Synthesis, Molecular Structure and Cytotoxicity of Molecular Materials Based on Water Soluble Half-Sandwich Rh(III) and Ir(III) Tetranuclear Metalla-Cycles
Abstract
:1. Introduction

2. Results and Discussion


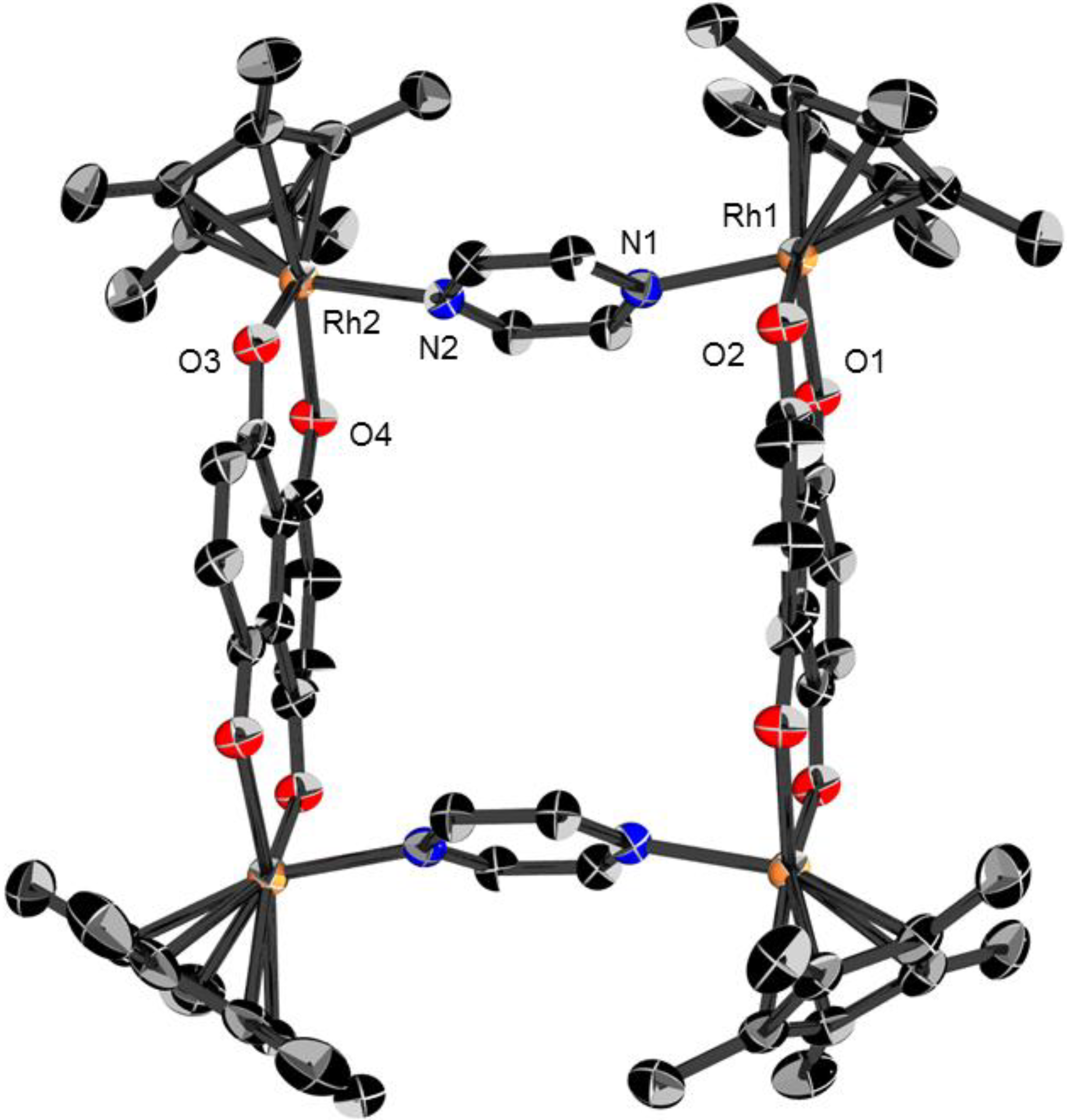
2.1. Molecular structures of 3, 5 and 6
| [3](CF3SO3)4·4CH2Cl2 | [5](CF3SO3)4·2CH2Cl2 | [6](CF3SO3)4·solvent | |
|---|---|---|---|
| Interatomic distances (Å) | |||
| M1-O1 | 2.065(3) | 2.030(4) | 2.073(5) |
| M1-O2 | 2.053(3) | 2.028(5) | 2.073(5) |
| M2-O3 | 2.061(3) | 2.034(5) | 2.069(5) |
| M2-O4 | 2.061(3) | 2.035(4) | 2.064(5) |
| M1-N1 | 2.150(3) | 2.099(5) | 2.116(6) |
| M2-N2 | 2.129(3) | 2.094(5) | 2.116(6) |
| M1-M2 (μ-dhnq) | 8.3831(5) | 8.283(1) | 8.4075(6) |
| M1-M2 (μ-N-ligand) | 6.9773(5) | 11.106(1) | 11.2437(7) |
| Angles (°) | |||
| O1-M1-O2 | 87.2(1) | 87.5(2) | 87.4(2) |
| O3-M2-O4 | 87.4(1) | 87.1(2) | 87.3(2) |
| N1-M1-O1 | 84.6(1) | 86.8(2) | 83.1(2) |
| N1-M1-O2 | 85.7(1) | 84.0(2) | 83.5(2) |
| N2-M2-O3 | 84.5(1) | 85.6(2) | 82.9(2) |
| N2-M2-O4 | 85.7(1) | 84.5(2) | 84.8(2) |
| Parameter | [3](CF3SO3)4·4CH2Cl2 | [5](CF3SO3)4·2CH2Cl2 | [6](CF3SO3)4·solvent |
|---|---|---|---|
| Chemical formula | C76H84Cl8F12N4O20Rh4S4 | C86H88Cl4F12N4O20Rh4S4 | C84H84F12Ir4N4O20S4 |
| Formula weight | 2424.95 | 2407.28 | 2594.59 |
| Crystal system | Monoclinic | Triclinic | Triclinic |
| Space group | P 21/c (no. 14) | P-1 (no. 2) | P-1 (no. 2) |
| Crystal color and shape | yellow block | green block | grey block |
| Crystal size | 0.22 × 0.18 × 0.17 | 0.16 × 0.15 × 0.13 | 0.21 × 0.20 × 0.16 |
| a (Å) | 12.3640(4) | 12.8157(9) | 12.5846(6) |
| b (Å) | 23.6046(6) | 14.8016(10) | 15.2071(7) |
| c (Å) | 16.8800(6) | 15.0871(10) | 15.4594(7) |
| α (°) | – | 90.206(5) | 89.590(4) |
| β (°) | 104.176(3) | 100.106(5) | 80.133(4) |
| γ (°) | – | 106.634(5) | 73.386(4) |
| V (Å3) | 4776.4(3) | 2695.2(3) | 2790.4(2) |
| Z | 2 | 1 | 1 |
| T (K) | 173(2) | 173(2) | 173(2) |
| Dc (g·cm−3) | 1.686 | 1.483 | 1.544 |
| μ (mm−1) | 1.080 | 0.860 | 4.906 |
| Scan range (°) | 1.70 ˂ θ ˂ 29.23 | 1.69 ˂ θ ˂ 29.30 | 1.72 ˂ θ ˂ 29.22 |
| Unique reflections | 12909 | 14597 | 14976 |
| Reflections used [I > 2σ(I)] | 9875 | 9188 | 10306 |
| Rint | 0.0908 | 0.1407 | 0.0623 |
| Final R indices [I > 2σ (I)]* | 0.0587, wR2 0.1202 | 0.0881, wR2 0.2094 | 0.0532, wR2 0.1163 |
| R indices (all data) | 0.0836, wR2 0.1299 | 0.1412, wR2 0.2423 | 0.0915, wR2 0.1274 |
| Goodness-of-fit | 1.039 | 1.103 | 1.001 |
| Max, Min Δρ (e Å−3) | 1.079, −1.341 | 1.775, −1.254 | 1.925, −2.579 |



2.2. Antiproliferative Activity
| IC50 (μM) | |||
|---|---|---|---|
| Compound | A2780 | A2780cisR | HEK293 |
| cisplatin | 1.26 ± 0.17 | 19.7 ± 3.00 | 6.55 ± 1.00 |
| 3 | 0.06 ± 0.01 | 0.19 ± 0.01 | 0.17 ± 0.01 |
| 4 | 0.07 ± 0.01 | 0.25 ± 0.05 | 0.09 ± 0.02 |
| 5 | 0.08 ± 0.01 | 0.20 ± 0.01 | 0.09 ± 0.02 |
| 6 | 0.13 ± 0.02 | 0.31 ± 0.04 | 0.11 ± 0.02 |
| 7 | 0.06 ± 0.01 | 0.18 ± 0.01 | 0.10 ± 0.01 |
| 8 | 0.17 ± 0.01 | 0.29 ± 0.03 | 0.10 ± 0.02 |
| Ru-analogue | 1.49 ± 0.11 | 1.94 ± 0.07 | 0.77 ± 0.03 |
3. Experimental Section
3.1. General
3.2. Synthesis of [(η5-C5Me5)2Rh2(μ-dhnq)Cl2] (1)
3.3. Synthesis of [(η5-C5Me5)2Ir2(μ-dhnq)Cl2] (2)
3.4. Synthesis of [(η5-C5Me5)4Rh4(μ-dhnq)2(μ-pyrazine)2](CF3SO3)4 {[3](CF3SO3)4}
3.5. Synthesis of [(η5-C5Me5)4Ir4(μ-dhnq)2(μ-pyrazine)2](CF3SO3)4 {[4](CF3SO3)4}
3.6. Synthesis of [(η5-C5Me5)4Rh4(μ-dhnq)2(μ-4,4′-bipyridine)2](CF3SO3)4 {[5](CF3SO3)4}
3.7. Synthesis of [(η5-C5Me5)4Ir4(μ-dhnq)2(μ-4,4′-bipyridine)2](CF3SO3)4 {[6](CF3SO3)4}
3.8. Synthesis of [(η5-C5Me5)4Rh4(μ-dhnq)2(μ-1,2-bis(4-pyridyl)ethane)2](CF3SO3)4 {[7](CF3SO3)4}
3.9. Synthesis of [(η5-C5Me5)4Ir4(μ-dhnq)2(μ-1,2-bis(4-pyridyl)ethane)2](CF3SO3)4 {[8](CF3SO3)4}
3.10. Cell Culture and Inhibition of Cell Growth
3.11. Single-Crystal X-ray Structure Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cook, T.R.; Vajpayee, V.; Lee, M.H.; Stang, P.J.; Chi, K.-W. Biomedical and biochemical applications of self-assembled metallacycles and metallacages. Acc. Chem. Res. 2013, 46, 2464–2474. [Google Scholar] [CrossRef]
- Therrien, B. Drug delivery by water-soluble organometallic cages. Top Curr. Chem. 2012, 319, 35–56. [Google Scholar] [PubMed]
- Smith, G.S.; Therrien, B. Targeted and multifunctional arene ruthenium chemotherapeutics. Dalton Trans. 2011, 40, 10793–10800. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Kang, S.C.; Chi, K.-W. Coordination-driven self-assembly of arene-ruthenium compounds. Eur. J. Inorg. Chem. 2013, 5222–5232. [Google Scholar]
- Hartinger, C.G.; Phillips, A.D.; Nazarov, A.A. Polynuclear ruthenium, osmium and gold complexes. The quest for innovative chemotherapeutics. Curr. Top Med. Chem. 2011, 11, 2688–2702. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Metzler-Nolte, N.; Dyson, P.J. Challenges and opportunities in the development of organometallic anticancer drugs. Organometallics 2012, 31, 5677–5685. [Google Scholar] [CrossRef]
- Mattsson, J.; Govindaswamy, P.; Renfrew, A.K.; Dyson, P.J.; Štěpnička, P.; Süss-Fink, G.; Therrien, B. Synthesis, molecular structure, and anticancer activity of cationic arene ruthenium metallarectangles. Organometallics 2009, 28, 4350–4357. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Zava, O.; Furrer, J.; Dyson, P.J.; Therrien, B. Anticancer activity of opened arene ruthenium metalla-assemblies. Dalton Trans. 2010, 39, 5272–5277. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Edafe, F.; Therrien, B. Anticancer activity of tetracationic arene ruthenium metalla-cycles. Dalton Trans. 2011, 40, 7172–7180. [Google Scholar] [CrossRef] [PubMed]
- Vajpayee, V.; Song, Y.H.; Yang, Y.J.; Kang, S.C.; Cook, T.R.; Kim, D.W.; Lah, M.S.; Kim, I.S.; Wang, M.; Stang, P.J.; et al. Self-assembly of cationic, hetero- or homonuclear ruthenium(II) macrocyclic rectangles and their photophysical, electrochemical, and biological studies. Organometallics 2011, 30, 6482–6489. [Google Scholar] [CrossRef] [PubMed]
- Vajpayee, V.; Lee, S.m.; Park, J.W.; Dubey, A.; Kim, H.; Cook, T.R.; Stang, P.J.; Chi, K.-W. Growth inhibitory activity of a bis-benzimidazole-bridged arene ruthenium metalla-rectangle and -prism. Organometallics 2013, 32, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Dubey, A.; Koo, H.J.; Vajpayee, V.; Cook, T.R.; Kim, H.; Kang, S.C.; Stang, P.J.; Chi, K.-W. Self-assembly of ambidentate pyridyl-carboxylate ligands with octahedral ruthenium metal centers: Self-selection for a single-linkage isomer and anticancer-potency studies. Chem. Eur. J. 2013, 19, 6709–6717. [Google Scholar] [CrossRef] [PubMed]
- Linares, F.; Galindo, M.A.; Galli, S.; Romero, M.A.; Navarro, J.A.R.; Barea, E. Tetranuclear coordination assemblies based on half-sandwich ruthenium(II) complexes: Noncovalent binding to DNA and cytotoxicity. Inorg. Chem. 2009, 48, 7413–7420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linares, F.; Procopio, E.Q.; Galindo, M.A.; Romero, M.A.; Navarro, J.A.R.; Barea, E. Molecular architecture of redox-active half-sandwich Ru(II) cyclic assemblies. Interactions with biomolecules and anticancer activity. CrystEngComm 2010, 12, 2343–2346. [Google Scholar] [CrossRef]
- Vajpayee, V.; Song, Y.H.; Lee, M.H.; Kim, H.; Wang, M.; Stang, P.J.; Chi, K.-W. Self-assembled arene-ruthenium-based rectangles for the selective sensing of multi-carboxylate anions. Chem. Eur. J. 2011, 17, 7837–7844. [Google Scholar] [CrossRef] [PubMed]
- Severin, K. Research in the laboratory of supramolecular chemistry: Functional nanostructures, sensors, and catalysts. Chimia 2011, 65, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Rochat, S.; Qian, X.; Severin, K. A simple assay for the fluorometric detection of lithium ions in aqueous solution. Chem. Eur. J. 2010, 16, 5013–5017. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Ferri, M.-G.; Hartinger, C.G.; Eichinger, R.E.; Stolyarova, N.; Severin, K.; Jakupec, M.A.; Nazarov, A.A.; Keppler, B.K. Influence of the spacer length on the in vitro anticancer activity of dinuclear ruthenium-arene compounds. Organometallics 2008, 27, 2405–2407. [Google Scholar] [CrossRef]
- Mendoza-Ferri, M.-G.; Hartinger, C.G.; Nazarov, A.A.; Kandioller, W.; Severin, K.; Keppler, B.K. Modifying the structure of dinuclear ruthenium complexes with antitumor activity. Appl. Organometal. Chem. 2008, 22, 326–332. [Google Scholar] [CrossRef]
- Liu, H.-K.; Sadler, P.J. Metal complexes as DNA intercalators. Acc. Chem. Res. 2011, 44, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Edafe, F.; Dyson, P.J.; Therrien, B. Anticancer activity of osmium metalla-rectangles. Dalton Trans. 2010, 39, 2816–2820. [Google Scholar] [CrossRef] [PubMed]
- Severin, K.; Bergs, R.; Beck, W. Bioorganometallic chemistry-transition metal complexes with α-amino acids and peptides. Angew. Chem. Int. Ed. 1998, 37, 1634–1654. [Google Scholar] [CrossRef]
- Herberhold, M.; Yan, H.; Milius, W.; Wrackmeyer, B. Metal-induced B-H activation: Addition of methyl acetylene carboxylates to Cp*Rh-, Cp*Ir-, (p-cymene)Ru-, and (p-cymene)Os half-sandwich complexes containing the chelating 1,2-dicarba-closo-dodecaborane-1,2-dithiolate ligand. Chem. Eur. J. 2000, 6, 3026–2032. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Dyson, P.J. Bioorganometallic chemistry—From teaching paradigms to medicinal applications. Chem. Soc. Rev. 2009, 38, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Garci, A.; Murray, B.S.; Dyson, P.J.; Fabre, G.; Trouillas, P.; Giannini, F.; Furrer, J.; Süss-Fink, G.; Therrien, B. Synthesis, molecular structure, computational study and in vitro anticancer activity of dinuclear thiolato-bridged pentamethylcyclopentadienyl Rh(II) and Ir(II) complexes. Dalton Trans. 2013, 42, 15457–15463. [Google Scholar] [CrossRef] [PubMed]
- Dorcier, A.; Ang, W.H.; Bolaño, S.; Gonsalvi, L.; Juillerat-Jeanneret, L.; Laurenczy, G.; Peruzzini, M.; Phillips, A.D.; Zanobini, F.; Dyson, P.J. In vitro evaluation of rhodium and osmium RAPTA analogues: The case for organometallic anticancer drugs not based on ruthenium. Organometallics 2006, 25, 4090–4096. [Google Scholar] [CrossRef]
- Hearn, J.M.; Romero-Canelón, I.; Qamar, B.; Liu, Z.; Hands-Portman, I.; Sadler, P.J. Organometallic iridium(III) anticancer complexes with new mechanisms of action: NCI-60 screening mitochondrial targeting, and apoptosis. ACS Chem. Biol. 2013, 8, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table: Towards new targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef]
- Geldmacher, Y.; Oleszak, M.; Sheldrick, W.S. Rhodium(III) and iridium(III) complexes as anticancer agents. Inorg. Chim. Acta 2012, 393, 84–102. [Google Scholar] [CrossRef]
- Han, Y.-F.; Jia, W.-G.; Yu, W.-B.; Jin, G.-X. Stepwise formation of organometallic macrocycles, prisms and boxes from Ir, Rh and Ru-based half-sandwich units. Chem. Soc. Rev. 2009, 38, 3419–3434. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-F.; Lin, Y.-J.; Jin, G.-X. Discrete half-sandwich Ir, Rh-based organometallic molecular boxes: Synthesis, characterization, and their properties. Dalton Trans. 2011, 40, 10370–10375. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lin, Y.-J.; Jin, G.-X. Stepwise formation of organometallic macrocycles and triangular prisms containing 2,2’-bisbenzimidazole ligands. Dalton Trans. 2013, 42, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Süss-Fink, G.; Neels, A.; Stoeckli-Evans, H. Mono-, di- and tetra-nuclear p-cymeneruthenium complexes containing oxalato ligands. J. Chem. Soc. Dalton Trans. 1997, 4345–4350. [Google Scholar]
- Pitto-Barry, A.; Barry, N.P.E.; Zava, O.; Deschenaux, R.; Therrien, B. Encapsulation of pyrene-functionalized poly(benzyl ether) dendrons into a water-soluble organometallic cages. Chem. Asian J. 2011, 6, 1595–1603. [Google Scholar] [PubMed]
- Huang, S.-L.; Lin, Y.-J.; Hor, T.S.A.; Jin, G.-X. Cp*Rh-based heterometallic metallarectangles: Size-dependent borromean link structures and catalytic acyl transfer. J. Am. Chem. Soc. 2013, 135, 8125–8128. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Furrer, J.; Therrien, B. In- and out-of-cavity interactions by modulating the size of ruthenium metallarectangles. Helv. Chim. Acta 2010, 93, 1313–1328. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Furrer, J.; Freudenreich, J.; Süss-Fink, G.; Therrien, B. Designing the host-guest properties of tetranuclear arene ruthenium metalla-rectangles to accommodate a pyrene molecule. Eur. J. Inorg. Chem. 2010, 725–728. [Google Scholar]
- Kang, J.W.; Moseley, K.; Maitlis, P.M. Pentamethylcyclopentadienylrhodium and -iridium halides. Synthesis and properties. J. Am. Chem. Soc. 1969, 91, 5970–5977. [Google Scholar] [CrossRef]
- White, C.; Yates, A.; Maitlis, P.M.; Heinekey, D.M. (η5-Pentamethylcyclopenta-dienyl)rhodium and -iridium compounds. Inorg. Synth. 1992, 29, 228–234. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for Windows—A version of ORTEP-III with a graphical interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Cryst. 2002, 58, 389–397. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gupta, G.; Murray, B.S.; Dyson, P.J.; Therrien, B. Synthesis, Molecular Structure and Cytotoxicity of Molecular Materials Based on Water Soluble Half-Sandwich Rh(III) and Ir(III) Tetranuclear Metalla-Cycles. Materials 2013, 6, 5352-5366. https://doi.org/10.3390/ma6115352
Gupta G, Murray BS, Dyson PJ, Therrien B. Synthesis, Molecular Structure and Cytotoxicity of Molecular Materials Based on Water Soluble Half-Sandwich Rh(III) and Ir(III) Tetranuclear Metalla-Cycles. Materials. 2013; 6(11):5352-5366. https://doi.org/10.3390/ma6115352
Chicago/Turabian StyleGupta, Gajendra, Benjamin S. Murray, Paul J. Dyson, and Bruno Therrien. 2013. "Synthesis, Molecular Structure and Cytotoxicity of Molecular Materials Based on Water Soluble Half-Sandwich Rh(III) and Ir(III) Tetranuclear Metalla-Cycles" Materials 6, no. 11: 5352-5366. https://doi.org/10.3390/ma6115352




