Carbon Dioxide Fluxes and Their Environmental Controls in a Riparian Forest within the Hyper-Arid Region of Northwest China
Abstract
:1. Introduction
2. Materials and Methods
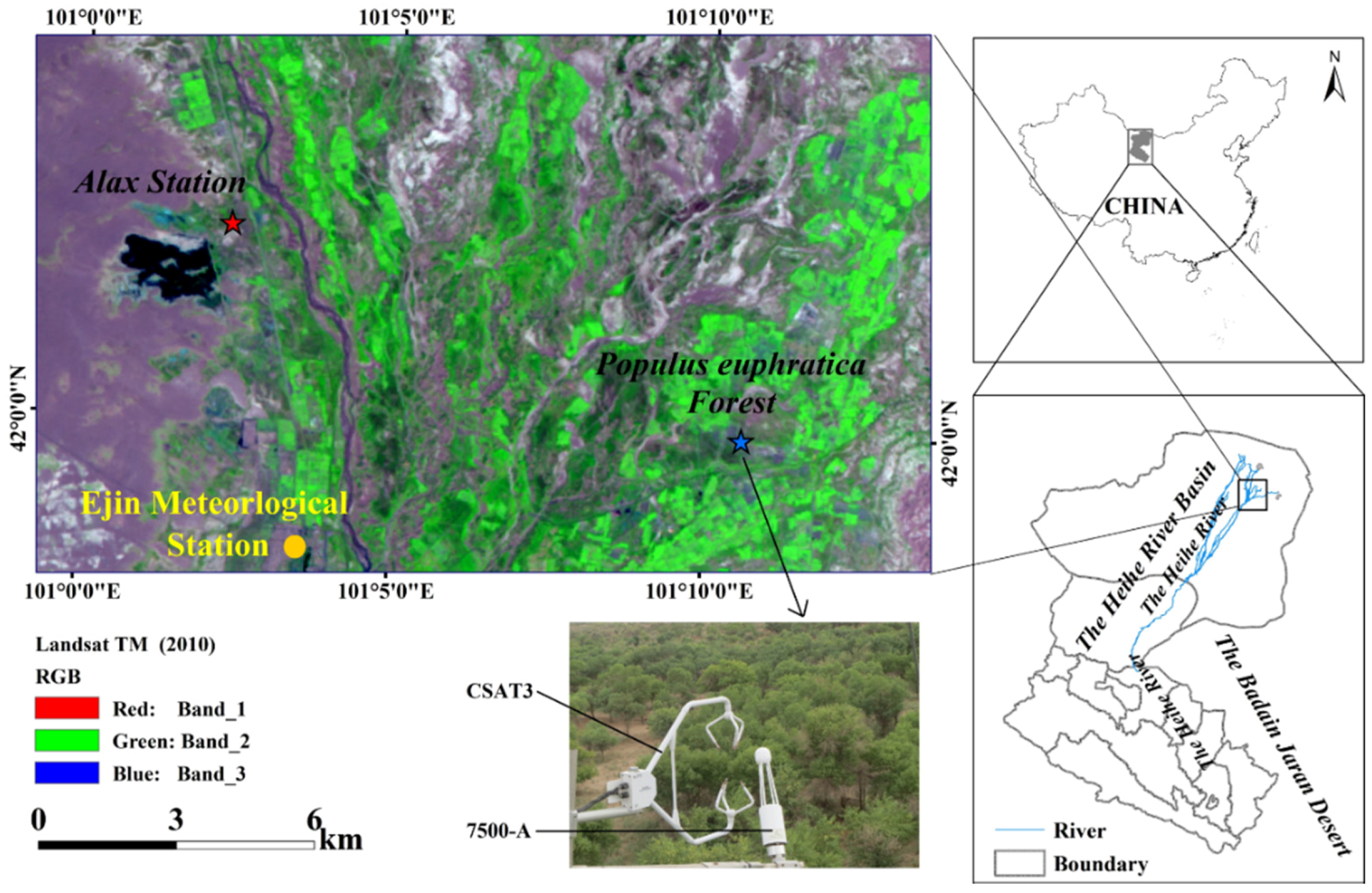
2.1. Site Description
2.2. Eddy Covariance and Meteorological Measurements
2.3. Eddy Covariance Flux Calculations and Data Quality Control
- (1)
- According to the empirical values of the CO2 fluxes and the statistical analyses of data, the scope of the threshold was defined, which ranged from approximately −60 to 60 μ mol m−2 s−1. All data outside of the threshold were removed;
- (2)
- Because of the complex structures, the eddy covariance instruments are susceptible to instability generated within the circuits, from dust and moisture in the air, precipitation, human operation, etc. Therefore, the spikes in the data appeared occasionally. For this reason, these spikes were filtered using the analysis of variance test [35] and 4 standard deviations were selected;
- (3)
- Because “photosynthesis” is unlikely to occur during the night, the occasional negative CO2 fluxes during the night were also filtered; and
- (4)
- A friction velocity (u*) threshold was used to filter the fluxes at night when the atmospheric turbulence was not well developed. The threshold for u* was estimated by the Moving Point Test as described in literature [36]. The flux data were discarded when the corresponding u* value was less than the threshold value.
2.4. Data Gaps Filling and NEE Partitioning
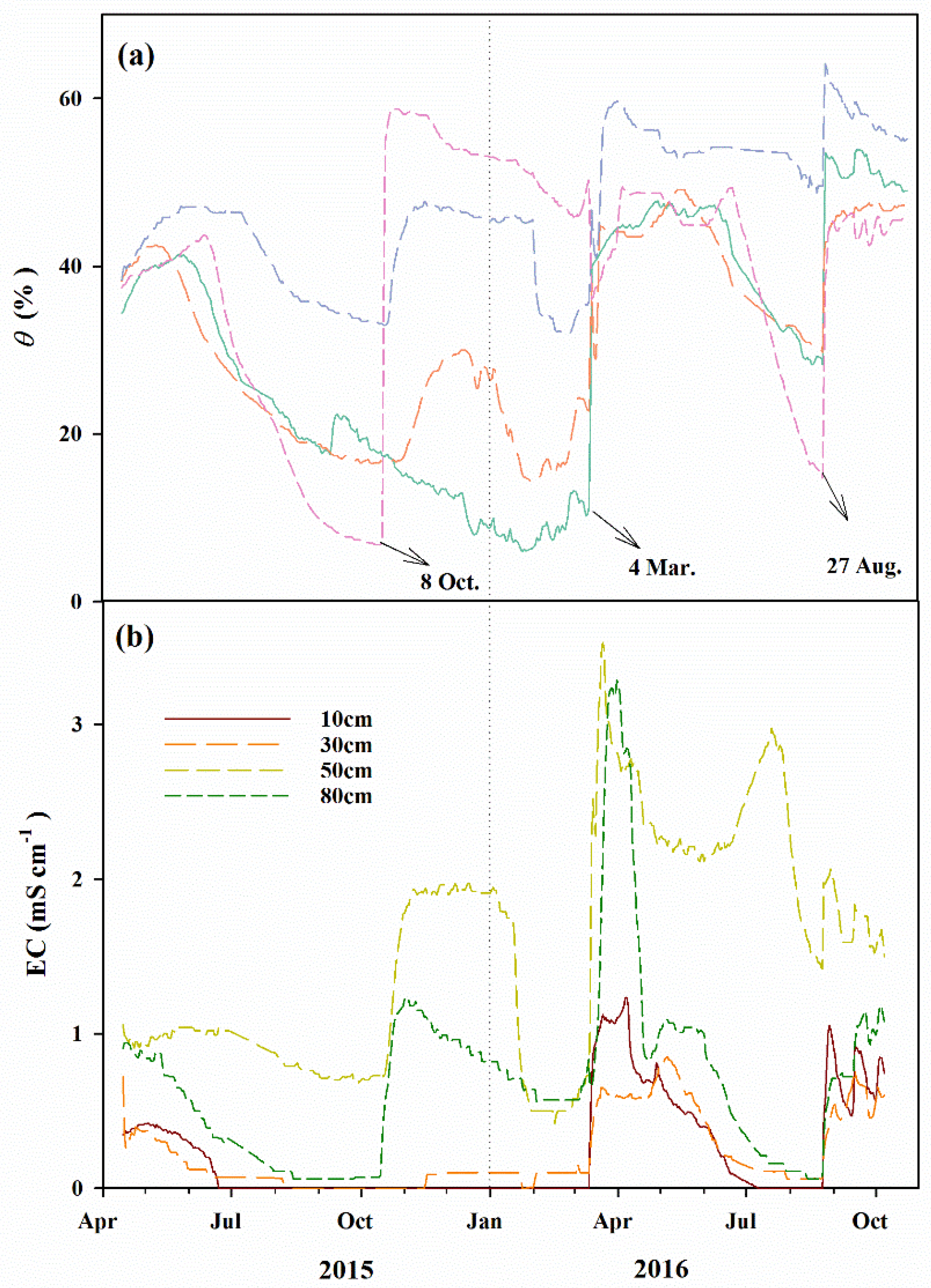
2.5. Groundwater Depth and Soil Moisture and Salt Measurement
2.6. Data Analysis
3. Results
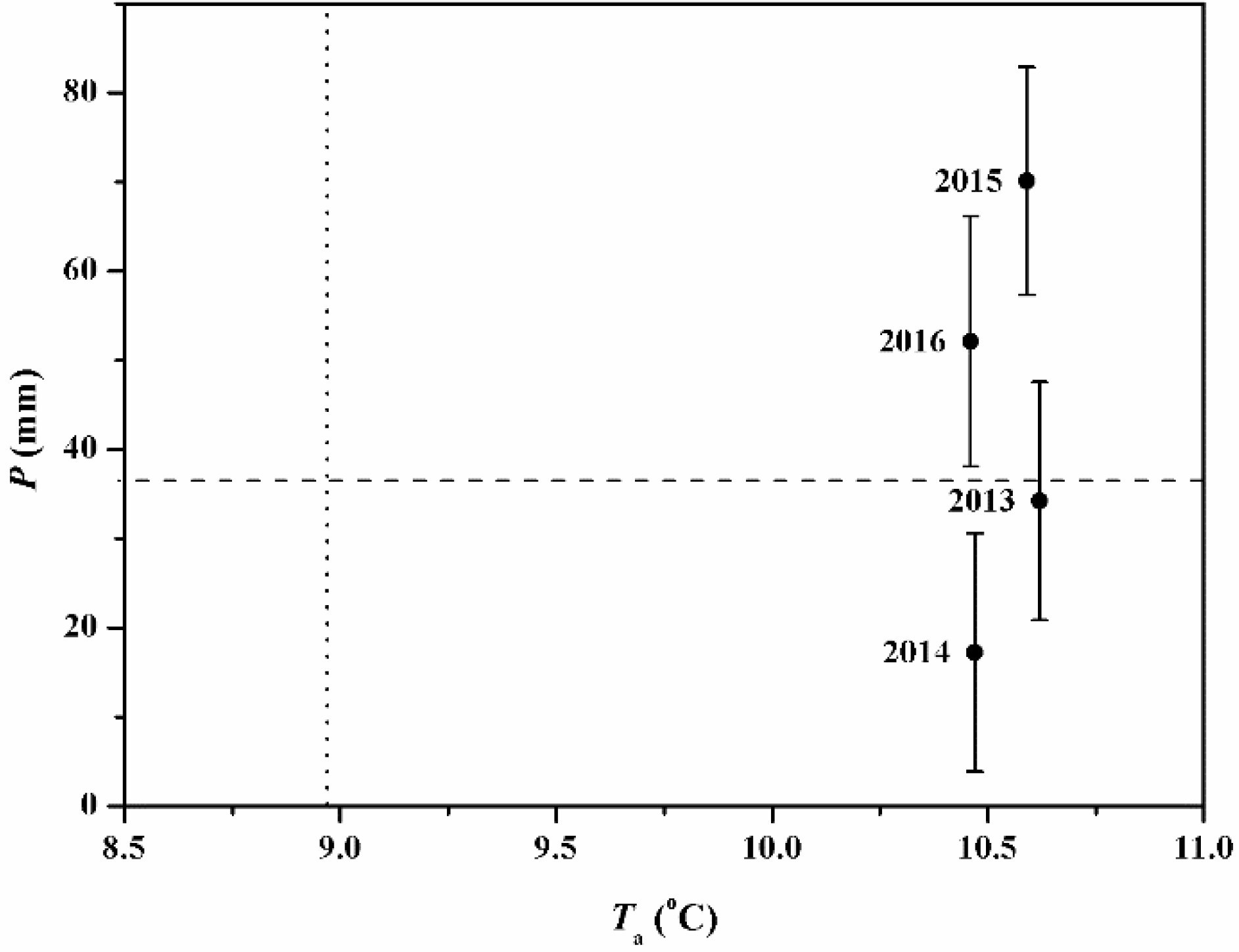
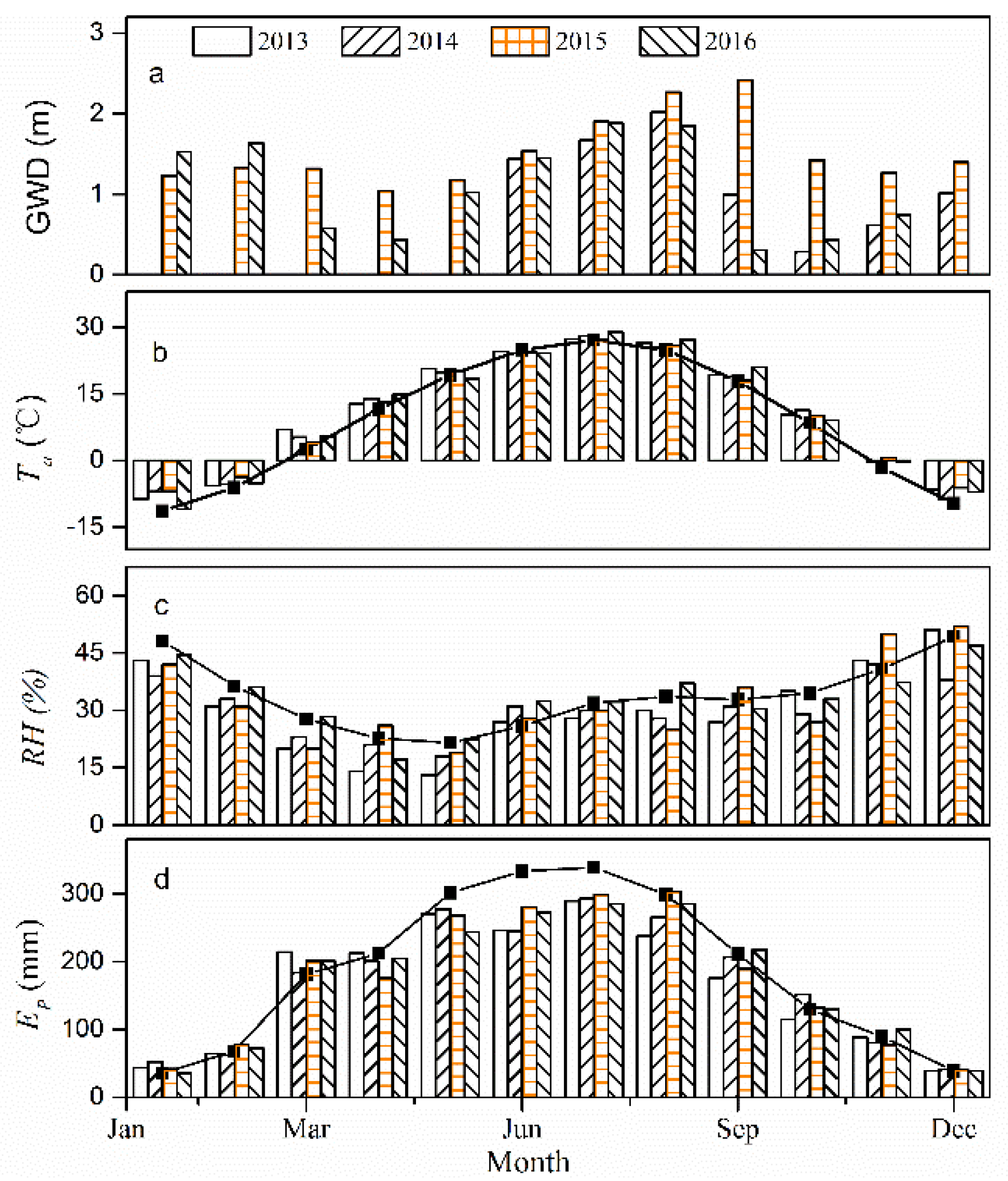
3.1. Weather Conditions
3.2. Multi-Scale Temporal Variations of Carbon Fluxes
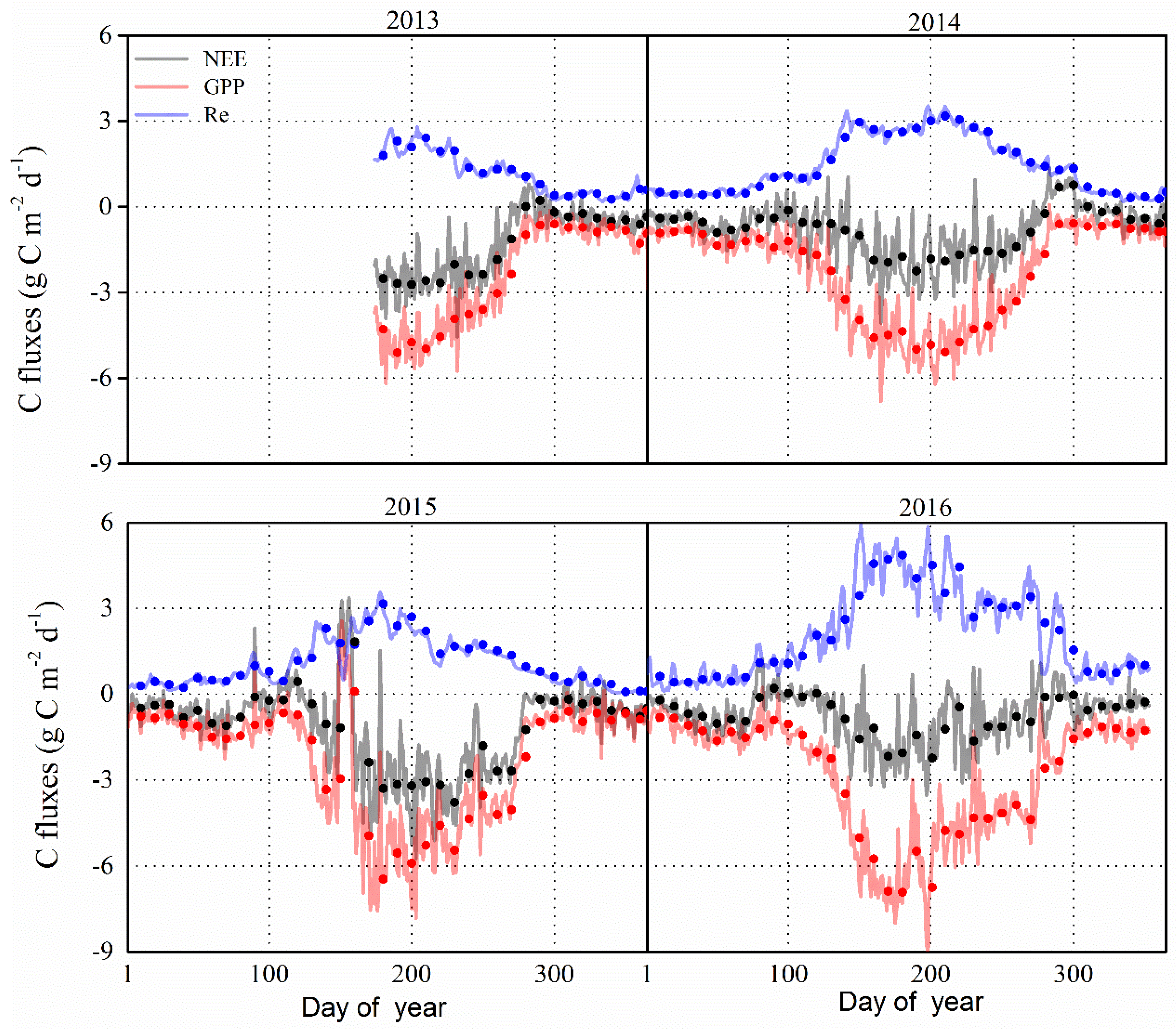
3.2.1. Daily Time Scale
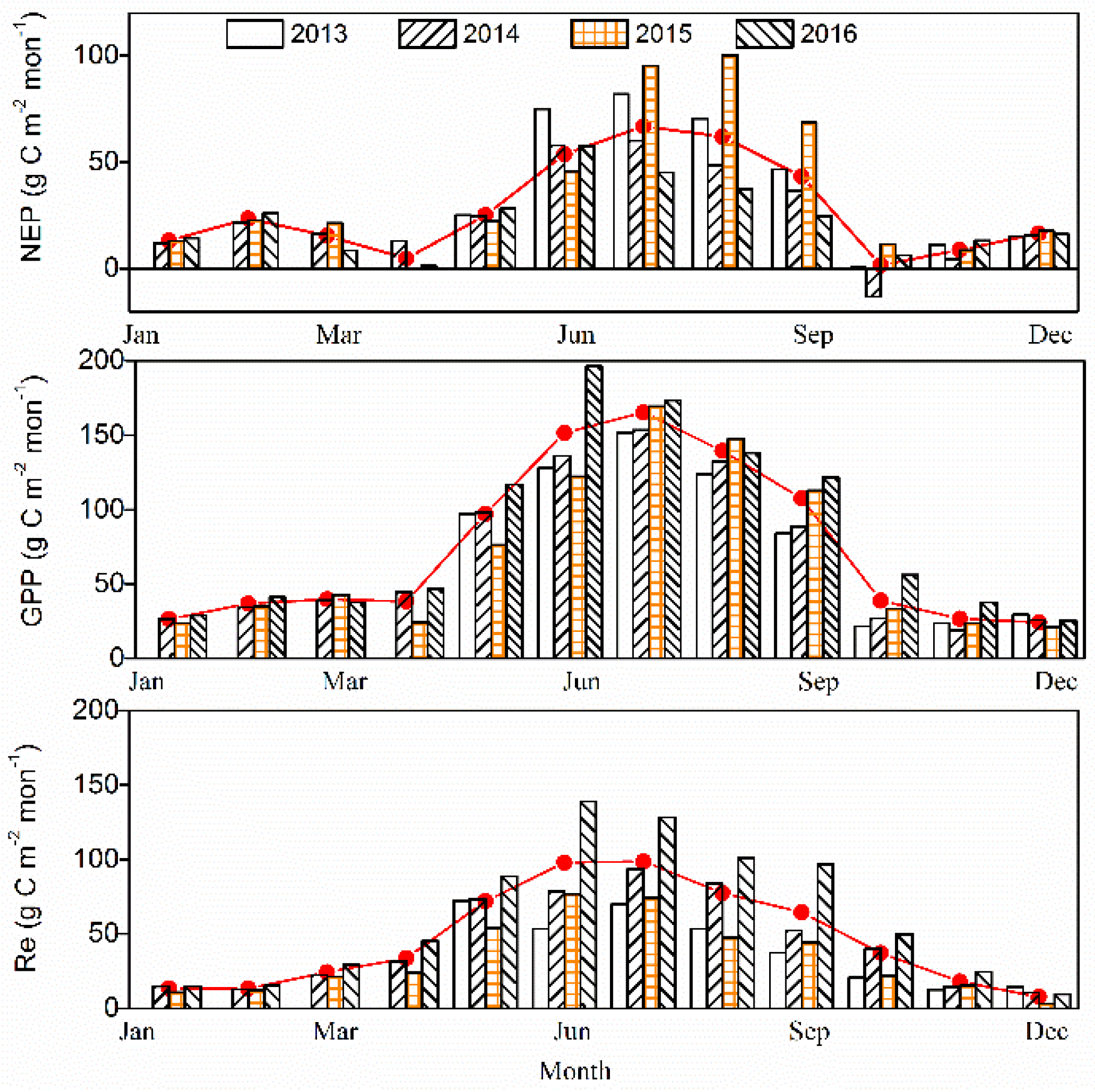
3.2.2. Monthly Time Scale
3.2.3. Annual Time Scale
3.3. Climatic Controls on Carbon Fluxes
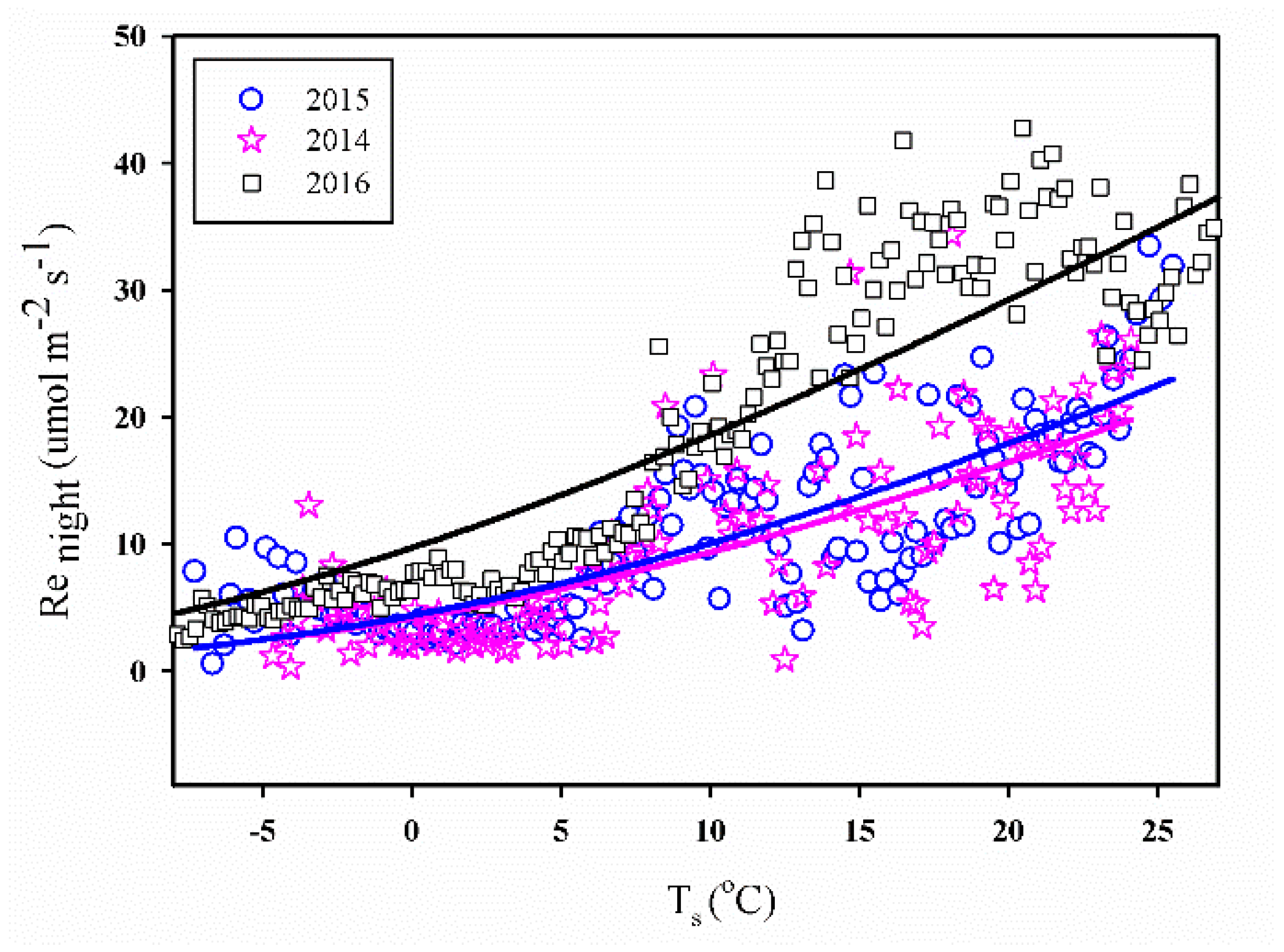
3.3.1. Ts vs. Re
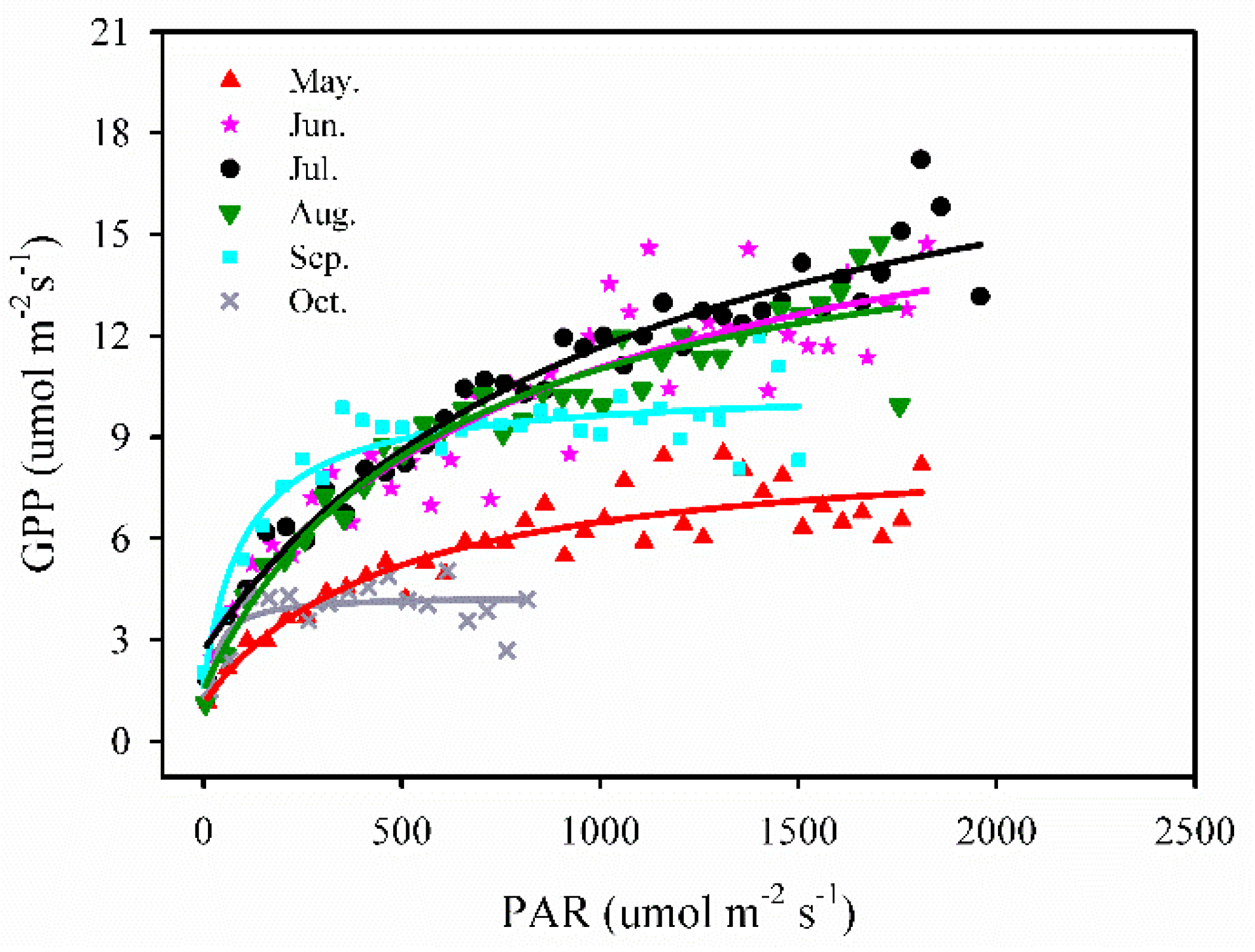
3.3.2. PAR vs. GPP
4. Discussion
4.1. CO2 Fluxes in the Riparian Forest
4.2. Controls on the Carbon Fluxes of Riparian Forest
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Le Quéré, C.; Andres, R.J.; Boden, T.; Conway, T.; Houghton, R.A.; House, J.I.; Marland, G.; Peters, G.P.; van der Werf, G.R.; Ahlström, A.; et al. The global carbon budget 1959–2011. Earth Syst. Sci. Data 2013, 5, 165–185. [Google Scholar] [CrossRef] [Green Version]
- Friedlingstein, P.; Meinshausen, M.; Arora, V.K.; Jones, C.D.; Anav, A.; Liddicoat, S.K.; Knutti, R. Uncertainties in CMIP5 climate projections due to carbon cycle feedbacks. J. Clim. 2014, 27, 511–526. [Google Scholar] [CrossRef]
- Schaphoff, S.; Lucht, W.; Gerten, D.; Sitch, S.; Cramer, W.; Prentice, I.C. Terrestrial biosphere carbon storage under alternative climate projections. Clim. Chang. 2006, 74, 97–122. [Google Scholar] [CrossRef]
- Ahlström, A.; Raupach, M.R.; Schurgers, G.; Smith, B.; Arneth, A.; Jung, M.; Reichstein, M.; Canadell, J.G.; Friedlingstein, P.; Jain, A.K. The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science 2015, 348, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Poulter, B.; Frank, D.; Ciais, P.; Myneni, R.B.; Andela, N.; Bi, J.; Broquet, G.; Canadell, J.G.; Chevallier, F.; Liu, Y.Y.; et al. Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 2014, 509, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.M.; Li, W.H.; Huang, X.; Zhu, C.G.; Ma, J.X. Assessment of the groundwater threshold of desert riparian forest vegetation along the middle and lower reaches of the Tarim river, China. Hydrol. Process. 2009. [Google Scholar] [CrossRef]
- Li, W.H.; Zhou, H.H.; Fu, A.H.; Chen, Y.P. Ecological response and hydrological mechanism of desert riparian forest in Inland River, northwest of China. Ecohydrology 2013, 6, 949–955. [Google Scholar] [CrossRef]
- Si, J.H.; Feng, Q.; Cao, S.; Yu, T.F.; Zhao, C.Y. Water use sources of desert riparian Populus euphratica forests. Environ. Monit. Assess. 2014, 186, 5469–5477. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Huxman, T.E.; Zitzer, S.F.; Charlet, T.N.; Housman, D.C.; Coleman, J.S.; Fenstermaker, L.K.; Seemann, J.R.; Nowak, R.S. Elevated CO2 increases productivity and invasive species success in an arid ecosystem. Nature 2000, 408, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.S.; Hanson, P.J.; Bolstad, P.; Barford, C.; Randolph, J.C.; Schmid, H.P.; Wilson, K.B. Biometric and eddy-covariance based estimates of annual carbon storage in five eastern North American deciduous forests. Agric. For. Meteorol. 2002, 113, 3–19. [Google Scholar] [CrossRef]
- Baldocchi, D.D.; Vogel, C.A.; Hall, B. Seasonal variation of carbon dioxide exchange rates above and below a boreal jack pine forest. Agric. For. Meteorol. 1997, 83, 147–170. [Google Scholar] [CrossRef]
- Baldocchi, D.D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob. Chang. Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef]
- Hirata, R.; Hirano, T.; Saigusa, N.; Fujinuma, Y.; Inukai, K.; Kitamori, Y.; Takahashi, Y.; Yamamoto, S. Seasonal and interannual variations in carbon dioxide exchange of a temperate larch forest. Agric. For. Meteorol. 2007, 147, 110–124. [Google Scholar] [CrossRef]
- Schimel, D.S.; House, J.I.; Hibbard, K.A.; Bousquet, P.; Ciais, P.; Peylin, P.; Braswell, B.H.; Apps, M.J.; Baker, D.; Bondeau, A.; et al. Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 2001, 414, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Amiro, B.D.; Barr, A.G.; Barr, J.G.; Black, T.A.; Bracho, R.; Brown, M.; Chen, J.; Clark, K.L.; Davis, K.J.; Desai, A.R.; et al. Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Williams, C.A.; Gu, H.; MacLean, R.; Masek, J.G.; Collatz, G.J. Disturbance and the carbon balance of us forests: A quantitative review of impacts from harvests, fires, insects, and droughts. Glob. Planet. Chang. 2016, 143, 66–80. [Google Scholar] [CrossRef]
- Ziemblińska, K.; Urbaniak, M.; Chojnicki, B.H.; Black, T.A.; Niu, S.; Olejnik, J. Net ecosystem productivity and its environmental controls in a mature scots pine stand in Northwestern Poland. Agric. For. Meteorol. 2016, 228–229, 60–72. [Google Scholar] [CrossRef]
- Houghton, R.A.; Skole, D.L.; Nobre, C.A.; Hackler, J.L.; Lawrence, K.T.; Chomentowski, W.H. Annual fluxes of carbon from deforestation and regrowth in the Brazilian Amazon. Nature 2000, 403, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Huete, A.; Cleverly, J.; Eamus, D.; Chevallier, F.; Joiner, J.; Poulter, B.; Zhang, Y.; Guanter, L.; Meyer, W.; et al. Drought rapidly diminishes the large net CO2 uptake in 2011 over Semi-Arid Australia. Sci. Rep. 2016, 6, 37747. [Google Scholar] [CrossRef] [PubMed]
- Kutsch, W.L.; Hanan, N.; Scholes, R.J.; Mchugh, I.; Kubheka, W.; Eckhardt, H.; Williams, C. Response of carbon fluxes to water relations in a savanna ecosystem in South Africa. Biogeosci. Discuss. 2008, 5, 2197–2235. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Z.Q.; Sun, G.; Fang, X.R.; Zha, T.G.; McNulty, S.; Chen, J.Q.; Jin, Y.; Noormets, A. Response of ecosystem carbon fluxes to drought events in a poplar plantation in northern china. For. Ecol. Manag. 2013, 300, 33–42. [Google Scholar] [CrossRef]
- Garcia-Franco, N.; Wiesmeier, M.; Goberna, M.; Martínez-Mena, M.; Albaladejo, J. Carbon dynamics after afforestation of semiarid shrublands: Implications of site preparation techniques. For. Ecol. Manag. 2014, 319, 107–115. [Google Scholar] [CrossRef]
- Kochendorfer, J.; Castillo, E.G.; Haas, E.; Oechel, W.C.; Paw, U.K.T. Net ecosystem exchange, evapotranspiration and canopy conductance in a riparian forest. Agric. For. Meteorol. 2011, 151, 544–553. [Google Scholar] [CrossRef]
- Scott, R.L.; Huxman, T.E.; Williams, D.G.; Goodrich, D.C. Ecohydrological impacts of woody-plant encroachment: Seasonal patterns of water and carbon dioxide exchange within a semiarid riparian environment. Glob. Chang. Biol. 2006, 12, 311–324. [Google Scholar] [CrossRef]
- Feng, Q.; Cheng, G.D. Current situation, problems and rational utilization of water resources in arid Northwestern China. J. Arid Environ. 1998, 40, 373–382. [Google Scholar]
- Hou, L.G.; Xiao, H.L.; Si, J.H.; Xiao, S.C.; Zhou, M.X.; Yang, Y.G. Evapotranspiration and crop coefficient of Populus euphratica Oliv forest during the growing season in the extreme arid region Northwest China. Agric. Water Manag. 2010, 97, 351–356. [Google Scholar] [CrossRef]
- Feng, Q.; Peng, J.Z.; Li, J.G.; Xi, H.Y.; Si, J.H. Using the concept of ecological groundwater level to evaluate shallow groundwater resources in hyperarid desert regions. J. Arid Land 2012, 4, 378–389. [Google Scholar] [CrossRef]
- Xi, H.Y.; Feng, Q.; Si, J.H.; Chang, Z.Q.; Cao, S.K. Impacts of river recharge on groundwater level and hydrochemistry in the lower reaches of Heihe River watershed, Northwestern China. Hydrogeol. J. 2010, 18, 791–801. [Google Scholar] [CrossRef]
- Guo, Q.L.; Feng, Q.; Li, J.L. Environmental changes after ecological water conveyance in the lower reaches of Heihe River, Northwest China. Environ. Geol. 2009, 58, 1387–1396. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Q.; Chen, L.J.; Yu, T.F. Significance and effect of ecological rehabilitation project in Inland river basins in Northwest China. Environ. Manag. 2013, 52, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Si, J.H.; Feng, Q.; Zhang, X.Y.; Chang, Z.Q.; Su, Y.H.; Xi, H.Y. Sap flow of Populus euphratica in a desert riparian forest in an extreme arid region during the growing season. J. Integr. Plant Biol. 2007, 49, 425–436. [Google Scholar] [CrossRef]
- Yu, T.F.; Feng, Q.; Si, J.H. Evapotranspiration of a Populus euphratica Oliv. forest and its controlling factors in the lower Heihe River basin, Northwest China. Sci. Cold Arid Reg. 2017, 9, 0175–0182. [Google Scholar]
- Moore, C.J. Frequency response corrections for eddy correlation systems. Bound.-Layer Meteorol. 1986, 37, 17–35. [Google Scholar] [CrossRef]
- Webb, E.K.; Pearman, G.I.; Leuning, R. Correction of flux measurements for density effects due to heat and water vapour transfer. Q. J. R. Meteorol. Soc. 1980, 106, 85–100. [Google Scholar] [CrossRef]
- Vickers, D.; Mahrt, L. Quality control and flux sampling problems for tower and aircraft data. J. Atmos. Ocean. Technol. 1997, 14, 512–526. [Google Scholar] [CrossRef]
- Papale, D.; Reichstein, M.; Aubinet, M.; Canfora, E.; Bernhofer, C.; Kutsch, W.; Longdoz, B.; Rambal, S.; Valentini, R.; Vesala, T.; et al. Towards a standardized processing of net ecosystem exchange measured with eddy covariance technique: Algorithms and uncertainty estimation. Biogeosciences 2006, 3, 571–583. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Fan, Y.; Li, H.; Miguez-Macho, G. Global patterns of groundwater table depth. Science 2013, 339, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Y.N.; Shen, Y.J.; Liu, Y.B.; Zhang, S.H. Analysis of changing pan evaporation in the arid region of Northwest China. Water Resour. Res. 2013, 49, 2205–2212. [Google Scholar] [CrossRef]
- Valentini, R.; Matteucci, G.; Dolman, A.J.; Schulze, E.D.; Rebmann, C.; Moors, E.J.; Granier, A.; Gross, P.; Jensen, N.O.; Pilegaard, K.; et al. Respiration as the main determinant of carbon balance in European forests. Nature 2000, 404, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, D.Y.; Goltz, S.M.; Davidson, E.A.; Lee, J.T.; Tu, K.; Valentine, H.T. Seasonal patterns and environmental control of carbon dioxide and water vapour exchange in an ecotonal boreal forest. Glob. Chang. Biol. 1999, 5, 891–902. [Google Scholar] [CrossRef]
- O’Grady, A.; Eamus, D.; Cook, P.; Lamontagne, S. Comparative water use by the riparian trees Melaleuca argentea and Corymbia bella in the wet-dry tropics of northern Australia. Tree Physiol. 2006, 26, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Guan, H.D.; Zhao, W.Z.; Yang, Y.T.; Li, S.B. Groundwater facilitated water-use efficiency along a gradient of groundwater depth in arid Northwestern China. Agric. For. Meteorol. 2017, 233, 235–241. [Google Scholar] [CrossRef]
- Yu, T.F.; Feng, Q.; Si, J.H.; Xi, H.Y.; Li, Z.X.; Chen, A.F. Hydraulic redistribution of soil water by roots of two desert riparian phreatophytes in Northwest China’s extremely arid region. Plant Soil 2013, 372, 297–308. [Google Scholar] [CrossRef]
- Jia, X.; Zha, T.S.; Gong, J.N.; Wang, B.; Zhang, Y.Q.; Wu, B.; Qin, S.G.; Peltola, H. Carbon and water exchange over a temperate semi-arid shrubland during three years of contrasting precipitation and soil moisture patterns. Agric. For. Meteorol. 2016, 228–229, 120–129. [Google Scholar] [CrossRef]
- Biederman, J.A.; Scott, R.L.; Goulden, M.L.; Vargas, R.; Litvak, M.E.; Kolb, T.E.; Yepez, E.A.; Oechel, W.C.; Blanken, P.D.; Bell, T.W.; et al. Terrestrial carbon balance in a drier world: The effects of water availability in Southwestern North America. Glob. Chang. Biol. 2016, 22, 1867–1879. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Tao, W.; Hong, S.Y.; Hua, S.J.; Wu, Z.Y.; Qiang, C.Z. Analysis of the characteristics of soil and groundwater salinity in the lower reaches of Heihe river. J. Glaciol. Geocryol. 2005, 27, 890–898. [Google Scholar]
- Wang, P.; Zhang, Y.C.; Yu, J.J.; Fu, G.B.; Ao, F. Vegetation dynamics induced by groundwater fluctuations in the lower Heihe river basin, Northwestern China. J. Plant Ecol. 2011, 4, 77–90. [Google Scholar] [CrossRef]
- Chen, Y.N.; Pang, Z.H.; Chen, Y.P.; Li, W.H.; Xu, C.; Hao, X.M.; Huang, X.; Huang, T.M.; Ye, Z.X. Response of riparian vegetation to water-table changes in the lower reaches of Tarim river, Xinjiang Uygur, China. Hydrogeol. J. 2008, 16, 1371–1379. [Google Scholar] [CrossRef]
- Chen, S.L.; Li, J.K.; Wang, S.S.; Hüttermann, A.; Altman, A. Salt, nutrient uptake and transport, and aba of Populus euphratica; a hybrid in response to increasing soil NACL. Trees 2001, 15, 186–194. [Google Scholar] [CrossRef]
- Zeng, F.J.; Yan, H.L.; Arndt, S.K. Leaf and whole tree adaptations to mild salinity in field grown Populus euphratica. Tree Physiol. 2009, 29, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Minkina, T.; Yaning, C.; Sushkova, S.; Chapligin, V.A.; Mandzhieva, S. A review on salinity adaptation mechanism and characteristics of Populus euphratica, a boon for arid ecosystems. Acta Ecol. Sin. 2016, 36, 497–503. [Google Scholar] [CrossRef]
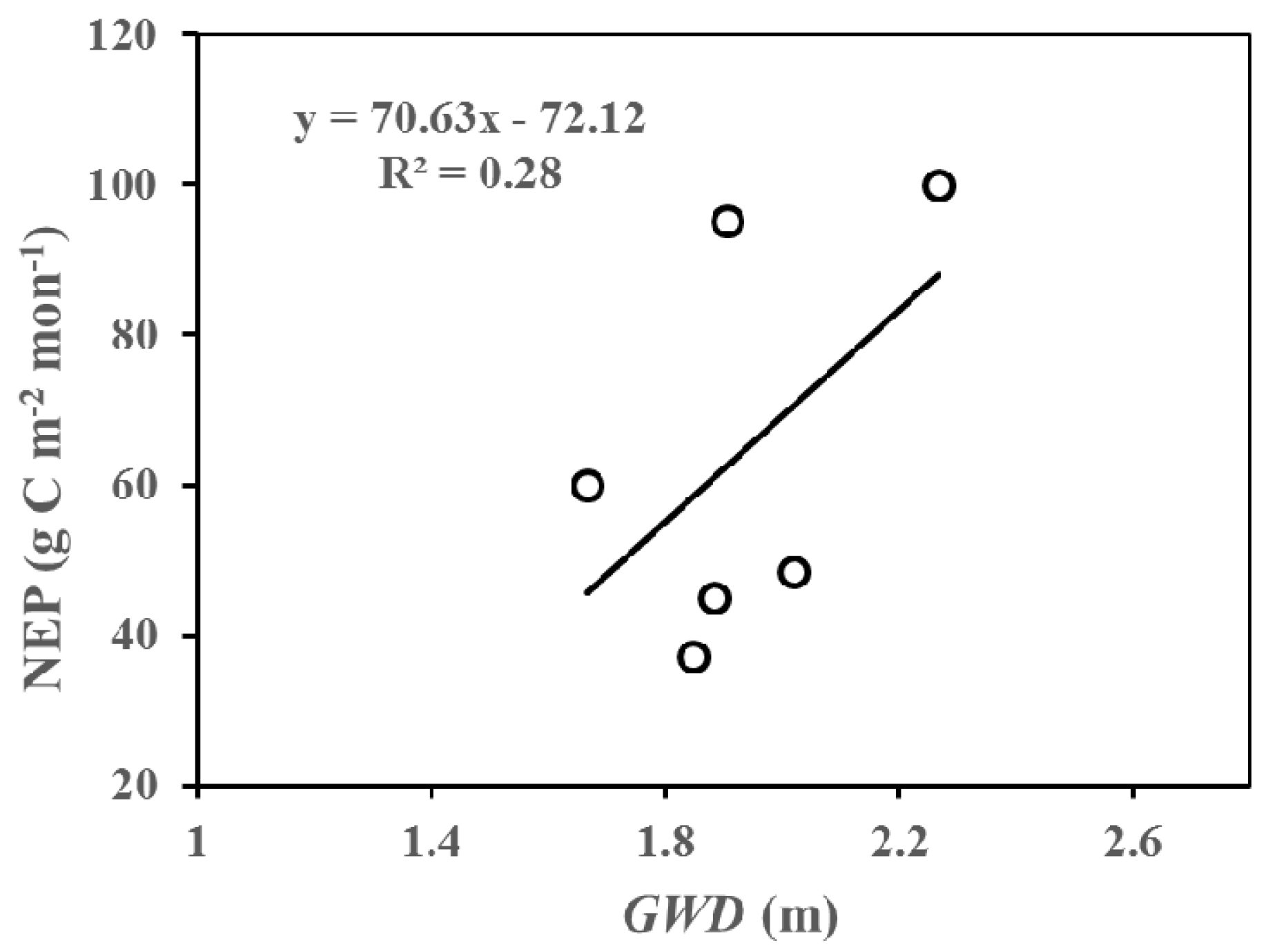
| Year | NEP | GPP | Re | GPP/Re | Ta | P | GWD |
|---|---|---|---|---|---|---|---|
| g C m−2 | °C | mm | m | ||||
| 2013 | 21.45 | 34.2 | 1.67 | ||||
| 2014 | 297 | 825 | 528 | 1.56 | 21.30 | 17.3 | 1.28 |
| 2015 | 427 | 834 | 405 | 2.05 | 20.98 | 70.1 | 1.79 |
| 2016 | 278 | 1019 | 742 | 1.37 | 21.46 | 52.1 | 1.16 |
| Average | 334 | 892 | 558 | 1.66 | 21.30 | 43.3 | 1.41 |
| Ta | VPD | PAR | GWD | Ep | |
|---|---|---|---|---|---|
| NEP | 0.598 ** | 0.333 ** | 0.395 ** | 0.525 ** | 0.268 ** |
| GPP | 0.663 ** | 0.524 ** | 0.426 ** | 0.390 ** | 0.458 ** |
| Re | 0.457 ** | 0.538 ** | 0.100 | 0.079 | 0.504 ** |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Feng, Q.; Yu, T.; Su, Y.; Deo, R.C. Carbon Dioxide Fluxes and Their Environmental Controls in a Riparian Forest within the Hyper-Arid Region of Northwest China. Forests 2017, 8, 379. https://doi.org/10.3390/f8100379
Ma X, Feng Q, Yu T, Su Y, Deo RC. Carbon Dioxide Fluxes and Their Environmental Controls in a Riparian Forest within the Hyper-Arid Region of Northwest China. Forests. 2017; 8(10):379. https://doi.org/10.3390/f8100379
Chicago/Turabian StyleMa, Xiaohong, Qi Feng, Tengfei Yu, Yonghong Su, and Ravinesh C. Deo. 2017. "Carbon Dioxide Fluxes and Their Environmental Controls in a Riparian Forest within the Hyper-Arid Region of Northwest China" Forests 8, no. 10: 379. https://doi.org/10.3390/f8100379







