Soil Organic Matter Accumulation and Carbon Fractions along a Moisture Gradient of Forest Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Scheme
2.3. Laboratory Analysis of Soil
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dorrepaal, E.; Sylvia, T.; van Logtestijn, R.S.P.; Swart, E.; Van de Weg, M.J.; Callaghan, T.V.; Aerts, R. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature 2009, 460, 616–619. [Google Scholar] [CrossRef]
- Fornara, D.A.; Steinbeiss, S.; McNamara, N.P.; Gleixner, G.; Oakley, S.; Poulton, P.R. Increases in soil organic carbon sequestration can reduce the global warming potential of long-term liming to permanent grassland. Glob. Chang. Biol. 2011, 17, 2762. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Fasham, M.J.R.; Goulden, M.L.; Heimann, M.; Jaramillo, V.J.; Kheshgi, H.S.; Le Quere, C.; Scholes, R.J.; Wallace, D.W.R. Climate change. The Scientific Basis IPCC. Chapter 3. In The Carbon Cycle and Atmospheric Carbon Dioxide; Cambridge University Press: Cambridge, UK, 2001; pp. 183–237. [Google Scholar]
- Stergiadi, M.; Van der Peck, M.; de Nijs, T.C.M.; Bierkens, M.F.P. Effect of climate change and land management on soil organic carbon dynamics and carbon leaching in northwestern Europe. Biogeosciences 2016, 13, 1519–1536. [Google Scholar] [CrossRef]
- Martin, M.P.; Wattenbach, M.; Smith, P.; Meersmans, J.; Jolivet, C.; Boulonne, L.; Arrouays, D. Spatial distribution of soil organic carbon stock in France. Biogeosciences 2011, 8, 1053–1065. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, P.B.; Duffy, S. Evaluation of Soil Organic Carbon and Soil Moisture Content from Agricultural Fileds in Mississipi. Open J. Soil Sci. 2013, 3, 81–90. [Google Scholar] [CrossRef]
- Hobley, E.U.; Wilson, B. The depth distribution of organic carbon in the soils of eastern Australia. Ecosphere 2016, 7, e01214. [Google Scholar] [CrossRef]
- Degórski, M. Influence of forest management into the carbon storage. Monit. Environ. 2005, 6, 75–83. [Google Scholar]
- Zwydak, M.; Brożek, S.; Lasota, J.; Małek, S. Reserve of Organic Carbon in Forest Soils of Lowlands in Poland. Pol. J. Environ. Stud. 2008, 17, 632–637. [Google Scholar]
- Ostrowska, A.; Porębska, G.; Kanafa, M. Carbon accumulation and Distribution in Profiles of Forest Soils. Pol. J. Environ. Stud. 2010, 19, 1307–1315. [Google Scholar]
- Stavi, I. Biochar use in forestry and tree-based agro-ecosystems for increasing climate change mitigation and adaptation. Int. J. Sustain. Dev. World Ecol. 2013, 20, 166–181. [Google Scholar] [CrossRef]
- Lasota, J.; Błońska, E. Forest Site Science in the Polish Lowlands and Highlands; Scientific Papers; University of Agriculture in Krakow: Kraków, Poland, 2013. [Google Scholar]
- Hassink, J. The capacity of soils to preserze organic C and N by their association with Clay and silt particles. Plant Soil 1997, 191, 77–87. [Google Scholar] [CrossRef]
- Six, J.; Paul, E.; Paustian, K. Stabilization Maechanisms of Soil Organic Matter: Implications for C—Saturation of Soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Wosten, J.H.; Lilly, A.; Nemes, A.; Le Bas, C. Development and use of a database of hydraulic properties of European soils. Geoderma 1999, 90, 169–185. [Google Scholar] [CrossRef]
- Bauer, J.; Herbst, M.; Huisman, J.A.; Weihermuller, L.; Vereecken, H. Sensitivity of simulated soil heterotrophic respiration to temperature and moisture reduction functions. Geoderma 2008, 145, 17–27. [Google Scholar] [CrossRef]
- Meersmans, J.; De Ridder, F.; Canters, F.; De Baets, S.; Van Molle, M. A multiple regression approach to assess the spatial distribution of Soil Organic Carbon (SOC) at the regional scale (Flanders, Belgium). Geoderma 2008, 143, 1–13. [Google Scholar] [CrossRef]
- Frouz, J.; Kalčík, J. Accumulation of soil organic carbon in relation to other soil characteristic during spontaneous succession in non-reclaimed colliery spoil heaps after brown coal mining near Sokolov (the Czech Republic). Ekológia 2006, 25, 388–397. [Google Scholar]
- Haynes, R.J. Labile organic matter fractions as central components of the quality of agricultural soils: An overview. Adv. Agron. 2005, 85, 221–268. [Google Scholar]
- Dębska, B.; Długosz, J.; Piotrowska-Długosz, A.; Banach-Szott, M. The impact of a bio-fertilizer on the soil organic matter status and carbon sequestration—Results from a field-scale study. J. Soils Sediments 2016, 16, 2335–2343. [Google Scholar] [CrossRef]
- Guimarães, D.V.; Gonzaga, M.I.S.; da Silva, T.O.; da Silva, T.L.; Dias, N.D.; Matias, M.I.S. Soil organic matter pools and carbon fractions in soil under different land uses. Soil Till. Res. 2013, 126, 177–182. [Google Scholar] [CrossRef]
- Błońska, E.; Lasota, J.; Piaszczyk, P.; Wiecheć, M.; Klamerus-Iwan, A. The effect of landslide on soil organic carbon stock and biochemical properties of soil. J. Soil Sendiments 2017. [Google Scholar] [CrossRef]
- World Reference Base (WRB). World Reference Base for Soil Resource; FAO: Rome, Italy, 2014. [Google Scholar]
- Dziadowiec, H.; Gonet, S. Przewodnik metodyczny do badań materii organicznej gleb. Prace Komisji Naukowych PTG 1999, 20, 42–43. [Google Scholar]
- Grossman, R.B.; Reinsch, T.G. Bulk density and linear extensibility. In Methods of Soil Analysis, Part 4; Dane, J.H., Topp, G.C., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 201–225. [Google Scholar]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Stendhal, J.; Johansson, M.B.; Eriksson, E.; Langvall, O. Soil organic carbon in Swedish spruce and pine forests-differences in stock levels and regional patterns. Silva Fennica 2010, 33, 5–21. [Google Scholar] [CrossRef]
- Prescott, P.C.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Gałka, B.; Kabała, C.; Łabaz, B.; Bogacz, A. Influence of stands with diversed share of Norway spruce in species structure on soils of various forest habitats in the Stołowe Mountains. Sylwan 2014, 158, 684–694. [Google Scholar]
- Gruba, P.; Mulder, J. Tree species affect cation exchange capacity (CEC) and cation binding properties of organic matter in acid forest soils. Sci. Total Environ. 2015, 511, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Błońska, E.; Lasota, J.; Gruba, P. Effect of temperate forest tree species on soil dehydrogenase and urease activities in relation to other properties of soil derived from loess and glaciofluvial sand. Ecol. Res. 2016, 31, 655–664. [Google Scholar] [CrossRef]
- Li, C.; Gao, S.; Zhang, J.; Zhao, L.; Wang, L. Moisture effect on soil humus characteristics in a laboratory incuba-tion experiment. Soil Water Res. 2016, 11, 37–43. [Google Scholar] [CrossRef]
- Turunen, J.; Tomppo, E.; Tolonen, K.; Reinikainen, A. Estimating carbon accumulation rates of undrained mires in Finland–application to boreal and subarctic regions. Holocene 2002, 12, 69–80. [Google Scholar] [CrossRef]
- Wellock, M.L.; Reidy, B.; Laperle, C.M.; Bolger, T.; Kiely, G. Soil organic carbon stocks of afforested peatlands in Irleand. Forestry 2011, 84, 441–451. [Google Scholar] [CrossRef]
- Walz, J.; Knoblauch, C.; Böhme, L.; Pfeiffer, E.M. Regulation of soil organic matter decomposition in permafrost-affected Siberian tundra soils—Impact of oxygen availability, freezing and thawing, temperature, and labile organic matter. Soil Biol. Biochem. 2017, 110, 34–43. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Reinhold, J. Stabilization of Soil Organic Matter: Association with Minerals or Chemical Recalcitrance? Biogeochemistry 2006, 77, 25. [Google Scholar] [CrossRef]
- Błońska, E. Effect of Stand Species Composition on the Enzyme Activity and Organic Matter Stabilization in Forest Soil; Scientific Papers of University of Agriculture in Krakow No. 527; University of Agriculture in Krakow: Kraków, Poland, 2015; Volume 404. [Google Scholar]
- Błońska, E.; Lasota, J.; Zwydak, M.; Piaszczyk, W. Stand mixing effect on enzyme activity and other soil properties. Soil Sci. Ann. 2016, 67, 173–178. [Google Scholar] [CrossRef]
- Osono, T.; Ono, Y.; Takeda, H. Fungal ingrowth on forest floor and decomposing needle litter of Chamaecyparis obtusa in relation to resource availability and moisture condition. Soil Biol. Biochem. 2003, 35, 1423–1431. [Google Scholar] [CrossRef]
- Jurgensen, M.; Ree, D.; Page-Dumroese, D.; Laks, P.; Collins, A.; Mroz, G.; Degórski, M. Wood strength loss as a measure of decomposition in northern forest mineral soil. Eur. J. Soil Biol. 2006, 42, 23–31. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol. Ecol. 2011, 78, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.L.M.; Nahas, E. Humic fractions of forest, pasture and maize crop soils resulting from microbial activity. Braz. J. Microbial. 2014, 45, 963–969. [Google Scholar] [CrossRef]
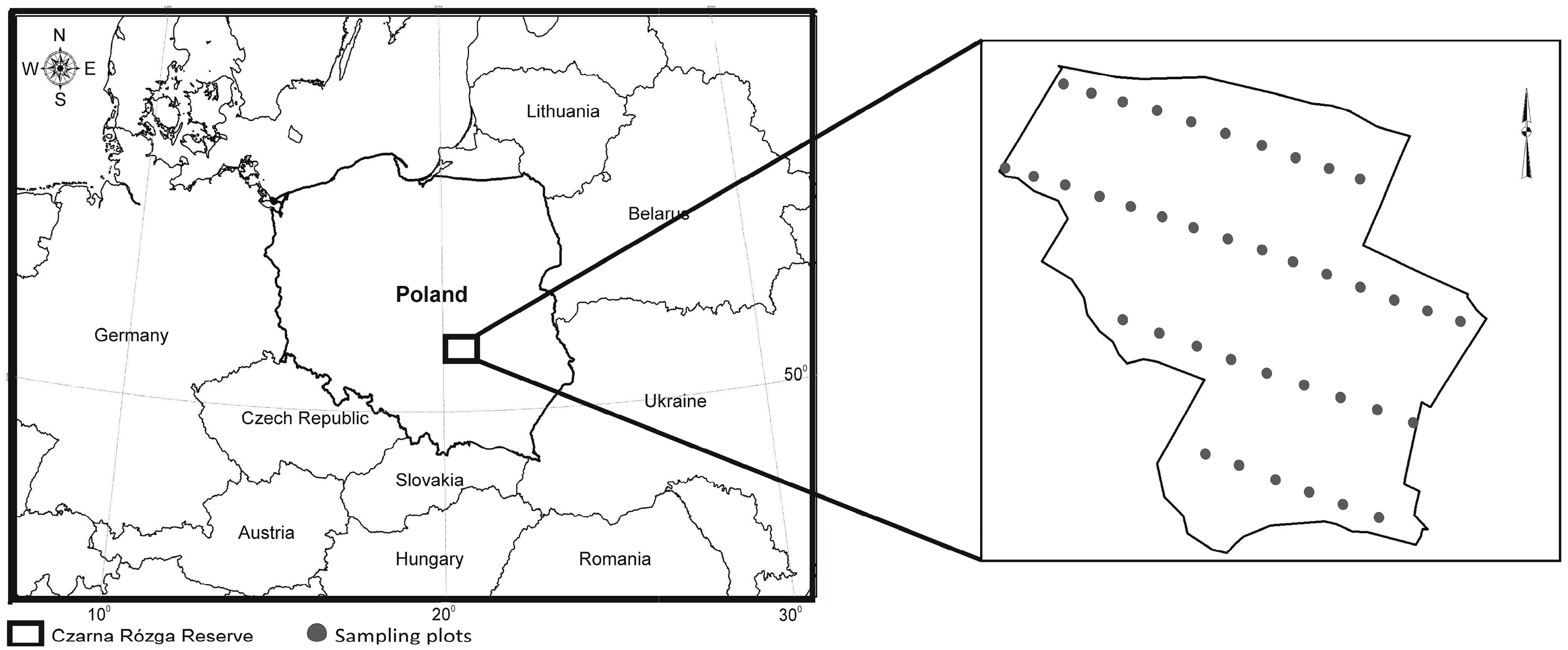
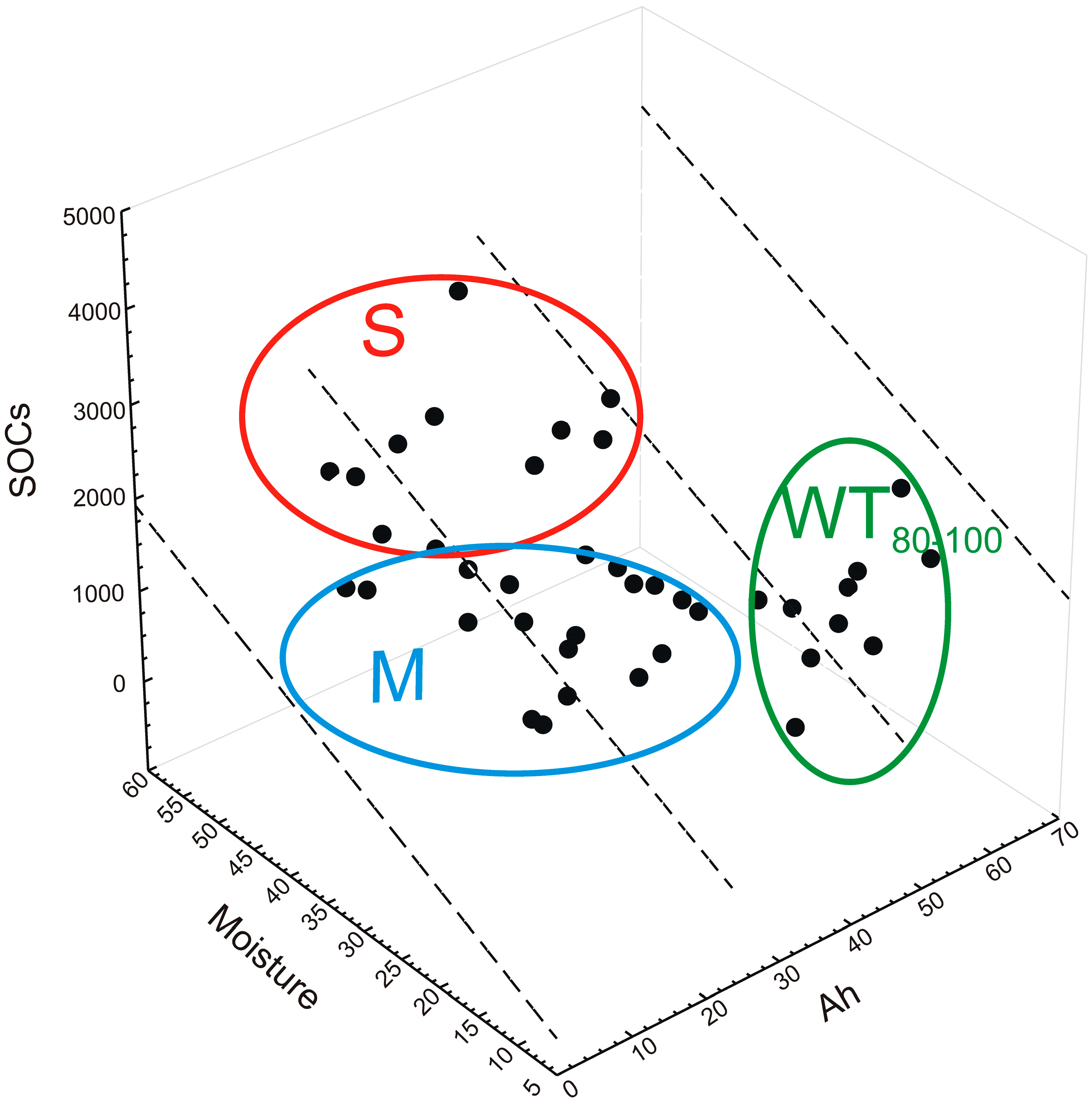
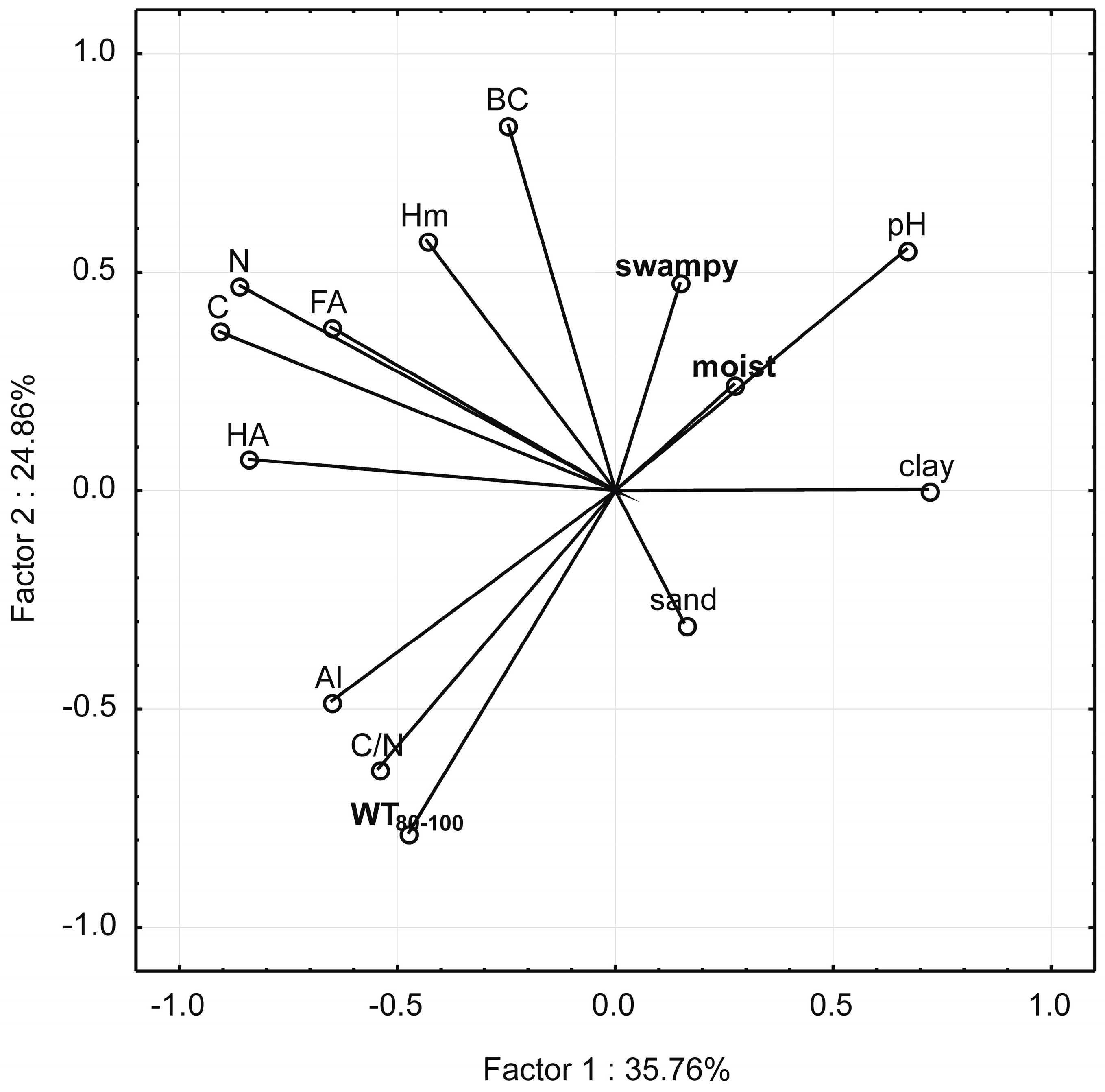
| Moisture Gradient | Plant Communities | Dominant Stand Species | Dominant Ground Cover Species | Type of Soil | Depth of Groundwater Level (in the Spring) (cm) | Humus Type |
|---|---|---|---|---|---|---|
| WT80–100 | Abietetum albae | Silver fir | Maianthemum bifolium Luzula pilosa Thiudium tamarescinum Polytrichum attenuatum | Podzols Stagnosols | 80–100 | mor |
| Moist | Tilio-Carpinetum ficarietosum | Common hornbeam, Common alder | Aegopodium podgraria Galeobdolon luteum Hepatica nobilis Impatiens noli-tangere | Gleysols | 40–50 | mull |
| Swampy | Fraxino-Alnetum | Common alder | Ficaria verna Valeriana simplicifolia Caltha palustris Carex remota | Mollic Gleysols | 0–30 | mull sticky |
| Moisture Gradient | Horizon | pH H2O | pH KCl | Ah | Aex | Al | P | Ca | K | Mg | Na | BC | BS | CECt | CECe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT80–100 | O | 3.85 b ± 0.37 | 3.04 b ± 0.35 | 49.68 a ± 18.74 | 10.14 a ± 3.43 | 306.98 ± 243.27 | 58.75 a ± 32.41 | 6.88 b ± 3.30 | 0.69 a ± 0.24 | 1.21 a ± 0.58 | 0.09a ± 0.08 | 8.89 b ± 3.84 | 16.49 b ± 8.82 | 58.58 a ± 20.86 | 19.03 a ± 6.07 |
| AE | 3.87 c ± 0.16 | 3.07 c ± 0.18 | 12.14 a ± 4.91 | 4.19 a ± 0.84 | 193.96 ± 79.65 | 8.67 a ± 4.90 | 0.94 b ± 0.32 | 0.05 a ± 0.03 | 0.13 b ± 0.09 | 0.02 b ± 0.01 | 1.13 b ± 0.41 | 7.15 b ± 4.57 | 13.26 a ± 6.07 | 5.32 b ± 1.82 | |
| G | 4.51 b ± 0.41 | 3.81 b ± 0.33 | 3.49 a ± 1.00 | 2.21 a ± 0.63 | 95.36 ± 41.21 | 61.46 a ± 46.18 | 0.36 b ± 0.19 | 0.01 b ± 0.01 | 0.03 b ± 0.02 | 0.01 b ± 0.01 | 0.41 b ± 0.37 | 10.03 b ± 6.19 | 3.91 b ± 0.87 | 2.62 b ± 0.54 | |
| Moist | A | 4.94 a ± 0.73 | 3.98 a ± 0.69 | 27.57 a,b ± 2.10 | 3.04 b ± 1.80 | 69.90 ± 9.37 | 19.18 b ± 12.18 | 19.95 a ± 12.86 | 0.36 b ± 0.29 | 1.6 a ± 1.15 | 0.09 a ± 0.06 | 22.04 a,b ± 14.22 | 45.65 a ± 20.06 | 49.61 a ± 31.46 | 25.08 a ± 14.23 |
| AG | 5.64 b ± 0.84 | 4.69 b ± 0.79 | 7.97 a,b ± 6.17 | 1.27 b ± 0.99 | 35.45 ± 68.05 | 3.85 b ± 3.69 | 16.07 a ± 14.54 | 0.06 a ± 0.04 | 0.93 a ± 0.87 | 0.06 a ± 0.04 | 17.12 a ± 12.15 | 60.75 a ± 23.38 | 25.10 a ± 19.31 | 18.39 a ± 15.02 | |
| G | 6.85 a ± 0.73 | 5.26 a ± 0.58 | 1.87 b ± 0.65 | 0.66 b ± 0.17 | 4.14 ± 5.25 | 1.27 b ± 0.72 | 8.30 a ± 5.07 | 0.07 a ± 0.05 | 0.60 a ± 0.40 | 0.04 a ± 0.02 | 9.01 a ± 5.52 | 76.92 a ± 15.04 | 10.88 a ± 5.61 | 9.67 a ± 5.44 | |
| Swampy | AM | 5.71 a ± 0.78 | 4.40 a ± 1.50 | 13.01 b ± 11.01 | 1.66 b ± 1.01 | 14.99 ± 5.84 | 30.70 a,b ± 28.43 | 28.73 a ± 15.79 | 0.34 b ± 0.28 | 1.74 a ± 1.12 | 0.10 a ± 0.07 | 30.90 a ± 16.96 | 68.52 a ± 18.79 | 43.92 a ± 25.71 | 32.57 a ± 17.45 |
| AG | 6.72 a ± 0.61 | 5.67 a ± 0.58 | 3.92 b ± 1.75 | 0.62 b ± 0.22 | 3.76 ± 6.59 | 8.86 a ± 5.88 | 21.73 a ± 12.85 | 0.10 a ± 0.08 | 1.09 a ± 0.72 | 0.07 a ± 0.05 | 22.99 a ± 13.05 | 82.32 a ± 12.04 | 26.92 a ± 14.75 | 23.62 a ± 13.45 | |
| G | 7.30 a ± 0.69 | 5.78 a ± 0.67 | 1.75 b ± 0.91 | 0.71 b ± 0.29 | 4.11 ± 1.01 | 5.09 a ± 4.47 | 10.59 a ± 7.06 | 0.12 a ± 0.07 | 0.70 a ± 0.53 | 0.04 a ± 0.02 | 11.40 a ± 7.59 | 83.53 a ± 9.73 | 13.16 a ± 8.22 | 12.11 a ± 7.47 |
| Moisture Gradient | Horizon | Sand Fraction | Silt Fraction | Clay | Sum of Fraction | BD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VCS | CS | MS | FS | VFS | Csi | Fsi | Sand | Silt | ||||
| WT80–100 | O | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.41 b ± 0.21 |
| AE | 0.4 a ± 0.3 | 15.5 a ± 8.8 | 30.5 a ± 2.6 | 15.0 a ± 4.0 | 9.2 a ± 2.4 | 10.9 a ± 3.7 | 14.6 a ± 4.5 | 3.8 a ± 1.3 | 70.7a ± 8.4 | 25.5 a ± 7.3 | 1.14 a ± 0.11 | |
| G | 1.3a ± 0.7 | 24.8 b ± 10.5 | 31.5 a ± 3.2 | 10.6 a ± 3.8 | 3.5 a ± 1.3 | 7.4 b ± 3.0 | 15.9 b ± 6.2 | 4.9 b ± 1.8 | 71.6a ± 9.1 | 23.4 b ± 7.6 | 1.34 a ± 0.01 | |
| Moist | A | 1.1a ± 1.0 | 10.9a ± 8.4 | 19.7 a ± 11.4 | 18.9 a ± 8.1 | 11.26 a ± 5.3 | 11.6 a ± 7.2 | 11.6a ± 9.1 | 2.7a ± 2.0 | 62.0a ± 30.1 | 23.3 a ± 15.7 | 0.83 a ± 0.25 |
| AG | 0.5 a ± 0.2 | 8.9 a ± 6.1 | 20.1 b ± 10.6 | 17.5 a ± 3.5 | 10.8 a ± 3.7 | 16.8 a ± 6.8 | 21.2 a ± 9.5 | 4.3 a ± 2.1 | 57.0 a ± 17.5 | 37.9 a ± 15.9 | 1.21 a ± 0.08 | |
| G | 0.3 a ± 0.1 | 7.8 a ± 5.7 | 23.7 a ± 11.5 | 14.4 a ± 3.2 | 4.9 a ± 2.0 | 14.0 a ± 6.5 | 27.1 a ± 10.9 | 7.7 a ± 2.9 | 51.1 b ± 18.8 | 41.1 a ± 16.3 | 1.35 a ± 0.02 | |
| Swampy | AM | 1.3 a ± 0.8 | 13.0 a ± 3.0 | 19.3 a ± 12.9 | 16.3 a ± 10.8 | 7.1 a ± 6.0 | 6.9 a ± 5.1 | 6.9 a ± 6.3 | 1.9 a ± 1.0 | 64.7 a ± 33.3 | 15.1 a ± 11.0 | 0.69 a,b ± 0.31 |
| AG | n.d. | 9.1 a ± 7.1 | 20.3 a,b ± 12.4 | 18.2 a ± 5.5 | 9.5 a ± 3.9 | 14.8 a ± 7.3 | 21.9 a ± 12.9 | 5.3 a ± 2.9 | 57.8 a ± 22.2 | 36.8 a ± 19.4 | 1.07 a ± 0.19 | |
| G | n.d. | 5.7 a ± 2.6 | 22.7 a ± 12.6 | 15.4 a ± 6.8 | 5.6 a ± 3.4 | 12.9 b ± 8.6 | 28.7 a ± 11.1 | 7.8 a ± 3.5 | 49.5 b ± 19.8 | 41.6 a ± 18.7 | 1.31 a ± 0.08 | |
| Moisture Gradient | Horizon | C | N | C/N | HA | FA | Hm | HA/HF | HA+HF/Hm | SOCs | CDI | Moisture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT80–100 | O | 241.87 a ± 74.21 | 12.45 a ± 5.28 | 19.6 b ± 2.8 | 11.9 a ± 6.78 | 4.43 a ± 2.28 | n.d. | 2.74 a ± 1.12 | n.d. | 14.09 a,b ± 2.98 | 0.837 | 16.43 c ± 3.42 |
| AE | 35.07 a ± 19.41 | 1.75 a ± 1.15 | 21.1 b ± 3.1 | 3.08 a ± 1.44 | 0.63 a ± 0.61 | 0.47 a ± 1.03 | 4.70 a ± 1.38 | 8.00 a ± 5.03 | ||||
| G | 4.87 a,b ± 2.01 | 0.24 a ± 0.07 | 20.3 b ± 2.5 | 0.81 a ± 0.44 | 0.82 a ± 0.45 | 0.3 a ± 0.03 | 1.05 a ± 1.10 | 54.30 a ± 22.90 | ||||
| Moist | A | 101.10 b ± 49.29 | 6.80 a ± 3.79 | 14.2 a ± 1.7 | 3.15 b ± 3.04 | 3.41 a ± 3.55 | 9.79 a ± 10.41 | 1.80 a ± 2.31 | 0.68 b ± 0.84 | 12.61 b ± 3.71 | 0.299 | 30.08 b ± 4.86 |
| AG | 24.29 a ± 13.80 | 1.84 a ± 1.04 | 13.2 a ± 1.2 | 1.37 b ± 0.73 | 1.69 a ± 2.10 | 1.89 a ± 2.53 | 2.25 b ± 1.96 | 1.63 b ± 1.25 | ||||
| G | 3.95 b ± 2.30 | 0.33 a ± 0.18 | 11.8 a ± 1.1 | 0.99 a ± 1.12 | 0.72 a ± 0.94 | 0.25 a ± 0.26 | 3.31 a ± 1.18 | 11.47 a ± 8.98 | ||||
| Swampy | AM | 135.96 a,b ± 107.11 | 10.09 a ± 7.02 | 12.8 a ± 1.6 | 4.67 a,b ± 3.55 | 2.91 a ± 3.72 | 5.51 a ± 7.63 | 3.84 a ± 2.41 | 1.40 a,b ± 0.68 | 19.02 a ± 1.82 | 0.329 | 46.85 a ± 3.28 |
| AG | 44.13 a ± 34.29 | 3.31 a ± 2.39 | 13.1 a ± 1.2 | 2.26 a,b ± 1.56 | 1.34 a ± 1.65 | 1.08 a ± 1.47 | 2.51 b ± 1.06 | 3.40 b ± 2.20 | ||||
| G | 8.51 a ± 4.31 | 0.64 a ± 0.51 | 12.8 a ± 1.6 | 0.79 a ± 0.72 | 0.99 a ± 0.90 | 0.31 a ± 0.28 | 2.98 a ± 1.47 | 16.18 a ± 12.45 |
| R2 | Equation Parameter | β | p | |
|---|---|---|---|---|
| C | 89% | Ah | 5.257 | 0.000001 |
| Moisture | 1.897 | 0.000001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błońska, E.; Lasota, J. Soil Organic Matter Accumulation and Carbon Fractions along a Moisture Gradient of Forest Soils. Forests 2017, 8, 448. https://doi.org/10.3390/f8110448
Błońska E, Lasota J. Soil Organic Matter Accumulation and Carbon Fractions along a Moisture Gradient of Forest Soils. Forests. 2017; 8(11):448. https://doi.org/10.3390/f8110448
Chicago/Turabian StyleBłońska, Ewa, and Jarosław Lasota. 2017. "Soil Organic Matter Accumulation and Carbon Fractions along a Moisture Gradient of Forest Soils" Forests 8, no. 11: 448. https://doi.org/10.3390/f8110448




