Synthesis and Characterization of Encapsulated Nanosilica Particles with an Acrylic Copolymer by in Situ Emulsion Polymerization Using Thermoresponsive Nonionic Surfactant
Abstract
:1. Introduction
2. Results and Discussion
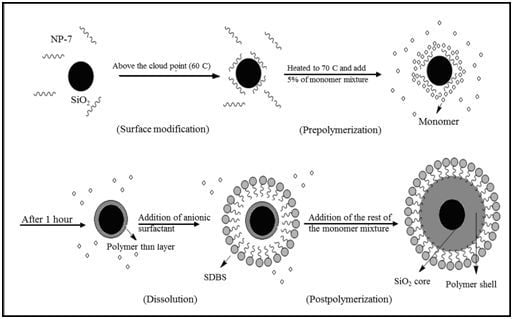
2.1. Encapsulation of Silica Nanoparticles with Acrylic Copolymer
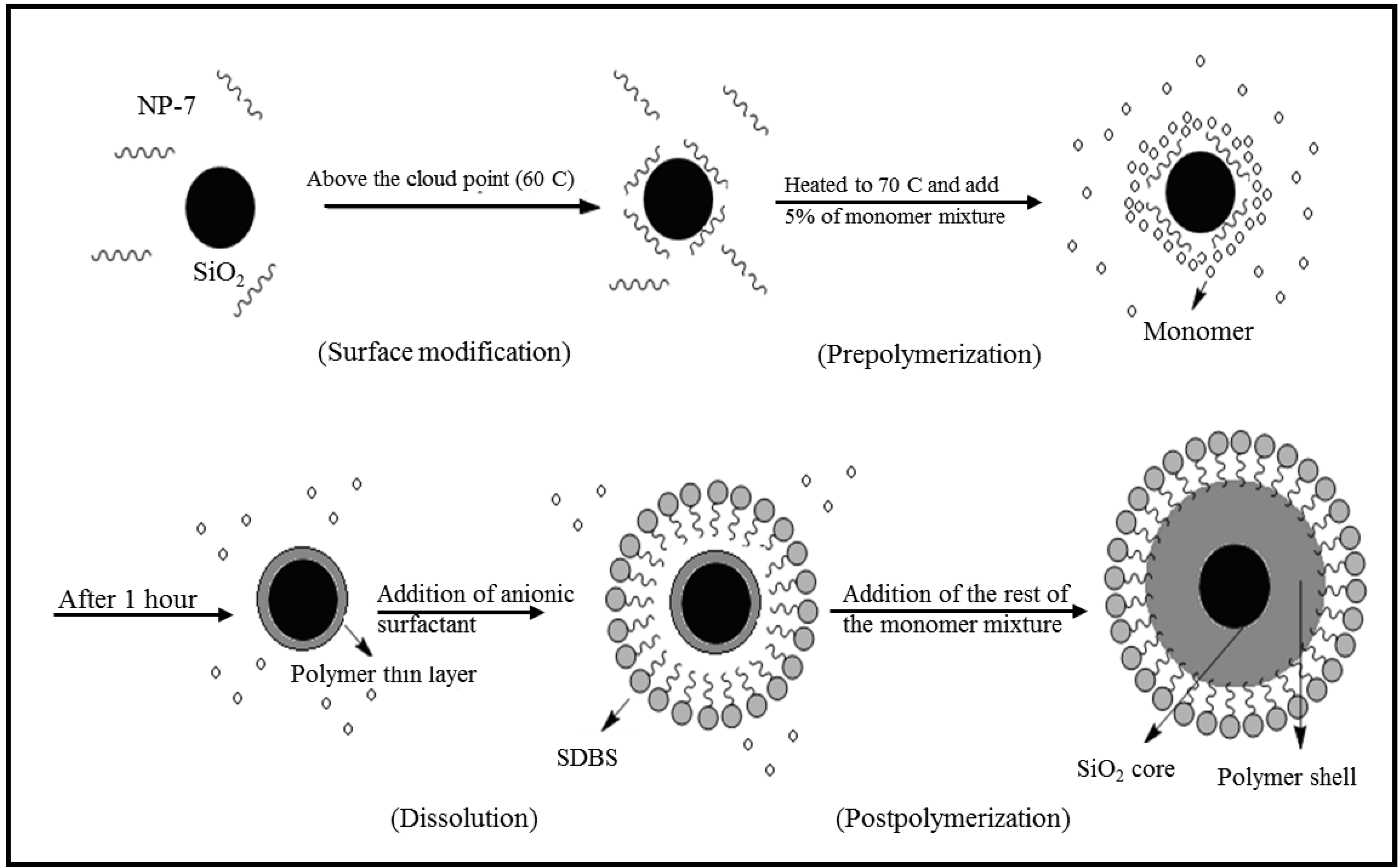
2.2. FTIR Analysis
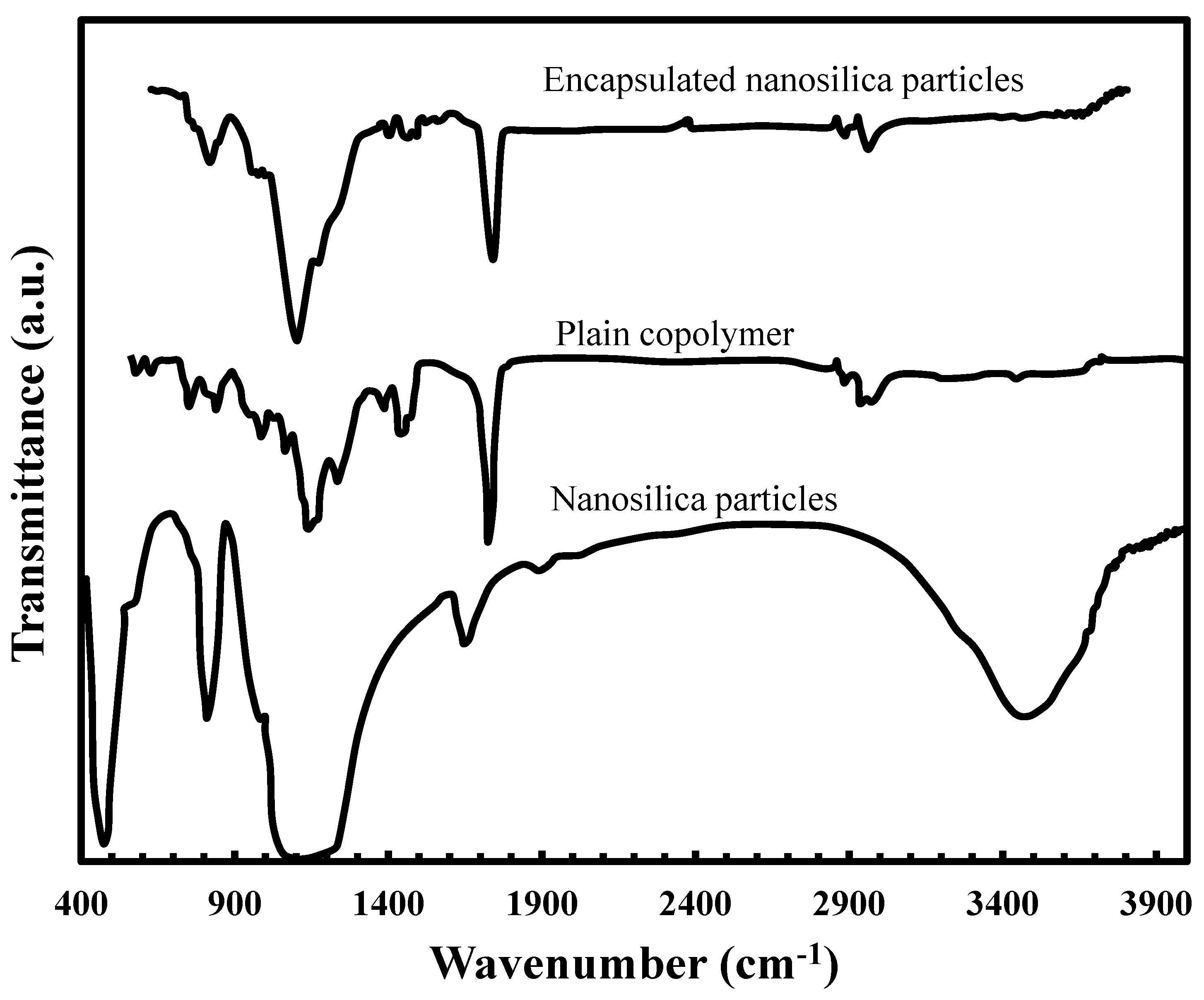
2.3. Particle Size Distribution
2.4. Morphological Studies

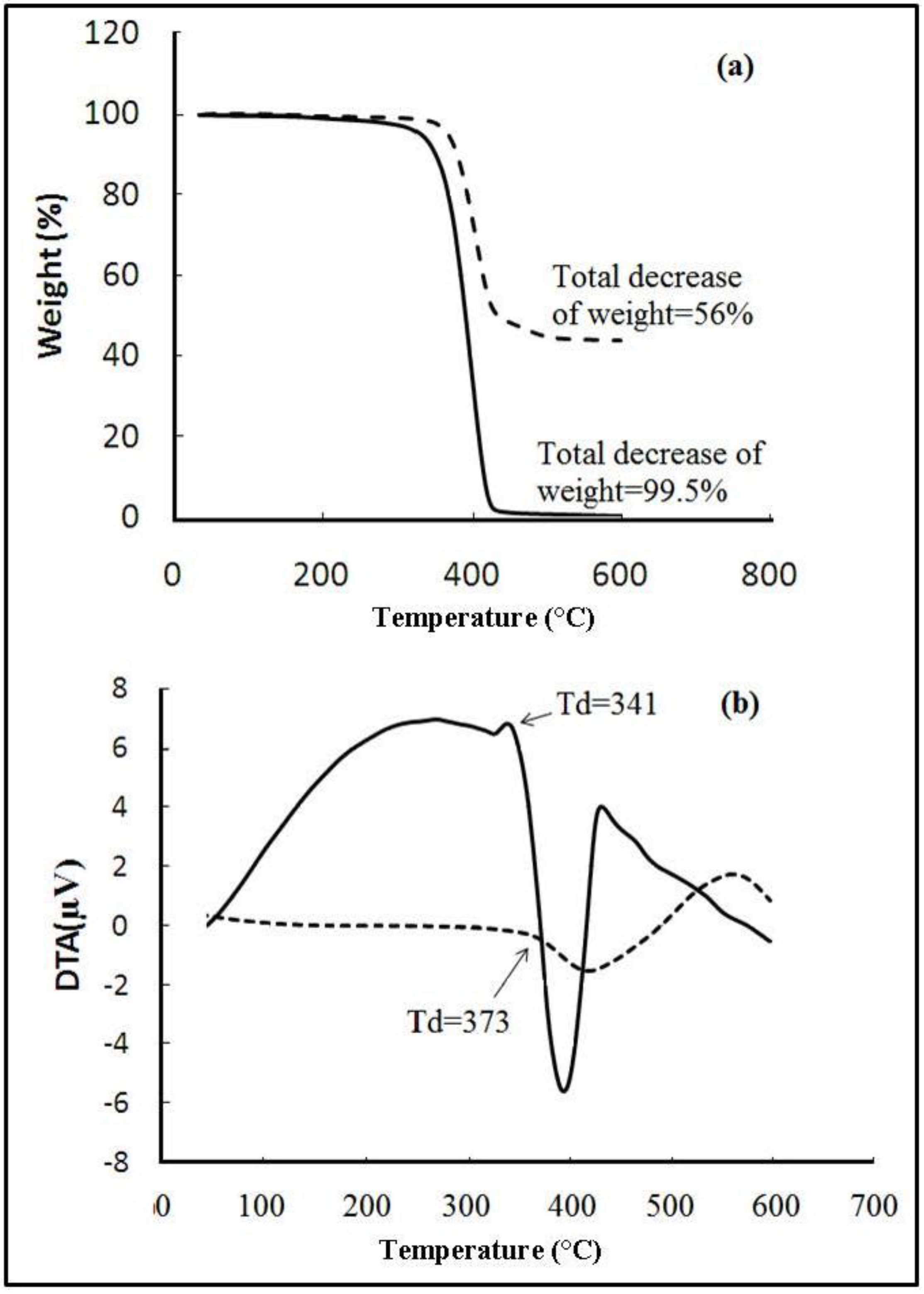
2.5. Thermal Analysis
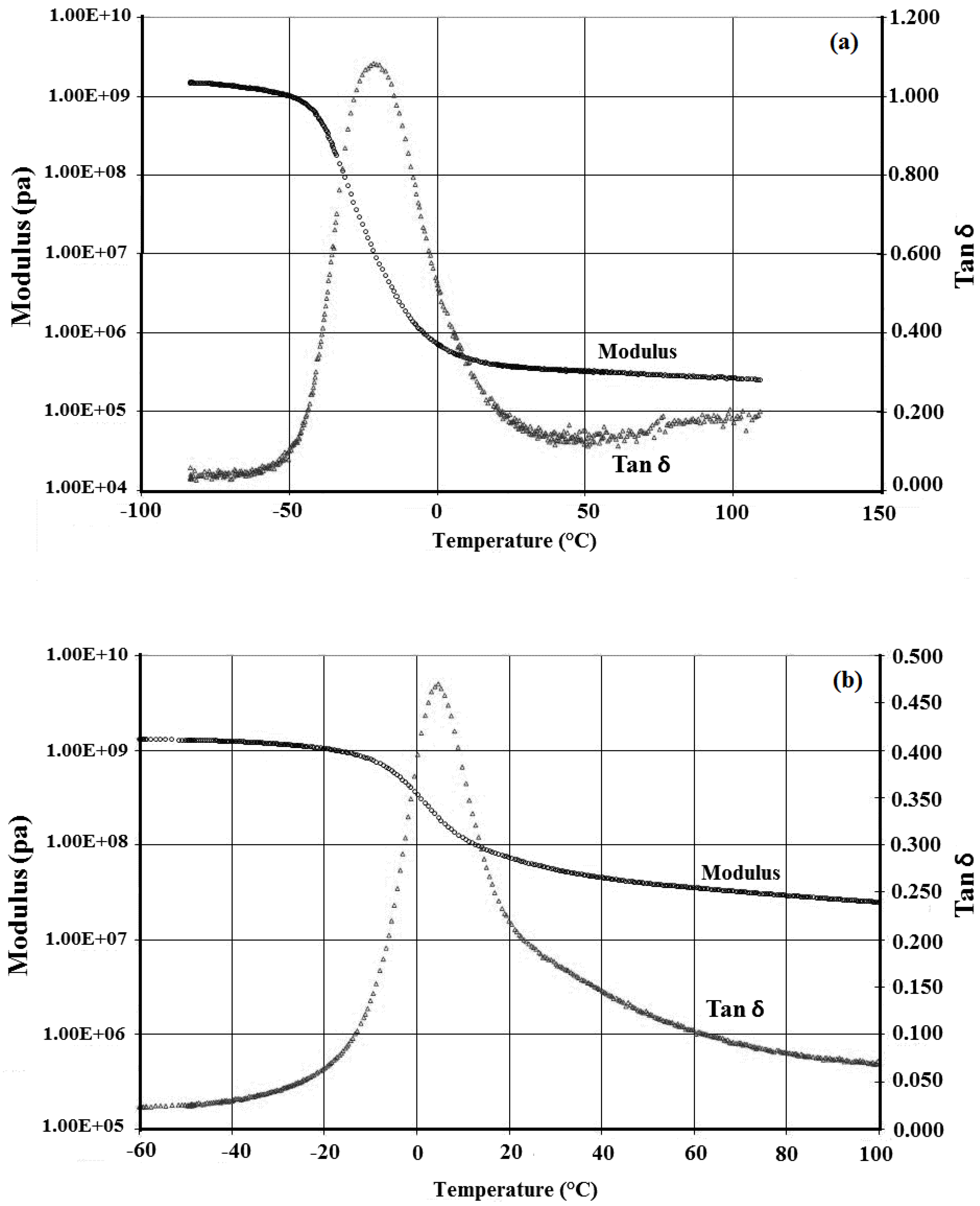
2.6. DMTA Analysis
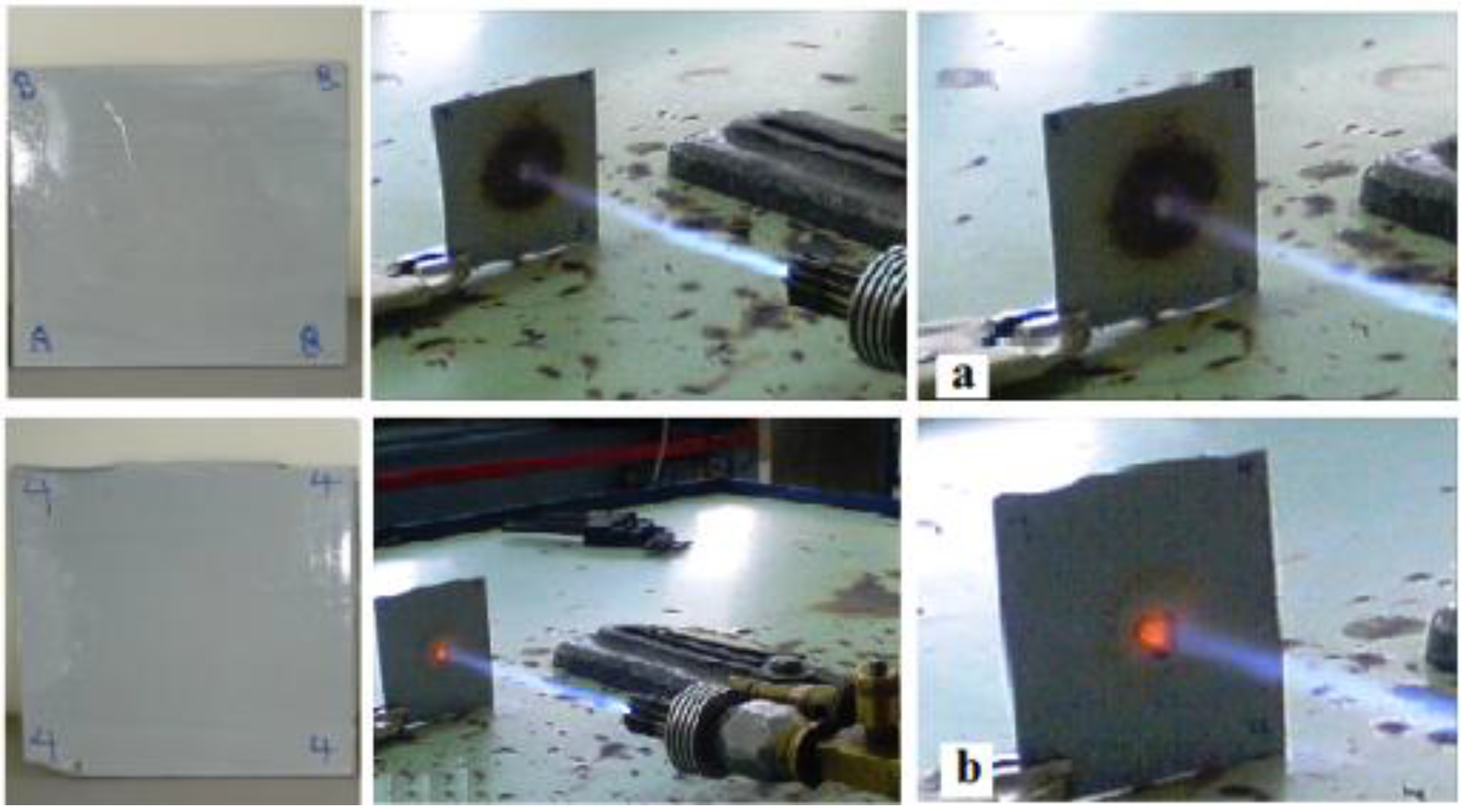
2.7. Flammability Test
3. Experimental Section
3.1. Materials
3.2. Preparation of the Nanocomposite
3.3. Sample Characterization
4. Conclusions
Conflict of Interest
References
- Ghosh, S.K. Functional Coatings; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Caris, C.H.M.; van Elven, L.P.M.; van Herk, A.M.; German, A.L. Polymerization of MMA at the surface of inorganic particles. Br. Polym. J. 1989, 21, 133–140. [Google Scholar] [CrossRef]
- Huang, C.; Partch, R.E.; Matijevic, E. Coating of uniform inorganic particles with polymers: II. Polyaniline on copper oxide. J. Colloid Interface Sci. 1995, 170, 275–283. [Google Scholar] [CrossRef]
- Li, X.; Sun, Z. Synthesis of magnetic polymer microspheres and application for immobilization of proteinase of balillus sublitis. J. Appl. Polym. Sci. 1995, 58, 1991–1997. [Google Scholar] [CrossRef]
- Oyama, H.T.; Sprycha, R.; Xie, Y.; Partch, R.E.; Matijevic, E. Coating of uniform inorganic particles with polymers. J. Colloid Interface Sci. 1993, 160, 298–303. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Pishvaei, M.; Kaffashi, B. Synthesis of latex based antibacterial acrylate polymer/nanosilver via in situ miniemulsion polymerization. Macromol. Res. 2011, 19, 243–249. [Google Scholar] [CrossRef]
- Huang, X.; Brittain, W.J. Synthesis and characterization of PMMA nanocomposites by suspension and emulsion polymerization. Macromolecules 2001, 34, 3255–3260. [Google Scholar] [CrossRef]
- Bakhshaee, M.; Pethrick, R.A.; Rashid, H.; Sherrington, D.C. Encapsulation of carbon black in suspension polymerized copolymers. Polym. Commun. 1985, 26, 185–192. [Google Scholar]
- Lee, J.; Hong, C.K.; Choe, S.; Shim, S.E. Synthesis of polystyrene/silica composite particles by soap-free emulsion polymerization using positively charged colloidal silica. J. Colloid Interface Sci. 2007, 310, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Chu, F.; Guyot, A.; Putaux, J.L.; Bourgeat-Lami, E. Silicone–polyacrylate composite latex particles. Particles formation and film properties. Polymer 2005, 46, 1331–1337. [Google Scholar] [CrossRef]
- Lee, M.S.; Jo, N.J. Coating of methyltriethoxysilane-modified colloidal silica on VTMS- and MTMS-modified particles. J. Sol-Gel Sci. Technol. 2002, 24, 175–180. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chen, C.Y.; Chen, W.C. Synthesis and characterization of organic-inorganic hybrid thin films from poly(acrylic) and monodispersed colloidal silica. Polymer 2003, 44, 593–601. [Google Scholar] [CrossRef]
- Guo, L.; Lee, H.J.; Beaucage, G.J. Structural analysis of poly (dimethylsiloxane) modified silica xerogels. J. Non-Cryst. Solids 1999, 243, 61–67. [Google Scholar] [CrossRef]
- Yabuta, T.; Bescher, E.P.; Mackenzie, J.D.; Tsuru, K.; Hayakawa, S.; Osaka, A. Synthesis of PDMS-based porous materials for bio- medical applications. J. Sol-Gel Sci. Technol. 2003, 26, 1219–1222. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.X.; Li, S.D.; Chen, Y.; Huang, M.F. Self-assembled natural rubber/silica nanocomposites: Its preparation and characterization. Compos. Sci. Technol. 2007, 67, 3130–3139. [Google Scholar] [CrossRef]
- Mammeri, F.; Rozes, L.; Bourhis, E.L.; Sanchez, C. Elaboration and mechanical characterization of nanocomposites thin films Part II. Correlation between structure and mechanical properties of SiO2-PMMA hybrid materials. J. Eur. Ceram. Soc. 2006, 26, 267–270. [Google Scholar] [CrossRef]
- Thomas, M.J.K.; Slipper, I.; Walunj, A.; Jain, A.; Favretto, M.E.; Kallinteri, P.; Douroumis, D. Inclusion of poorly soluble drugs in highly ordered mesoporous silica Nanoparticles. Int. J. Pharm. 2010, 387, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Douroumis, D.; Onyesom, I.; Maniruzzaman, M.; Mitchell, J. Mesoporous silica nanoparticles in nanotechnology. J. Crit. Rev. Biotechnol. 2012, 33, 229–245. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, H.T.; Chen, X.; Chen, Z.M.; Cui, Z.C.; Yang, B. Monodisperse silica-polymer core-shell microspheres via surface grafting and emulsion polymerization. Macromol. Mater. Eng. 2003, 288, 380–385. [Google Scholar] [CrossRef]
- Tissot, I.; Novat, C.; Lefebvre, F.; Bourgeat-Lami, E. Hybrid latex particles coated with silica. Macromolecules 2001, 34, 5737–5739. [Google Scholar] [CrossRef]
- Reculusa, S.; Poncet-Legrand, C.; Ravaine, S.; Minogotaud, C.; Duguet, E.; Bourgeat-Lami, E. Syntheses of raspberrylike silica/polystyrene materials. Chem. Mater. 2002, 14, 2354–2359. [Google Scholar] [CrossRef]
- Derro, A.; Reculusa, S.; Bourgeat-Lami, E.; Duguet, E.; Ravaine, S. Synthesis of hybrid colloidal particles: From snowman-like to raspberry-like morphologies. Colloids Surf. A 2006, 284, 78–84. [Google Scholar]
- Barthlet, S.; Mingotaud, C.; Bourgeat-Lami, E.; Duguet, E.; Ravaine, R. Synthesis of daisy-shaped and multipod-like silica/polystyrene nanocomposites. Nano Lett. 2004, 4, 1677–1682. [Google Scholar] [CrossRef]
- Barthlet, C.; Hickey, A.J.; Carins, D.B.; Armes, S.P. Synthesis of novel polymer–silica colloidal nanocomposites via free-radical polymerization of vinyl monomers. Adv. Mater. 1999, 11, 408–410. [Google Scholar] [CrossRef]
- Schmid, A.; Fujii, S.; Armes, S.P. Polystyrene-silica nanocomposite particles via alcoholic dispersion polymerization using a cationic azo initiator. Langmuir 2006, 22, 4923–4927. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Fedynshyn, T.H.; Sinta, R.; Matijevic, E. Encapsulation of nanosized silica by in situ polymerization of tert-butyl acrylate monomer. Langmuir 2000, 16, 9031–9034. [Google Scholar] [CrossRef]
- Percy, M.J.; Amalvy, J.I.; Randall, D.P.; Armes, S.P. Synthesis of vinyl polymer-silica colloidal nanocomposites prepared using commercial alcoholic silica sols. Langmuir 2004, 20, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Arai, K.; Miyamoto, M.; Kimura, Y. Preparation of spherical nanocomposites consisting of silica core and polyacrylate shell by emulsion polymerization. J. Appl. Polym. Sci. 2005, 99, 659–669. [Google Scholar] [CrossRef]
- Mizutani, T.; Arai, K.; Miyamoto, M.; Kimura, Y. Application of silica-containing nano-composite emulsion to wall paint: A new environmentally safe paint of high performance. Prog. Org. Coat. 2006, 55, 276–283. [Google Scholar] [CrossRef]
- Ahn, S.H.; Kim, S.H.; Lee, S.G. Surface-modified silica nanoparticle—Reinforced poly(ethylene 2,6-naphthalate). J. Appl. Polym. Sci. 2004, 94, 812–818. [Google Scholar] [CrossRef]
- Kim, H.C.; Dubois, G. Dekker Encyclopedia of Nanoscience and Nanotechnology; Taylor & Francis: New York, NY, USA, 2005. [Google Scholar]
- Zhu, A.; Cai, A.; Yu, Z.; Zhou, W. Film characterization of poly(styrene-butylacrylate-acrylic acid)–silica nanocomposite. J. Colloid Interface Sci. 2008, 322, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.Z.; Zhang, M.Q.; Ruan, W.H. Surface modification of nanoscale fillers for improving properties of polymer. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; Lang, J. Encapsulation of inorganic particles by dispersion polymerization in polar media: Effect of silica size and concentration on the morphology of silica—Polystyrene composite particles. J. Colloid Interface Sci. 1999, 210, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Corcos, F.; Bourgeat-Lami, E.; Novat, C.; Lang, J. Poly(styrene-b-ethylene oxide) copolymers as stabilizer for the synthesis of silica-polystyrene core-shell particles. Colloid Polym. Sci. 1999, 277, 1142–1151. [Google Scholar] [CrossRef]
- Edmondson, S.; Osborne, V.L.; Huck, W.T.S. Polymer brushes via surface-initiated polymerizations. Chem. Soc. Rev. 2004, 33, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, B.; Ranjan, R.; Brittain, W.J. Surface initiated polymerizations from silica nanoparticles. Soft Matter 2006, 2, 386–396. [Google Scholar] [CrossRef]
- Ghannam, L.; Parvole, J.; Laruelle, G.; Francois, J.; Billon, L. Surface-initiated nitroxide-mediated polymerization: A tool for hybrid inorganic/organic nanocomposites “in situ” synthesis. Polym. Int. 2006, 55, 1199–1207. [Google Scholar] [CrossRef]
- Wu, T.M.; Chu, M.S. Preparation and characterization of thermoplastic vulcanizate/silica nanocomposites. J. Appl. Polym. Sci. 2005, 98, 2058–2063. [Google Scholar] [CrossRef]
- Lai, Y.H.; Kuo, M.C.; Huang, J.C.; Chen, M. On the PEEK composites reinforced by surface-modified nano-silica. Mater. Sci. Eng. A 2007, 458, 158–169. [Google Scholar] [CrossRef]
- Ding, X.F.; Zhao, J.Z.; Liu, Y.H.; Zhang, H.B.; Wang, Z.C. Silica nanoparticles encapsulated by polystyrene via surface grafting and in situ emulsion polymerization. Mater. Lett. 2004, 58, 3126–3130. [Google Scholar] [CrossRef]
- Mahdavian, A.R.; Ashjari, M.; Makoo, A.B. Preparation of poly (styrene–methyl methacrylate)/SiO2 composite nanoparticles via emulsion polymerization. An investigation into the compatiblization. Eur. Polym. J. 2007, 43, 336–344. [Google Scholar] [CrossRef]
- Tang, J.C.; Lin, G.L.; Yang, H.C.; Jiang, G.J.; Chen-Yang, Y.W. Polyimide-silica nanocomposites exhibiting low thermal expansion coefficient and water absorption from surface-modified silica. J. Appl. Polym. Sci. 2007, 104, 4096–4105. [Google Scholar] [CrossRef]
- Dashtizadeh, A.; Abdouss, M.; Mahdavi, H.; Khorassani, M. Acrylic coatings exhibiting improved hardness, solvent resistance and glossiness by using silica nano-composites. Appl. Surf. Sci. 2011, 257, 2118–2125. [Google Scholar] [CrossRef]
- Liu, M.; Gan, L.; Chen, L.; Zhu, D.; Xu, Z.; Hao, Z.; Chen, L. A novel liposome-encapsulated hemoglobin/silica nanoparticle as an oxygen carrier. Int. J. Pharm. 2012, 427, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.; Ma, J.H.; Du, Z.; Wang, W.; Wu, J. A simple method for synthesis of thermal responsive silica nanoparticle/PNIPAAm hybrids. Powder Technol. 2013, 233, 47–51. [Google Scholar] [CrossRef]
- Salami-Kalajahi, M.; Haddadi-Asl, V.; Roghani-Mamaqani, H. Study of kinetics and properties of polystyrene/silica nanocomposites prepared via in situ free radical and reversible addition-fragmentation chain transfer polymerizations. Sci. Iran. 2012, 19, 2004–2011. [Google Scholar] [CrossRef]
- Feng, L.; He, L.; Ma, Y.; Wang, Y. Grafting poly(methyl methacrylate) onto silica nanoparticle surfaces via a facile esterification reaction. Mater. Chem. Phys. 2009, 116, 158–163. [Google Scholar] [CrossRef]
- Qiu, S.; Yang, L.; Zhang, Y.; Xu, Y. Modification of silica with PMMA via ultrasonic irradiation and its application for reinforcement of polyacrylates. Particuology 2013, in press. [Google Scholar]
- Chang, G.; He, L.; Zheng, W.; Pan, A.; Liu, J.; Li, Y.; Cao, R. Well-defined inorganic/organic nanocomposite by nano silica core-poly(methyl methacrylate/butylacrylate/trifluoroethyl methacrylate) shell. J. Colloid Interface Sci. 2013, 396, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hashemi-Nasab, R.; Mirabedini, S.M. Effect of silica nanoparticles surface treatment on in situ polymerization of styrene—Butyl acrylate latex. Prog. Org. Coat. 2013, 76, 1016–1023. [Google Scholar]
- Abd El-Wahab, H.; Abd El-Fattah, M.; Gabr, M.Y. Preparation and characterization of flame retardant solvent base and emulsion paints. Prog. Org. Coat. 2010, 69, 272–277. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yazdimamaghani, M.; Pourvala, T.; Motamedi, E.; Fathi, B.; Vashaee, D.; Tayebi, L. Synthesis and Characterization of Encapsulated Nanosilica Particles with an Acrylic Copolymer by in Situ Emulsion Polymerization Using Thermoresponsive Nonionic Surfactant. Materials 2013, 6, 3727-3741. https://doi.org/10.3390/ma6093727
Yazdimamaghani M, Pourvala T, Motamedi E, Fathi B, Vashaee D, Tayebi L. Synthesis and Characterization of Encapsulated Nanosilica Particles with an Acrylic Copolymer by in Situ Emulsion Polymerization Using Thermoresponsive Nonionic Surfactant. Materials. 2013; 6(9):3727-3741. https://doi.org/10.3390/ma6093727
Chicago/Turabian StyleYazdimamaghani, Mostafa, Tannaz Pourvala, Elaheh Motamedi, Babak Fathi, Daryoosh Vashaee, and Lobat Tayebi. 2013. "Synthesis and Characterization of Encapsulated Nanosilica Particles with an Acrylic Copolymer by in Situ Emulsion Polymerization Using Thermoresponsive Nonionic Surfactant" Materials 6, no. 9: 3727-3741. https://doi.org/10.3390/ma6093727