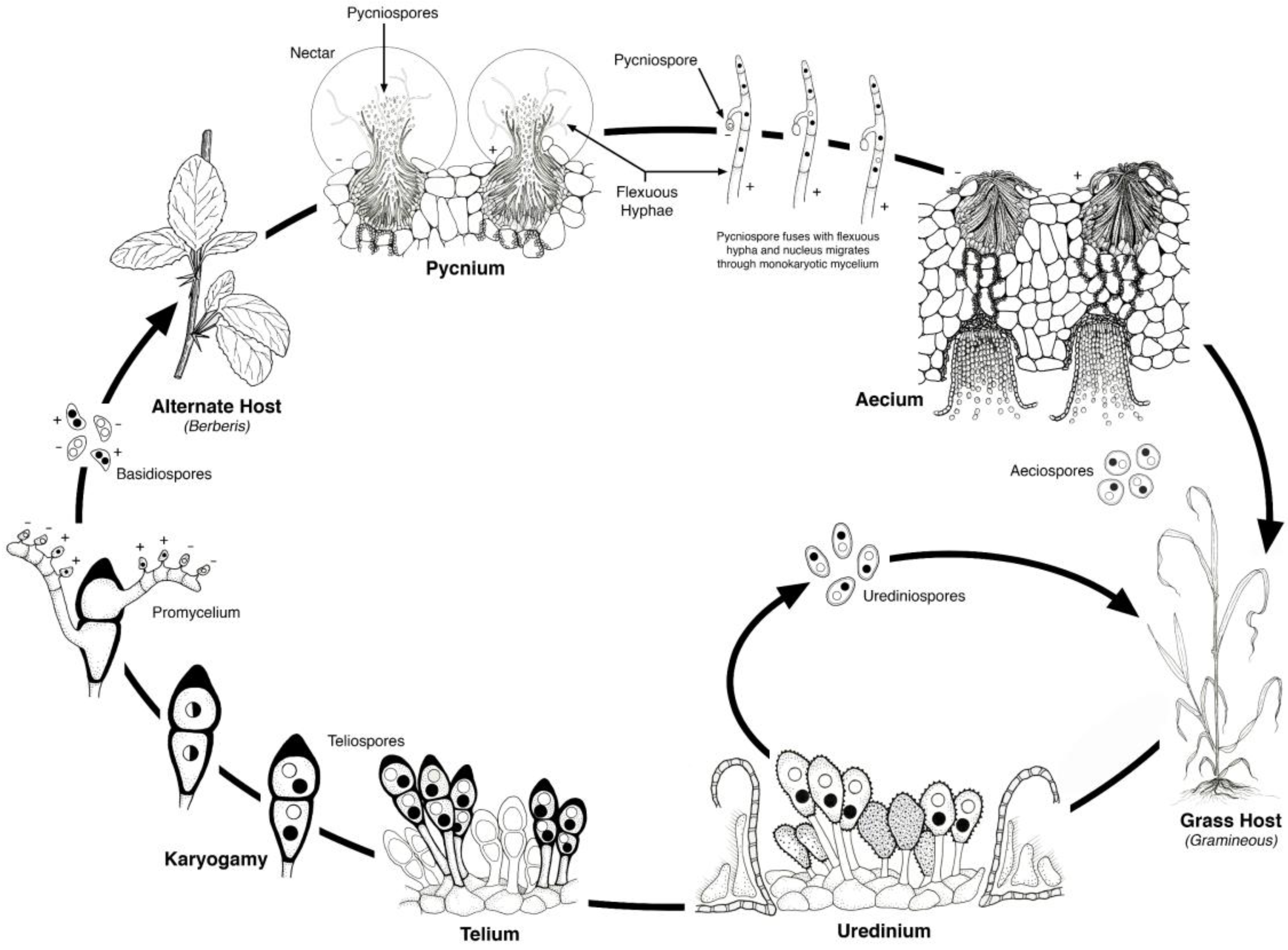
2. Life Cycle
The cereal rust fungi,
P. graminis—causal pathogen of stem rust on wheat, oat, barley, and rye;
P. striiformis—causal pathogen of wheat stripe rust;
P. triticina—causal pathogen of wheat leaf rust, and
P. coronata—casual pathogen of oat crown rust, complete their life cycles on two taxonomically unrelated hosts, and are thus heteroecious rusts. The cereal rusts are macrocyclic and have five distinct stages of teliospores, basidiospores, and urediniospores on cereal hosts, and pycniospores and aeciospores on the alternate hosts [
6] (
Figure 2). The urediniospores have a dikaryotic nuclear condition and are capable of cycling continuously on the cereal hosts. Teliospores develop in the uredinial infections and the dikaryotic nuclei merge to form a diploid nucleus. The teliospores germinate and undergo meiosis producing four haploid basidiospores that are forcibly ejected into the air. The basidiospores then must land on and infect the alternate host. The haploid infections on the alternate host occur on the upper leaf surface and are of two mating types that give rise to the pycnial structures within which flexous hyphae produce haploid pycniospores. The pycniospores are often carried by insects to other pycnial infections where combinations of pycniospores and flexuous hyphae of opposite mating type result in plasmogamy, the fusion of two genetically distinct cells that restores the dikaryotic nuclear condition. The dikaryotic aecium develops on the underside of the leaf surface and within these are chains of aeciospores. When the aecium has matured, the aeciospores are released and wind dispersed to infect their cereal host. Uredinial infections then follow, which can then cycle continuously or develop into teliospores, thus completing the life cycle.
The sexual cycle of the cereal rusts is dependent on the presence of a suitable alternate host. Common barberry,
Berberis vulgaris, the alternate host of
P. graminis and
P. striiformis was eradicated from large regions of North America from the 1920s to 1970 [
7]. As a result stem rust and stripe rust diseases in North America are found usually as uredinial infections on cereal crops or grasses. Some pycnia and aeciospores of wheat stem rust are found on barberry in the Pacific Northwest region of Washington and Idaho [
8]. In the Midwest aeciospores from barberry plants usually infect rye and are not pathogenic to wheat. The alternate host for
P. triticina,
Thalictrum speciosissimum L., is native to southern Europe and southwest Asia and does not occur naturally in North America [
9], although the synonym
T. glaucum is sold in garden nursery stores. There are no reports of pycnia or aeciospores produced on either
T. speciosissimum or
T. glaucum in North America. Native North American species of
Thalictrum are resistant to basidiospore infection by
P. triticina [
10]. As a result,
P. triticina is found exclusively as uredinial infections on wheat in North America and in most places worldwide. Aeciospores produced on
Thalictrum spp. in North Americaresult from basidiospore infection from other
Puccinia spp., usually do not infect wheat and have ITS DNA sequences that are highly related to uredinial collections from
Elymus glaucus. The only cereal rust in North America with a widespread compatible alternate host is
P. coronata. [
11]. Common buckthorn
Rhamnus cathartica is an invasive woody shrub commonly found in the eastern and Midwestern states and adjacent provinces in Canada. Pycnia and aeciospores of
P. coronata are commonly seen on buckthorn in the spring and early summer in these regions.
Figure 2.
Life cycle of a heteroecious, macrocyclic cereal rust. The alternate host for stem rust—Puccinia graminis is Berberis vulgaris (barberry); for leaf rust—Puccinia triticina, Thalictrum speciosissimum (meadow rue); for crown rust—Puccinia coronata, Rhamnus cathartica (buckthorn). Illustration by Jackie Morrison, USDA-ARS.
Figure 2.
Life cycle of a heteroecious, macrocyclic cereal rust. The alternate host for stem rust—Puccinia graminis is Berberis vulgaris (barberry); for leaf rust—Puccinia triticina, Thalictrum speciosissimum (meadow rue); for crown rust—Puccinia coronata, Rhamnus cathartica (buckthorn). Illustration by Jackie Morrison, USDA-ARS.
Uredinial infections of cereal rusts are widespread in North America.
P. triticina is found on wheat throughout the eastern and southern states, and the southern and northern Great Plains region of the US and into Canada. Of the three rusts on wheat,
P. triticina (leaf rust) is the most commonly found in North America [
12]. Winter wheat in the southern states and southern Great Plains region is infected by urediniospores in the fall months. The infections subsequently develop allowing the rust to survive the winter dormancy period of the wheat. Uredinial infections can often be seen in winter wheat in February and March in Texas and the Gulf Coast states. As the winter wheat breaks dormancy and develops through growth stages to eventual maturity, urediniospore production increases with the warmer temperatures [
3]. The urediniospores are wind dispersed in the southerly winds to infect wheat crops developing further north [
13]. Eventually leaf rust uredinial infections can be found on spring wheat in Minnesota by mid June. Many winter wheat cultivars are susceptible to leaf rust, this allows a large population of
P. triticina to overwinter over a large geographic region on an annual basis. In contrast
P. graminis (stem rust) on wheat is found more rarely, as many winter and spring wheat cultivars are highly resistant to the current races of stem rust in North America [
14]. Cultivar resistance combined with the effects of barberry eradication has reduced the uredinial population size of
P. graminis infections on wheat [
15] and also reduced the number of races that are detected annually.
Puccinia coronata (crown rust) can be found almost wherever oats are grown in the eastern and Midwestern states due to the presence of buckthorn and the overwintering of
P. coronata on oat in southern regions where the alternate host is not present.
3. Pathogen Variation
Variation in cereal rusts has traditionally been assessed by testing isolates on host genotypes that differ for resistance. The early differentials for wheat stem rust [
16] and wheat leaf rust [
17] were collections of wheat cultivars and varieties that had different infection types on seedling plants to the most commonly found isolates of both rust pathogens. These early differential sets were genetically undefined as there was no information on the resistance genes present in the cultivars or varieties. Many of the early differentials had more than one resistance gene and also had genes in common which reduced their effectiveness in distinguishing rust races. Later as knowledge was gained about the genetics of rust resistance in wheat [
18], cultivars with identified single genes for rust resistance were used as differentials. As an increased number of rust resistance genes were characterized in wheat germplasm, single resistance genes were transferred into single wheat genotype backgrounds by backcrossing (
Figure 3) [
19]. Isogenic differential lines have been developed for all three rusts of wheat and also for oat crown rust. Single gene differentials that are not isogenic lines are still used in Australia for wheat stem rust and wheat leaf rust virulence surveys [
20].
Virulence surveys of the three wheat rusts and oat crown rust are conducted annually in the US, Canada, Australia and other countries as well. Races of rust are determined based on qualitative infection types of the isolates on each differential line that are classified as avirulent or virulent. The goals of the surveys are to determine pathogenic variation present in the rust populations and to detect any new races of rust that may pose a threat to the commonly grown cultivars. Furthermore, rust isolates obtained from the surveys can be used in testing cereal germplasm for rust resistance. The most common stem rust races are avirulent to the most wheat cultivars and do not represent any threat to wheat production, yet some winter wheat cultivars are susceptible to these avirulent races. Occasionally stem rust races with increased virulence are detected; however these races have not increased in frequency. In contrast many different races of
P. triticina are found in North America; in the US between 50 and 60 races are identified annually [
21].
P. coronata is likely the most variable of the cereal rusts in North America due to the prevalence of buckthorn and the production of sexually derived aeciospores with new combinations of virulence on an annual basis [
22].
Figure 3.
Infection types of a single Puccinia triticina race on Thatcher backcross lines of wheat with single leaf rust resistance genes. Infection type 0; to 2+ are considered low-resistant; infection type 3 to 4 are considered high-susceptible. Photo by James A. Kolmer, USDA-ARS.
Figure 3.
Infection types of a single Puccinia triticina race on Thatcher backcross lines of wheat with single leaf rust resistance genes. Infection type 0; to 2+ are considered low-resistant; infection type 3 to 4 are considered high-susceptible. Photo by James A. Kolmer, USDA-ARS.
Since rusts are obligate parasites, any resistance genes in host cultivars that curtail or eliminate rust reproduction will place tremendous selection pressure on variants that are virulent to the resistance genes. The widespread use of wheat cultivars with different leaf rust resistance genes has resulted in different leaf rust races being found in the major wheat production regions of the US [
12]. Soft red winter wheat cultivars are grown in the southern states, eastern states, and in the Ohio Valley region. The soft red winter wheat cultivars in these states commonly have leaf rust resistance genes
Lr9,
Lr11,
Lr18 or
Lr26, which have selected for races with virulence to these genes. Similarly the hard red winter wheat cultivars grown in the southern-mid Great Plains region from Texas to Nebraska have selected for races with virulence to
Lr17,
Lr24,
Lr26, and
Lr39/41 due to the presence of these genes in the hard red winter wheat cultivars. The hard red spring wheat cultivars grown in Minnesota, North Dakota and South Dakota have resistance genes
Lr2a,
Lr16,
Lr23, and
Lr21. Leaf rust races with virulence to these genes are commonly found in the Northern Great Plains. Rust races selected by host resistance in one region can also be wind blown into adjacent regions. Leaf rust races with virulence to resistance genes in the hard red winter wheat cultivars are also found in the southeastern states and in the spring wheat region of the northern plains, even though cultivars in these regions did not directly select for these races.
In the last 20 years DNA based markers have been used to characterize cereal rust populations, and thus have given new insights into the origin and spread of rust genotypes. DNA markers are not directly selected by host genotypes, and are thus unbiased markers that can be used for population genetic analyses. Markers based on random amplified DNA polymorphism (RAPD) [
23], amplified fragment length polymorphism (AFLP) [
24] and simple sequence repeat (SSR) polymorphism [
25] have been used and developed for studies of
P. triticina populations in North America. Although the markers differ in ability to detect polymorphism among different groups of
P. triticina, the results between the different studies were very consistent. Of the three markers, SSRs likely offer the optimal combination of sufficient levels of polymorphism, ability to distinguish multiple alleles at single loci, ease of scoring allele molecular weights, and repeatability across experiments (
Figure 4). Currently in North America, there are six groups of
P. triticina races based on SSR genotype groupings (
Figure 5) [
26]. Isolates within the SSR groups are identical or highly related for SSR genotype, and are also related for virulence to key leaf rust resistance genes. Isolates in different SSR groups are distinct for SSR genotype and are usually highly different for virulence as well. Two SSR groups that are found throughout the Great Plains region and the southern and eastern states, NA-3 and NA-5, account for 95% of the isolates in the current
P. triticina population. One small group of isolates, NA-2 is found mainly in the soft white wheat regions of upstate New York and Washington State. Isolates from California, Arizona, and Mexico that are virulent to durum wheat are also distinct for SSR group and are in NA-6. The
P. triticina population in the US and worldwide has characteristics of highly clonal populations as evidenced by high levels of observed heterozygosity relative to expected levels under random mating, high levels of linkage disequilibrium between SSR markers, and a general correlation between virulence and SSR genotypes. If aeciospores derived from sexual recombination contributed regularly to epidemic development it would be expected that observed heterozygosity levels would be close to expected levels, most SSR markers would be in equilibrium, and that there would be no relationship between virulence and SSR genotype.
Molecular markers and virulence have also been used to characterize worldwide
P. triticina populations in order to determine their genetic relationships and possible migration between continents. Collections of
P. triticina from Uruguay, Argentina, Chile, and Brazil were highly similar or in a few cases identical for SSR genotype to the NA3 and NA5 SSR groups in North America [
27]. Isolates in the NA3 SSR group and the related SA3 group in South America were likely introduced from the same origin, as both were detected and become common in the mid and late 1990s in North America and South America, respectively. Similarly collections of
P. triticina from durum wheat in Europe, South America, Mexico and the Middle East were nearly identical for SSR genotype and for virulence, which implied a common origin of these isolates [
28].
Figure 4.
Amplification products of a pair of simple sequence repeat primers in Puccinia triticina. Bands with dots are alleles. B = isolate from bread wheat; D = isolate from durum wheat.
Figure 4.
Amplification products of a pair of simple sequence repeat primers in Puccinia triticina. Bands with dots are alleles. B = isolate from bread wheat; D = isolate from durum wheat.
Figure 5.
Neighbor joining plot of groups of Puccinia triticina isolates based on simple sequence repeat (SSR) genotypes in North America. Size of circles are approximately proportional to the number of isolates in each SSR group in the annual survey data from the US. Values between groups represent RST distance.
Figure 5.
Neighbor joining plot of groups of Puccinia triticina isolates based on simple sequence repeat (SSR) genotypes in North America. Size of circles are approximately proportional to the number of isolates in each SSR group in the annual survey data from the US. Values between groups represent RST distance.
4. Leaf Rust Resistance in Wheat
To date, 71 leaf rust resistance genes in wheat have been mapped to chromosome location and given gene designations [
29]. Most resistance genes are effective in seedling plants and remain effective through the adult plant stage. Genes such as
Lr1,
Lr10, and
Lr21 are good examples of race specific resistance genes that are effective in seedling and adult plants [
18]. These genes condition very low infection types of hypersensitive flecks or minute uredinia surrounded by necrosis and chlorosis to avirulent rust isolates (
Figure 3). These genes have conserved motifs that code for nucleotide binding site (NBS) and leucine repeat rich (LRR) proteins typical of other plant disease resistance genes [
30,
31]. Leaf rust resistance genes were initially characterized in wheat
T. aestivum (Lr1,
Lr2a,
Lr3,
Lr10,
Lr11), and later in wheat related species such as
T. tauschii (Lr21),
Aegilops elongatum (Lr24),
A. umbellulata (Lr9), and common rye,
Secale cereale (Lr26) [
32]. Race specific seedling resistance genes have been proven to be very vulnerable to selection and increase of virulent races in rust populations. Many wheat cultivars were resistant when first released, but as leaf rust races with virulence were selected and increased in frequency, the resistance in the cultivars was eroded. Selection of virulent races can occur relatively quickly in the winter wheat region where leaf rust overwinters on a regular basis. Race specific resistance genes that are optimally expressed in the adult plant stage, but poorly expressed in seedlings have also been characterized. Some like
Lr12 and
Lr13 were derived from wheat (
Figure 6), while others such as
Lr22a and
Lr37 were derived from
T. tauschii and
A. ventricosa, respectively. As in the case of the seedling resistance genes, races with virulence to these adult plant resistance genes have eroded their effectiveness.
Some other genes express a partial type of resistance that is manifested by fewer uredinia of variable size that are surrounded by varying amounts of chlorosis [
33]. This type resistance is optimally expressed in adult plants, as seedlings can be susceptible. A key characteristic of these genes is that they confer resistance to all known races of
P. triticina, with no demonstrated race specificity, although these genes singly do not provide complete resistance that is manifested by hypersensitive infection types with no uredinia produced. These genes have provided long-term durable resistance since virulent forms of
P. triticina have not yet been detected. The best known and characterized of these genes is
Lr34 [
34] that is found in wheat germplasm around the world [
35] (
Figure 6). Wheat cultivars with
Lr34 also have a distinct phenotype of leaf tip necrosis that is expressed independently of rust infection.
Lr34 has a resistance phenotype of smaller and fewer uredinia compared to a susceptible genotype and codes for an ABC drug resistant transporter protein [
36]. Other leaf rust resistance genes
Lr46,
Lr67, and
Lr68 also confer adult plant-partial resistance [
37,
38,
39], however these genes have not yet been cloned and sequenced.
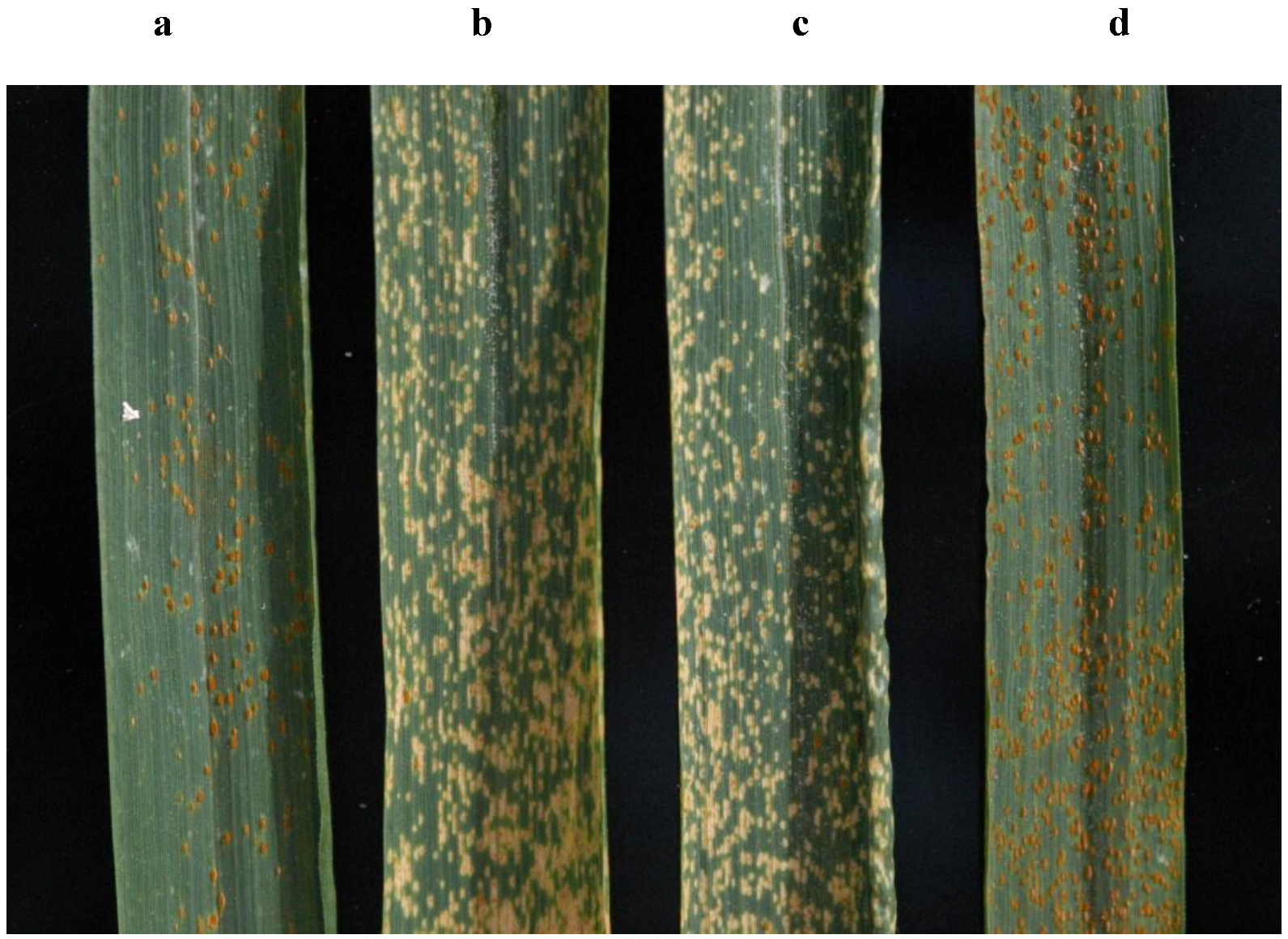
Figure 6.
Flag leaves of Thatcher wheat lines expressing resistance to Puccinia triticina (a) Thatcher+Lr34 (b) Thatcher+Lr12 (c) Thatcher+Lr13 (d) Thatcher. Photo by James A. Kolmer, USDA-ARS.
Figure 6.
Flag leaves of Thatcher wheat lines expressing resistance to Puccinia triticina (a) Thatcher+Lr34 (b) Thatcher+Lr12 (c) Thatcher+Lr13 (d) Thatcher. Photo by James A. Kolmer, USDA-ARS.
Due to the highly variable nature of
P. triticina, durable leaf rust resistance in wheat cultivars has been difficult to achieve. In the US, many of the common winter and spring wheat cultivars are leaf rust susceptible due to selection of virulent races. Although isolates with virulence to
Lr34 have not been detected, cultivars that have only
Lr34 do not have high levels of resistance. Additional effective resistance genes are required for wheat genotypes to be highly resistant. Certain combinations of genes have provided long lasting resistance. Hard red spring wheat cultivars with combinations of
Lr13,
Lr16,
Lr23 and
Lr34 have had moderate to high levels of resistance for over 30 years [
21]. Wheat genotypes developed at CIMMYT with combinations of adult plant genes
Lr34,
Lr46, and
Lr68 have shown long lasting resistance. Only by development and selection of wheat genotypes with combinations of effective genes can cultivars with durable leaf rust resistance be released.
5. Testing Wheat Germplasm for Leaf Rust Resistance
At the USDA-ARS Cereal Disease Laboratory wheat germplasm from across the US is tested for leaf rust resistance in seedling plants in greenhouse tests and as adult plants in field plots. The goal of the germplasm testing program is to postulate which seedling leaf rust resistance genes may be present in the breeding lines and to determine if the breeding lines have resistance to the most common races of leaf rust in the US.
Entries from the advanced hard red winter wheat nurseries from the Great Plains region, the advanced soft red winter wheat nurseries from the eastern and southern US, and the hard red spring wheat nurseries from the northern Great Plains are tested annually as seedlings with different leaf rust races. The entries are inoculated with rust when the primary leaves are fully emerged, usually at 8–9 days after planting. An oil/urediniospore mixture is atomized onto the seedlings using specially designed inoculators with positive air pressure [
40]. The plants are dried for an hour to allow the oil to evaporate and then are placed in dew chambers for an overnight incubation period. The plants are then treated with a dilute fertilizer solution and placed in greenhouses at temperatures of 18–25 °C, with supplemental metal halide lighting. At 10–12 days after inoculation the plants are evaluated for infection type using a 0–4 scale (
Figure 3). Low infection types of 0 to 2
+ can vary from immune responses with no visible sign of rust infection, to small necrotic flecks with minute uredinia, and small to moderate sized uredinia surrounded by chlorosis. High infection types of 3 to 4 are characterized by moderate to large uredinia that lack any necrosis or chlorosis. The germplasm entries are inoculated with leaf rust races that differ for avirulence to the key leaf rust resistance genes that are present in each of the different market classes of wheat. The leaf rust resistance genes in the entries can be postulated by comparing their low infection types to the low infection types of a set Thatcher wheat lines that differ for single leaf rust resistance genes. Based on gene-for-gene specificity [
41,
42] the germplasm entries and Thatcher lines that have the same pattern of high and low infection to the different leaf rust races must have at least one leaf rust resistance gene in common. Generally up to 10 different races are sufficient to postulate the leaf rust resistance genes in the different wheat classes.
Using these methods hundreds to thousands of germplasm lines can be evaluated annually in greenhouse tests. Results of the gene postulation tests are sent directly to the coordinators of the individual nurseries, who then in turn distribute the results to the cooperators and contributors of germplasm. Descriptions of released cultivars that include the gene postulation results are published in the Journal of Plant Registrations. Gene postulation tests are also done on released cultivars to ensure that some data is available for all of the commonly grown winter and spring wheat cultivars in the southern, eastern and Great Plains regions. An accessible database of the leaf rust resistance gene postulations for current cultivars is posted at the USDA-ARS Cereal Disease Laboratory website.
In addition to rust phenotyping the entries in the various wheat nurseries are also tested for molecular markers that are closely linked to key leaf rust resistance genes. This work is done at the USDA-ARS wheat genotyping laboratories in Raleigh NC, Manhattan KS, Fargo ND, and Pullman WA. Some leaf rust resistance genes such as
Lr26 and
Lr37 [
43] are present on large translocations from wheat related species. These translocations do not undergo recombination with the homologous wheat chromosome, thus markers for the translocations can serve as a proxy for the resistance gene. Other genes have linked SSR markers that recombine at a frequency with the resistance gene that is related to the distance between the resistance gene and the marker. Markers more closely linked to the resistance gene are obviously more useful than markers at greater distances that show greater recombination. The ultimate marker is based on the known sequence of the resistance gene. Since
Lr21 and
Lr34 have been isolated and sequenced perfect PCR based markers for both of these genes have been developed.
Wheat breeding lines are also tested for leaf rust resistance in field plots in St. Paul MN. A mixture of leaf rust susceptible wheat cultivars are planted as a rust spreader in rows 3 to 4 feet wide that span the research plots. The germplam entries are planted in four to five foot rows perpendicular to the spreader rows. The spreader rows are inoculated with a mixture of common leaf rust races using an oil/urediniospore mixture when the plants have finished tillering, usually about a month after planting for spring wheat types. Light, hand carried battery powered atomizers designed for application of pesticides on a small scale, are used to apply the oil/spore mixture directly to spreader rows. Inoculation is optimal when the overnight temperature and humidity predict a dew period, or if rain is imminent. Leaf rust spores once applied to plant leaves have remained viable for over seven days in greenhouse tests, so a dew period within a few days of inoculation allows the spores to germinate and infect the spreader row plants. Uredinia can be seen on the spreader row plants within 7 to 8 days following a dew period. Urediniospores produced on the spreader rows will then move into the entries. In the spring wheat plots, uredinia begin to appear on the flag leaves of the entries within 2 to 3 weeks after inoculation (
Figure 7).
The entries are evaluated when at least 70% of the area of the flag leaves of susceptible check lines are covered by uredinia, which for spring wheats is usually about one month after inoculation. The percentage coverage of uredinia can be estimated with standardized diagrams that show different levels of rust uredinia [
5]. The infection response based on the presence of hypersensitive flecks, necrosis and chlorosis associated with the uredinia is also recorded. A large number of lines can be evaluated daily by estimating the percent of the flag leaf covered by uredinia (severity) and the type of resistance response from fully susceptible (large uredinia with no chlorosis or necrosis) to highly resistant (small hypersensitive flecks with no uredinia). Wheat lines with intermediate resistance are usually characterized by small to moderate size uredinia associated with chlorosis and necrosis.
Figure 7.
Plots of wheat germplasm lines inoculated with Puccinia triticina. Photo courtesy of Xiuling Zhang, University of Minnesota.
Figure 7.
Plots of wheat germplasm lines inoculated with Puccinia triticina. Photo courtesy of Xiuling Zhang, University of Minnesota.