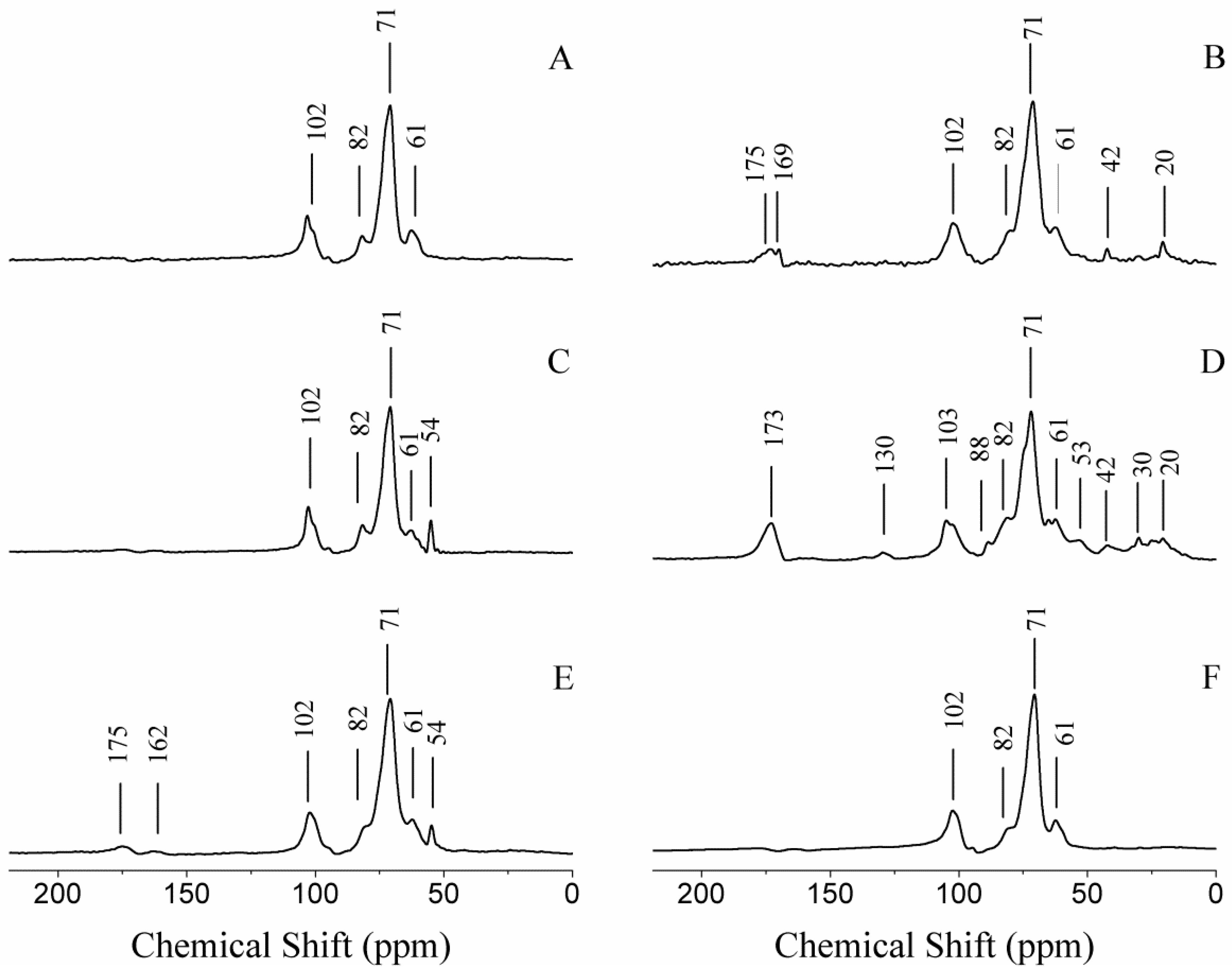
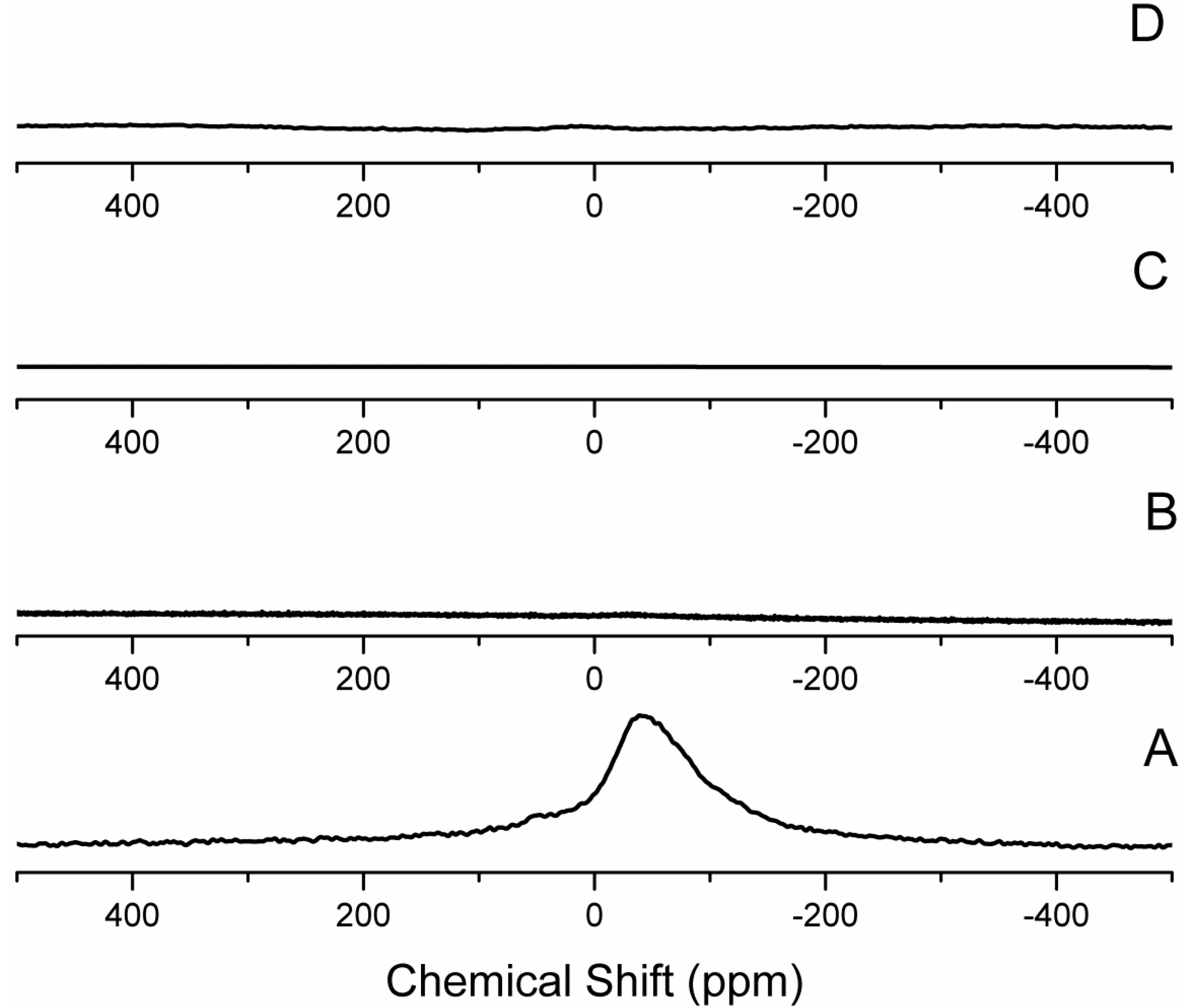
2.5. Chemical Analysis
| Element | Na+ MMT | CG-MMT 3:1 | NG-MMT 3:1 | NG-MMT 6:1 |
|---|---|---|---|---|
| Al (wt %) | 9.666 | 5.067 | 4.527 | 3.638 |
| Ca (wt %) | 0.047 | 0.075 | 0.119 | 0.162 |
| Fe (wt %) | 2.614 | 1.308 | 1.222 | 0.987 |
| K (wt %) | 0.110 | 0.063 | 0.250 | 0.273 |
| Mg (wt %) | 1.199 | 0.645 | 0.622 | 0.535 |
| Na (wt %) | 1.649 | 0.082 | 0.431 | 0.305 |
| Si (wt %) | 28.69 | 12.84 | 11.47 | 9.25 |
| Cu wt (μg/g) | 6.3 | 9.3 | 19.1 | 12.7 |
| Mn wt (μg/g) | 90.4 | 44.3 | 42.6 | 39.8 |
| Zn wt (μg/g) | 92.0 | 61.3 | 68.9 | 60.0 |
| Element wt (μg/g) | CG | NG |
|---|---|---|
| Al | 1.85 | 3.56 |
| Ca | 381.0 | 518.2 |
| Fe | 19.5 | 28.8 |
| K | 1357 | 1886 |
| Mg | 234 | 293 |
| Na | 4732 | 147 |
| Si | 4.62 | 10.94 |
| Cu | 1.31 | 1.14 |
| Mn | 1.261 | 2.025 |
| Zn | 3.95 | 4.52 |
3. Experimental Section
3.1. Materials
3.2. Preparation of Guar-Montmorillonite Nanocomposites (CG-MMT 3:1, NG-MMT 3:1, NG-MMT 6:1)
3.3. Preparation of NG Control
3.4. Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Komarneni, S. Feature article. Nanocomposites. J. Mater. Chem. 1992, 2, 1219–1230. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; van Meerbeek, A. Clay mineral- and organoclay-polymer nanocomposite. In Handbook of Clay Science, 1st ed.; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 583–621. [Google Scholar]
- Wegner, T.H.; Jones, E.P. A fundamental review of the relationships between nanotechnology and lignocellulosic biomass. In The Nanoscience and Technology of Renewable Biomaterials; Lucia, L.A., Rojas, O.J., Eds.; Wiley: Chichester, UK, 2009. [Google Scholar]
- Bitinis, N.; Hernandez, M.; Verdejo, R.; Kenny, J.M.; Lopez-Manchado, M.A. Recent advances in clay/polymer nanocomposites. Adv. Mater. 2011, 23, 5229–5236. [Google Scholar] [CrossRef] [PubMed]
- Corcione, C.E.; Frigione, M. Characterization of nanocomposites by thermal analysis. Materials 2012, 5, 2960–2980. [Google Scholar] [CrossRef]
- Sanchez, C.; Julian, B.; Belleville, P.; Popall, M. Applications of hybrid organic-inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Campbell, K.; Craig, D.Q.M.; McNally, T. Poly(ethylene glycol) layered silicate nanocomposites for retarded drug release prepared by hot-melt extrusion. Int. J. Pharm. 2008, 363, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kaygusuv, H.; Erim, F.B. Alginate/BSA/montmorillonite composites with enhanced protein entrapment and controlled release efficiency. React. Funct. Polym. 2013, 73, 1420–1425. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Figueiras, A.; Veiga, F.; de Freitas, R.M.; Nunes, L.C.; da Silva Filho, E.C.; da Silva Leite, C.M. The systems containing clays and clay minerals from modified drug release: A review. Colloids Surf. B Biointerfaces 2013, 103, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, I.; Aguzzi, C.; Sandri, G.; Bonferoni, M.C.; Mori, M.; Cerezo, P.; Sánchez, R.; Viseras, C.; Caramella, C. In vitro biocompatibility and mucoadhesion of montmorillonite chitosan nanocomposite: A new drug delivery. Appl. Clay Sci. 2012, 55, 131–137. [Google Scholar] [CrossRef]
- Seema, M.D. Clay–polymer nanocomposites as a novel drug carrier: Synthesis, characterization and controlled release study of Propranolol Hydrochloride. Appl. Clay Sci. 2013, 80–81, 85–92. [Google Scholar] [CrossRef]
- Aguzzi, C.; Cerezo, P.; Viseras, C.; Caramella, C. Use of clays as drug delivery systems: Possibilities and limitations. Appl. Clay Sci. 2007, 36, 22–36. [Google Scholar] [CrossRef]
- Wang, X.Y.; Du, Y.M.; Luo, J.W. Biopolymer/montmorillonite nanocomposite: Preparation, drug-controlled release property and cytotoxicity. Nanotechnology 2008, 19, 1–7. [Google Scholar] [PubMed]
- Cypes, S.H.; Saltzman, W.M.; Giannelis, E.P. Organosilicate-polymer drug delivery systems: Controlled release and enhanced mechanical properties. J. Control. Release 2003, 90, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.F.; Fu, Y.T. Effect of montmorillonite on the swelling behavior and drug-release behavior of nanocomposite hydrogels. J. Appl. Polym. Sci. 2003, 89, 3652–3660. [Google Scholar] [CrossRef]
- Yuan, Q.; Shah, J.; Hein, S.; Misra, R.D.K. Controlled and extended drug release behavior of chitosan-based nanoparticle carrier. Acta Biomater. 2010, 6, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Rhim, J.W.; Ng, P.K.W. Natural biopolymer-based nanocomposite films for packaging applications. Crit. Rev. Food Sci. Nutr. 2007, 47, 411–433. [Google Scholar] [CrossRef]
- Tang, X.Z; Kumar, P.; Alavi, S.; Sandeep, K.P. Recent advances in biopolymers and biopolymer-based nanocomposites for food packaging materials. Crit. Rev. Food Sci. Nutr. 2012, 52, 426–442. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Misra, M.; Drzal, L.T.; Mohanty, A.K. “Green” nanocomposites from cellulose acetate bioplastic and clay: Effect of eco-friendly triethyl citrate plasticizer. Biomacromolecules 2004, 5, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite. Chem. Mater. 2003, 15, 3774–3780. [Google Scholar] [CrossRef]
- Darder, M.; Colilla, M.; Ruiz-Hitzky, E. Chitosan-clay nanocomposites: Application as electrochemical sensors. Appl. Clay Sci. 2005, 28, 199–208. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.R.; Darder, M.; Aranda, P. Functional biopolymer nanocomposites based on layered solids. J. Mater. Chem. 2005, 15, 3650–3662. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, Y.H.; Szeto, Y.S.; Liao, L.B. A novel method to prepare chitosan/montmorillonite nanocomposites in the presence of hydroxy-aluminum oligomeric cations. Compos. Sci. Technol. 2008, 68, 2917–2921. [Google Scholar] [CrossRef]
- Delhom, C.D.; White-Ghoorahoo, L.A.; Pang, S.S. Development and characterization of cellulose/clay nanocomposites. Compos. Part B Eng. 2010, 41, 475–481. [Google Scholar] [CrossRef]
- Levy, N.; Garti, N.; Magdassi, S. Flocculation of bentonite suspensions with cationic guar. Colloids Surf. A 1995, 97, 91–99. [Google Scholar] [CrossRef]
- Srivastava, M.; Kapoor, V.P. Seed gallactomannans: An overview. Chem. Biodivers. 2005, 2, 295–317. [Google Scholar] [CrossRef]
- Tiraferri, A.; Chen, K.L.; Sethi, R.; Elimelech, M. Reduced aggregation and sedimentation of zero-valent iron nanoparticles in the presence of guar gum. J. Colloid Interface Sci. 2008, 324, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gliko-Kabir, I.; Penhasi, A.; Rubinstein, A. Characterization of crosslinked guar by thermal analysis. Carbohydr. Res. 1999, 316, 6–13. [Google Scholar] [CrossRef]
- Guégan, R. Intercalation of a nonionic surfactant (C10E3) bilayer into a Na-Montmorillonite clay. Langmuir 2010, 26, 19175–19180. [Google Scholar]
- Carrado, K.A.; Decarreau, A.; Petit, S.; Bergaya, F.; Lagaly, G. Synthetic clay minerals and purification of natural clays. In Handbook of Clay Science, 1st ed.; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 115–139. [Google Scholar]
- Defontaine, G.; Barichard, A.; Letaief, S.; Feng, C.Y.; Matsuura, T.; Detellier, C. Nanoporous polymer-clay hybrid membranes for gas separation. J. Colloid Interface Sci. 2010, 343, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.L.; Bassett, W.A.; Wu, T.C. Dehydration and hydration of montmorillonite at elevated-temperatures and pressures monitored using synchrotron-radiation. Am. Miner. 1994, 79, 683–691. [Google Scholar]
- Hower, W.F. Adsorption of surfactants on montmorillonite. Clays Clay Miner. 1970, 18, 97–105. [Google Scholar] [CrossRef]
- Mania, E.; Heidecker, M.J.; Nakajima, H.; Costache, M.C.; Wilkie, A.C. PET nanocomposites using nanoclays modified with thermally stable surfactants. In Thermally Stable and Flame Retardant Polymer Nanocomposites; Mittal, V., Ed.; Cambridge University Press: New York, NY, USA, 2011; pp. 99–118. [Google Scholar]
- Wu, T.M.; Wu, C.Y. Biodegradable poly(lactic acid)/chitosan-modified montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2006, 91, 2198–2204. [Google Scholar] [CrossRef]
- Martín, Z.; Jimenez, I.; Gomez, M.A.; Ade, H.W.; Kilcoyne, D.A.; Hernadez-Cruz, D. Spectromicroscopy study of intercalation and exfoliation in polypropylene/montmorillonite nanocomposites. J. Phys. Chem. B 2009, 113, 11160–11165. [Google Scholar] [CrossRef] [PubMed]
- Bhiwankar, N.N.; Weiss, R.A. Melt intercalation/exfoliation of polystyrene-sodium-montmorillonite nanocomposites using sulfonated polystyrene ionomer compatibilizers. Polymer 2006, 47, 6684–6691. [Google Scholar] [CrossRef]
- Ma, J.; Xu, H.; Ren, J.H.; Yu, Z.Z.; Mai, Y.W. A new approach to polymer/montmorillonite nanocomposites. Polymer 2003, 44, 4619–4624. [Google Scholar] [CrossRef]
- Fajnor, V.S.; Jesenak, K. Differential thermal analysis of montmorillonite. J. Therm. Anal. 1996, 46, 489–493. [Google Scholar] [CrossRef]
- Girgis, B.S.; Elbarawy, K.A.; Felix, N.S. Dehydration kinetics of some smectites: A thermogravimetric study. Thermochim. Acta 1987, 111, 9–19. [Google Scholar] [CrossRef]
- Xi, Y.F.; Ding, Z.; He, H.P.; Frost, R.L. Structure of organoclays—An X-ray diffraction and thermogravimetric analysis study. J. Colloid Interface Sci. 2004, 277, 116–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langier-Kuzniarowa, A. Thermal analysis of organo-clay complexes. In Organo-Clay Complexes and Interactions; Yariv, S., Cross, H., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 273–344. [Google Scholar]
- Letaief, S.; Detellier, C. Application of thermal analysis for the characterization of intercalated and grafted organo-kaolinite nanohybrid materials. J. Therm. Anal. Calorim. 2011, 104, 831–839. [Google Scholar] [CrossRef]
- Liu, R.; Frost, R.L.; Martens, W.N.; Yuan, Y. Synthesis, characterization of mono, di and tri alkyl surfactant intercalated wyoming montmorillonite for the removal of phenol from aqueous systems. J. Colloid Interface Sci. 2008, 327, 287–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; He, H.P.; Frost, R.L.; Xi, Y.F. Changes in the surfaces on ddoab organoclays adsorbed with paranitrophenol—An XRD, TEM and TG study. Mater. Res. Bull. 2008, 43, 3318–3326. [Google Scholar] [CrossRef] [Green Version]
- Parfitt, R.L.; Greenland, D.J. Adsorption of polysaccharide by montmorillonite. Soil Sci. Soc. Am. J. 1970, 34, 862–866. [Google Scholar] [CrossRef]
- Chenlo, F.; Moreira, R.; Silva, C. Rheological properties of aqueous dispersions of tragacanth and guar gums at different concentrations. J. Texture Stud. 2010, 41, 396–415. [Google Scholar] [CrossRef]
- Adams, J.M.; McCabe, R.W. Clay minerals as catalysts. In Handbook of Clay Science, 1st ed.; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherland, 2006; pp. 541–481. [Google Scholar]
- Letaief, S.; Aranda, P.; Fernandez-Saavedra, R.; Margeson, J.C.; Detellier, C.; Ruiz-Hitzky, E. Poly(3,4-ethylenedioxythiophene)-clay nanocomposites. J. Mater. Chem. 2008, 18, 2227–2233. [Google Scholar] [CrossRef]
- Debon, S.J.J.; Tester, R.F. In vitro binding of calcium, iron and zinc by non-starch polysaccharides. Food Chem. 2001, 73, 401–410. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). San Joaquin Soil Baseline Trace Element Concentrations. Certificate of Analysis: Standard Reference Material 2709; NIST: Gaithersburg, MD, USA, 2003.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mansa, R.; Detellier, C. Preparation and Characterization of Guar-Montmorillonite Nanocomposites. Materials 2013, 6, 5199-5216. https://doi.org/10.3390/ma6115199
Mansa R, Detellier C. Preparation and Characterization of Guar-Montmorillonite Nanocomposites. Materials. 2013; 6(11):5199-5216. https://doi.org/10.3390/ma6115199
Chicago/Turabian StyleMansa, Rola, and Christian Detellier. 2013. "Preparation and Characterization of Guar-Montmorillonite Nanocomposites" Materials 6, no. 11: 5199-5216. https://doi.org/10.3390/ma6115199




