Genetic Variation in Quercus acutissima Carruth., in Traditional Japanese Rural Forests and Agricultural Landscapes, Revealed by Chloroplast Microsatellite Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling of Plant Populations
2.2. Chloroplast SSR Analysis
2.3. Genetic Data Analysis
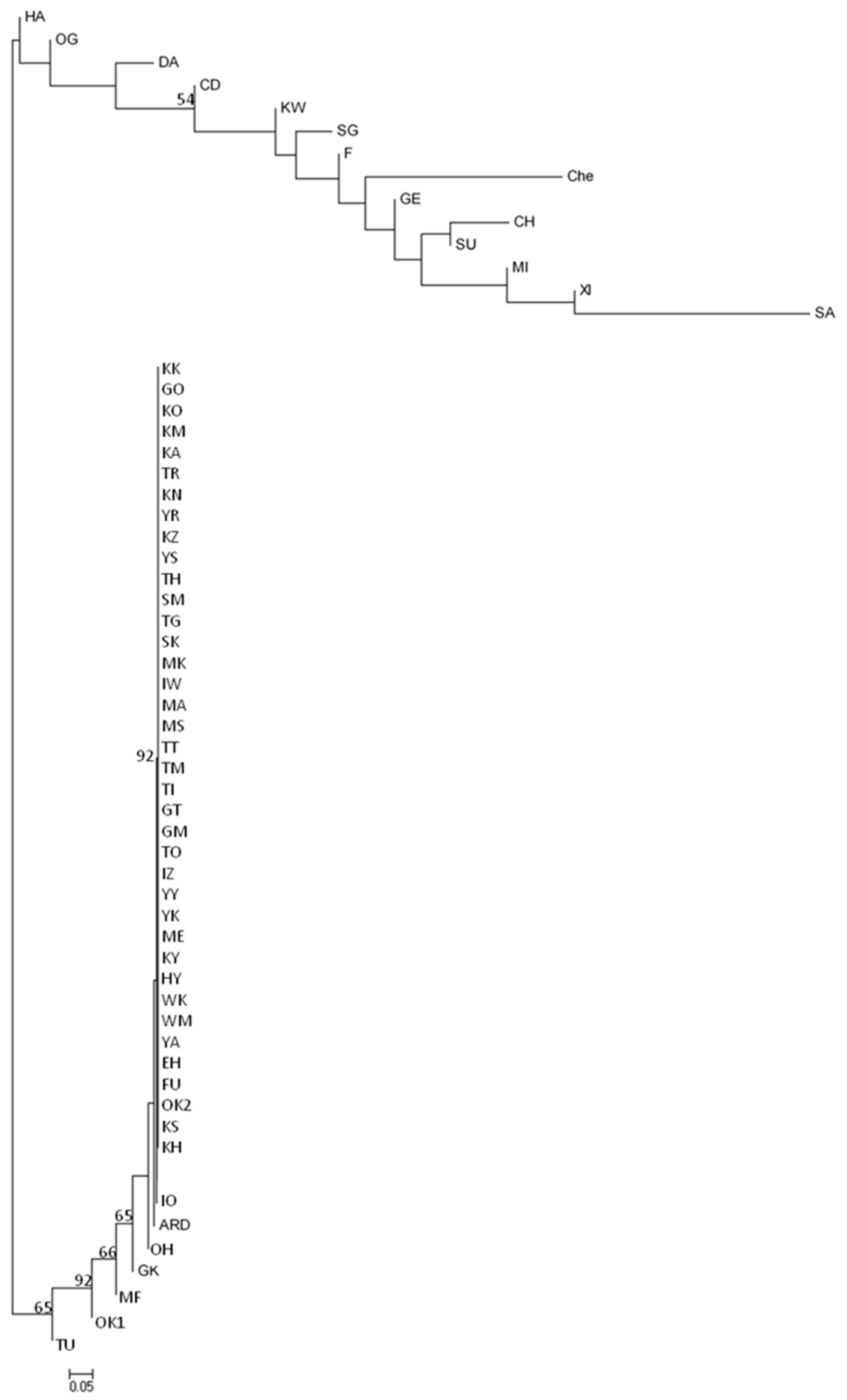
3. Results
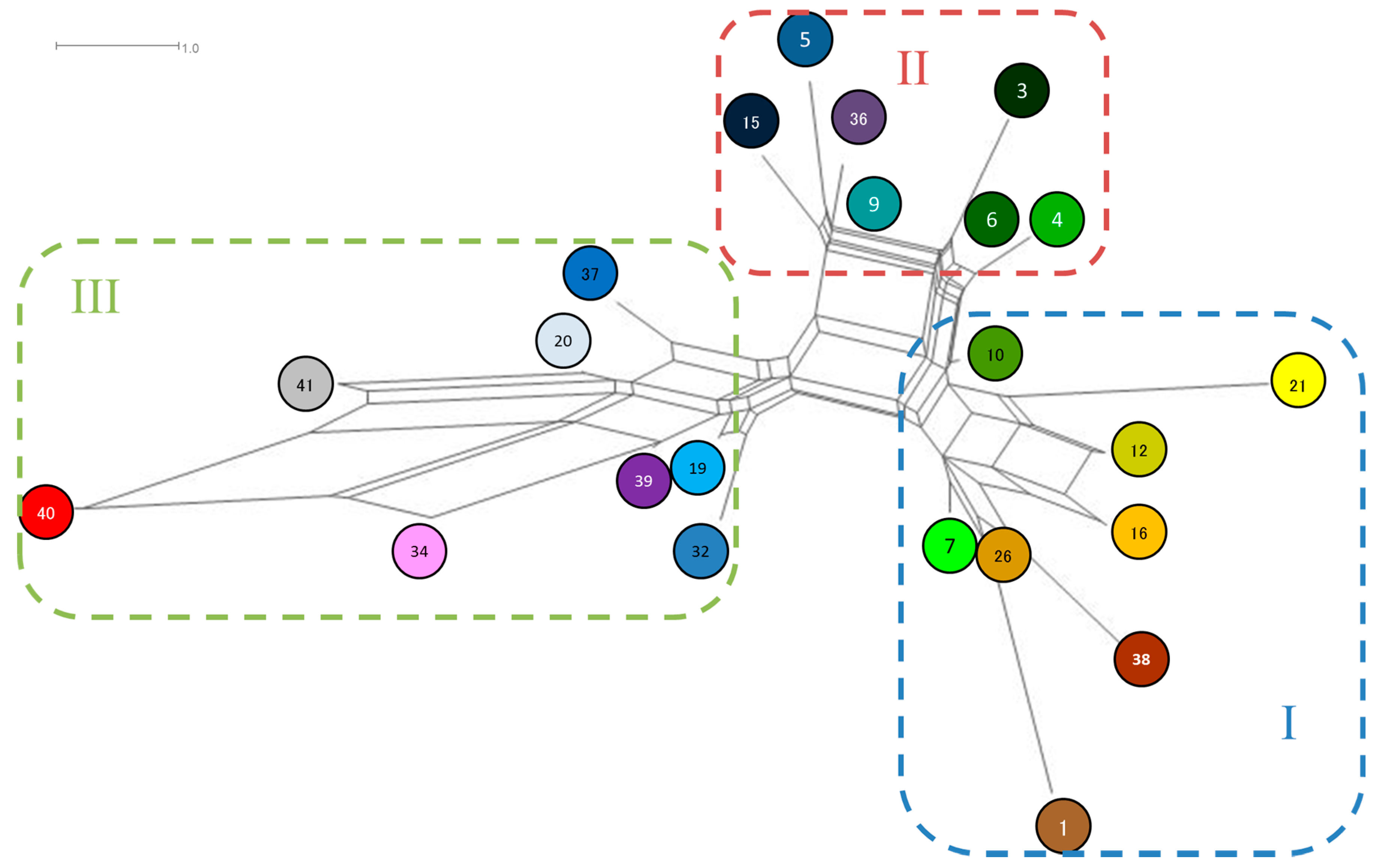
3.1. Haplotypes
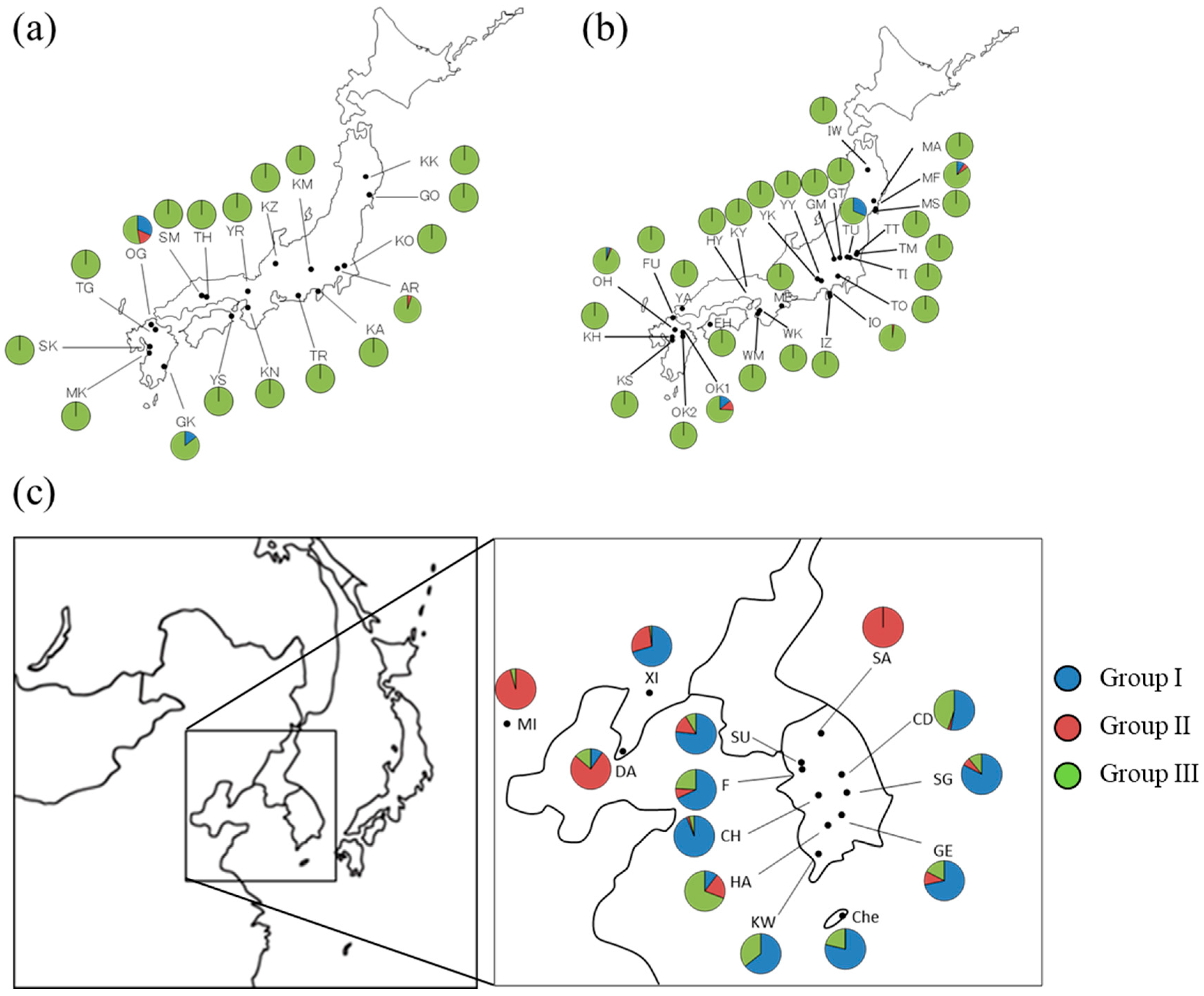
3.2. Geographical Distribution of Haplotypes of Q. acutissima
3.3. Genetic Diversity within Populations and Differentiation between Populations of Q. acutissima
3.4. Genetic Relationship between Populations of Q. acutissima
4. Discussion
4.1. Genetic Characteristics of Q. acutissima Natural Populations in Northeast Asia
4.2. Genetic Structure and Population Differentiation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Baldoni, L.; Tosti, N.; Ricciolini, C.; Belaj, A.; Arcioni, S.; Pannelli, G.; Germana, M.; Mulas, M.; Porceddu, A. Genetic structure of wild and cultivated olives in the central Mediterranean Basin. Ann. Bot. 2006, 98, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Fineschi, S.; Taurchini, D.; Villani, F.; Vendramin, G.G. Chloroplast DNA polymorphism reveals little geographical structure in Castanea sativa Mill. (Fagaceae) throughout southern European countries. Mol. Ecol. 2000, 9, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Gunn, B.; Baudouin, L.; Olsen, K. Independent origins of cultivated coconut (Cocos nucifera L.) in the old world tropics. PLoS ONE 2011, 6, e21143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, A.O.; Ziegenhaben, B.; Dam, B.C.; Csaikl, U.M.; Coart, E.; Degen, B.; Burg, K.; Vries, S.M.G.; Petit, R.J. Chloroplast DNA variation of oaks in western central Europe and genetic consequences of human influences. For. Ecol. Manag. 2002, 156, 147–166. [Google Scholar] [CrossRef]
- Mohanty, A.; Martin, J.P.; Aguinaglade, I. A population genetic analysis of chloroplast DNA in wild populations of Prunus avium L. in Europe. Heredity 2001, 87, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Besnard, G.; Khadari, B.; Baradat, P.; Bervill, A. Olea europaea (Oleaceae) phylogeography based on chloroplast DNA polymorphism. Theor. Appl. Genet. 2002, 104, 1353–1361. [Google Scholar] [PubMed]
- Magri, D.; Fineschi, S.; Bellarosa, R.; Buonamici, A.; Sebastiani, F.; Schirone, B.; Simeone, M.C.; Vendramin, G.G. The distribution of Quercus suber chloroplast haplotypes matches the palaogeographical history of the western Mediterranean. Mol. Ecol. 2007, 16, 5259–5266. [Google Scholar] [CrossRef] [PubMed]
- Dumolin, S.; Demesure, B.; Petit, R.J. Inheritance of chloroplast and mitochondrial genomes in pedunculate oak investigated with an efficient PCR method. Theor. Appl. Genet. 1995, 91, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.J.; Kremer, A.; Wagner, D. Geographic structure of chloroplast DNA polymorphisms in European oaks. Theor. Appl. Genet. Int. J. Plant Breed. Res. 1993, 87, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Rajora, O.P.; Dancik, B.P. Chloroplast DNA inheritance in Populus. Theor. Appl. Genet. 1992, 84, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Heuertz, M.; Fineschi, S.; Anzidei, M.; Pastorelli, R.; Salvini, D.; Paule, L.; Frascaria-Lacoste, N.; Hardy, O.J.; Vekemans, X.; Vendramin, G.G. Chloroplast DNA variation and postglacial recolonization of common ash (Faxinus excelsio L.) in Europe. Mol. Ecol. 2004, 13, 3437–3452. [Google Scholar] [CrossRef] [PubMed]
- Palme, A.E.; Su, Q.; Rautenberg, A.; Manni, F.; Lascoux, M. Postglacial recolonization and cpDNA variation of silver birch, Betula pendula. Mol. Ecol. 2003, 12, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.; Peakall, R. Chloroplast simple sequence repeats (cpSSRs): Technical resources and recommendations for expanding cpSSR discovery and applications to a wide array of plant species. Mol. Ecol. Res. 2009, 9, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, F.; Tsuda, Y.; Fineschi, S.; Bruschi, P.; Magri, D.; Zhelev, P.; Paule, L.; Simeone, M.; González-Martínez, S.; Vendramin, G. Combining molecular and fossil data to infer demographic history of Quercus cerris: Insights on European eastern glacial refugia. J. Biogeogr. 2016, 43, 679–690. [Google Scholar] [CrossRef]
- Provan, J.; Powell, W.; Hollingswoth, P.M. Chloroplast microsatellites: New tools for studies in plant ecology and evolution. Trends Ecol. Evol. 2001, 16, 142–147. [Google Scholar] [CrossRef]
- Grivet, D.; Deguilloux, M.F.; Petet, R.J.; Sork, V.L. Contrasting patterns of historical colonization in white oaks (Quercus spp.) in California and Europe. Mol. Ecol. 2006, 15, 4085–4093. [Google Scholar] [CrossRef] [PubMed]
- Marsico, T.D.; Hellmann, J.J.; Romero-Severson, J. Patterns of seed dispersal and pollen flow in Quercus garryana (Fagaceae) following post-glacial climatic changes. J. Biogeogr. 2009, 36, 929–941. [Google Scholar] [CrossRef]
- Mohammad-Panah, N.; Shabanian, N.; Khadivi, A.; Rahmani, M.S.; Emami, A. Genetic structure of gall oak (Quercus infectoria) characterized by nuclear and chloroplast SSR markers. Tree Genet. Genomes 2017, 13, 70–81. [Google Scholar] [CrossRef]
- Iida, S. Quantitative analysis of acorn transportation by rodents using magnetic locator. Vegetatio 1996, 124, 39–43. [Google Scholar] [CrossRef]
- Satake, Y.; Hara, H.; Watari, S.; Tominari, T. Wild Flowers of Japan—Woody Plants; Heibonsha Ltd.: Tokyo, Japan, 1989. (In Japanese) [Google Scholar]
- Kurata, S. Trip for Plants and Literature; Chikyu-Sha: Tokyo, Japan, 1976. (In Japanese) [Google Scholar]
- Fukamachi, K.; Oku, H.; Rackham, O. A comparitive stydy on tees and hedgerows in Japan and England. In Landscape Interfaces; Palang, H., Fry, G., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; pp. 53–69. [Google Scholar]
- Tsukada, M. Map of vegetation during the Last Glacial Maximum in Japan. Quat. Res. 1985, 23, 369–381. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Liu, C.; Xia, T.; Zhang, Q.; Fang, Y. Phylogeography of the temperate tree species Quercus acutissima in China: Inferences from chloroplast DNA variations. Biochem. Syst. Ecol. 2015, 63, 190–197. [Google Scholar] [CrossRef]
- Yasuda, Y.; Miyoshi, N. Illustration Vegetation History of Japan; Asakura Pub.: Tokyo, Japan, 1998. (In Japanese) [Google Scholar]
- Shimaji, K.; Itoh, T. Comprehensive List of Unearthed Wood Product from the Remains in Japan; Yuzankaku Pub.: Tokyo, Japan, 1988. (In Japanese) [Google Scholar]
- Suzuki, M. Japanese and Wood Culture; Yasaka Shobo: Tokyo, Japan, 2002. (In Japanese) [Google Scholar]
- Nakamura, K. Historical Study on Shitake Mushroom Culture; Tohsen Pub.: Tokyo, Japan, 1983. (In Japanese) [Google Scholar]
- Abrams, M.D.; Copenheaver, C.A.; Terazawa, K.; Umeki, K.; Takiya, M.; Akashi, N. A 370-year dendroecological history of an old-growth Abies-Acer-Quercus forest in Hokkaido, northern Japan. Can. J. For. Res. 1999, 29, 1891–1899. [Google Scholar] [CrossRef]
- Sakai, T.; Tanaka, H.; Shibata, M.; Suzuki, W.; Nomiya, H.; Kanazashi, T.; Iida, S.; Nakashizuka, T. Riparian disturbance and community structure of a Quercus-Ulmus forest in central Japan. Plant Ecol. 1999, 140, 99–109. [Google Scholar] [CrossRef]
- Kanno, M.; Yokoyama, J.; Suyama, Y.; Ohyama, M.; Itoh, T.; Suzuki, M. Geographical distribution of two haplotypes of chloroplast DNA in four oak species (Quercus) in Japan. J. Plant Res. 2004, 117, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Okaura, T.; Quang, N.D.; Ubukata, M.; Harada, K. Phylogeographic structure and late Quaternary population history of the Japanese oak Quercus mongolica var. crispula and related species revealed by chloroplast DNA variation. Genes Genet. Syst. 2007, 82, 465–477. [Google Scholar] [PubMed]
- Ohsawa, T.; Tsuda, Y.; Saito, Y.; Ide, Y. The genetic structure of Quercus crispula in northeastern Japan as revealed by nuclear simple sequence repeat loci. J. Plant Res. 2011, 124, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.L.; Oishi, R.; Miyashita, N.; Nara, K.; Nakaya, H.; Wu, B.Y.; Zhou, Z.H.; Hogetsu, T. Genetic structure and reproduction dynamics of Salix reinii during primary succession on Mount Fuji, as revealed by nuclear and chloroplast microsatellite analysis. Mol. Ecol. 2003, 12, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Deguilloux, M.F.; Dumolin-Lapegue, S.; Gielly, L.; Grivet, D.; Petit, R.J. A set of primers for the amplification of chloroplast microsatellites in Quercus. Mol. Ecol. Notes 2003, 3, 24–27. [Google Scholar] [CrossRef]
- Pons, O.; Petit, R.J. Mesuring and testing genetic differentiation with orderd versus unorderd allels. Genetics 1996, 14, 1237–1245. [Google Scholar]
- Estoup, A.; Jarne, P.; Cornuet, J.M. Homoplasy and mutation model at microsatellite loci and their consequences for population genetics analysis. Mol. Ecol. 2002, 11, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.J.; El Mousadik, A.; Pons, O. Identifying populations for conservation on the basis of genetic markers. Conserv. Biol. 1998, 12, 844–855. [Google Scholar] [CrossRef]
- Nei, M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. USA 1973, 70, 3321–3323. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, M. A measure of population subdivision based on microsatellite allele frequencies. Genetics 1995, 139, 457–462. [Google Scholar] [PubMed]
- Hedric, P.W. A standardized genetic differentiation measure. Evolution 2005, 59, 1633–1638. [Google Scholar] [CrossRef]
- Bryant, D.; Moulton, V. NeighborNet: An agglomerative method for the construction of planar phylogenetic networks. In Algorithms in Bioinformatics, Proceedings of WABI, Rome, Italy, 17–21 September 2002; Guigó, R., Gusfield, D., Eds.; Springer: Berlin, Germany, 2002; Volume 2452, pp. 375–391. [Google Scholar]
- Huson, D.H.; Bryant, D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.B.; Linares, A.R.; Feldman, M.W.; Cavallisforza, L.L. Genetic absolute dating based on microsatellites and origin of modern humans. Proc. Natl. Acad. Sci. USA 1995, 92, 6720–6727. [Google Scholar] [CrossRef]
- Langella, O. Population 1.2.30. Logiciel de Génétique des Populations. Laboratoire Populations, Génétique et Évolution, CNRS UPR 9034, Gif-sur-Yvette. Available online: http://www.cnrs-gif.fr/pge/ (accessed on 2 October 2009).
- Parks, D.H.; Mankowski, T.; Zangooei, S.; Porter, M.S.; Armanini, D.G.; Baird, D.J.; Langille, M.G.I.; Beiko, R.G. GenGIS 2: Geospatial Analysis of Traditional and Genetic Biodiversity, with New Gradient Algorithms and an Extensible Plugin Framework. PLoS ONE 2013, 8, e69885. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Ide, Y. Chloroplast DNA phylogeography of Betula maximowicziana, a long-lived pioneer tree species and noble hardwood in Japan. J. Plant Res. 2010, 123, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Heuertz, M.; Carnevale, S.; Fineschi, S.; Sebastiani, F.; Hausman, J.F.; Paule, L.; Vendramin, G.G. Chloroplast DNA phylogeography of European ashes, Fraxinus sp. (Oleaceae): Roles of hybridization and life history traits. Mol. Ecol. 2006, 15, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Tsuda, Y.; Uchiyama, K.; Fukuda, T.; Ide, Y. Genetic structure of Quercus variabilis in Japan. For. Genet. Tree Breed. 2017. in print. (In Japanese with English Summary). [Google Scholar]
- Sugahara, K.; Kaneko, Y.; Ito, S.; Yamanaka, K.; Sakio, H.; Hoshizaki, K.; Suzuki, W.; Yamanaka, N.; Setoguchi, H. Phylogeography of Japanese horse chestnut (Aesculus turbinata) in the Japanese Archipelago based on chloroplast DNA haplotypes. J. Plant Res. 2011, 124, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.S.; Fastie, C.; Hurtt, G.; Jackson, S.T.; Johnson, C.; King, G.A.; Lewis, M.; Lynch, J.; Pacala, S.; Prentice, C.; et al. Reid’s Paradox of Rapid Plant Migration: Dispersal theory and interpretation of paleoecological records. BioScience 1998, 48, 13–24. [Google Scholar] [CrossRef]
- Hashizume, H. Flowering habit in young trees of Quercus acutissima Carr. and Quercus serrata Thunb. Hardwood Res. 1983, 2, 49–54, (In Japanese with English Summary). [Google Scholar]
- Toda, T.; Fujimoto, Y.; Nishimura, K.; Maeda, T. Reserch of 18 year old plantation Quercus acutissima established by imported seed. Annu. Rep. Kyushu Reg. Breed. 1986, 13, 100–106. (In Japanese) [Google Scholar]
- Aguinagalde, I.; Hampe, A.; Mohanty, A.; Martin, J.P.; Duminil, J.; Petit, R.J. Effects of life-history traits and species distribution on genetic structure at maternally inherited markers in European trees and shrubs. J. Biogeogr. 2005, 32, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Duminil, J.; Fineschi, S.; Hampe, A.; Jordano, P.; Salvini, D.; Vendramin, G.G.; Petit, R.J. Can population genetic sturcture be predicted from life-history traits? Am. Nat. 2007, 169, 662–672. [Google Scholar] [PubMed]
- Sugiura, N.; Tang, D.; Kurokochi, H.; Saito, Y.; Ide, Y. Genetic structure of Quercus gilva Blume in Japan as revealed by chloroplast DNA sequences. Botany 2015, 93, 873–880. [Google Scholar] [CrossRef]
- Tomaru, N.; Takahashi, M.; Tsumura, Y.; Takahashi, M.; Ohba, K. Intraspecific variation and phylogeographic patterns of Fagus crenata (Fagaceae). Am. J. Bot. 1998, 85, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Okaura, T.; Harada, K. Phylogeographical structure revealed by chloroplast DNA variation in Japanese beech (Fagus crenata Blume). Heredity 2002, 88, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, M.; Yoshimaru, H.; Saito, H.; Katsuki, T.; Kawahara, T.; Kitamura, K.; Shi, F.; Kaji, M. Phylogeography of a northeast Asian spruce, Picea jezoensis, inferred from genetic variation observed in organelle DNA markers. Mol. Ecol. 2007, 16, 3393–3405. [Google Scholar] [CrossRef] [PubMed]
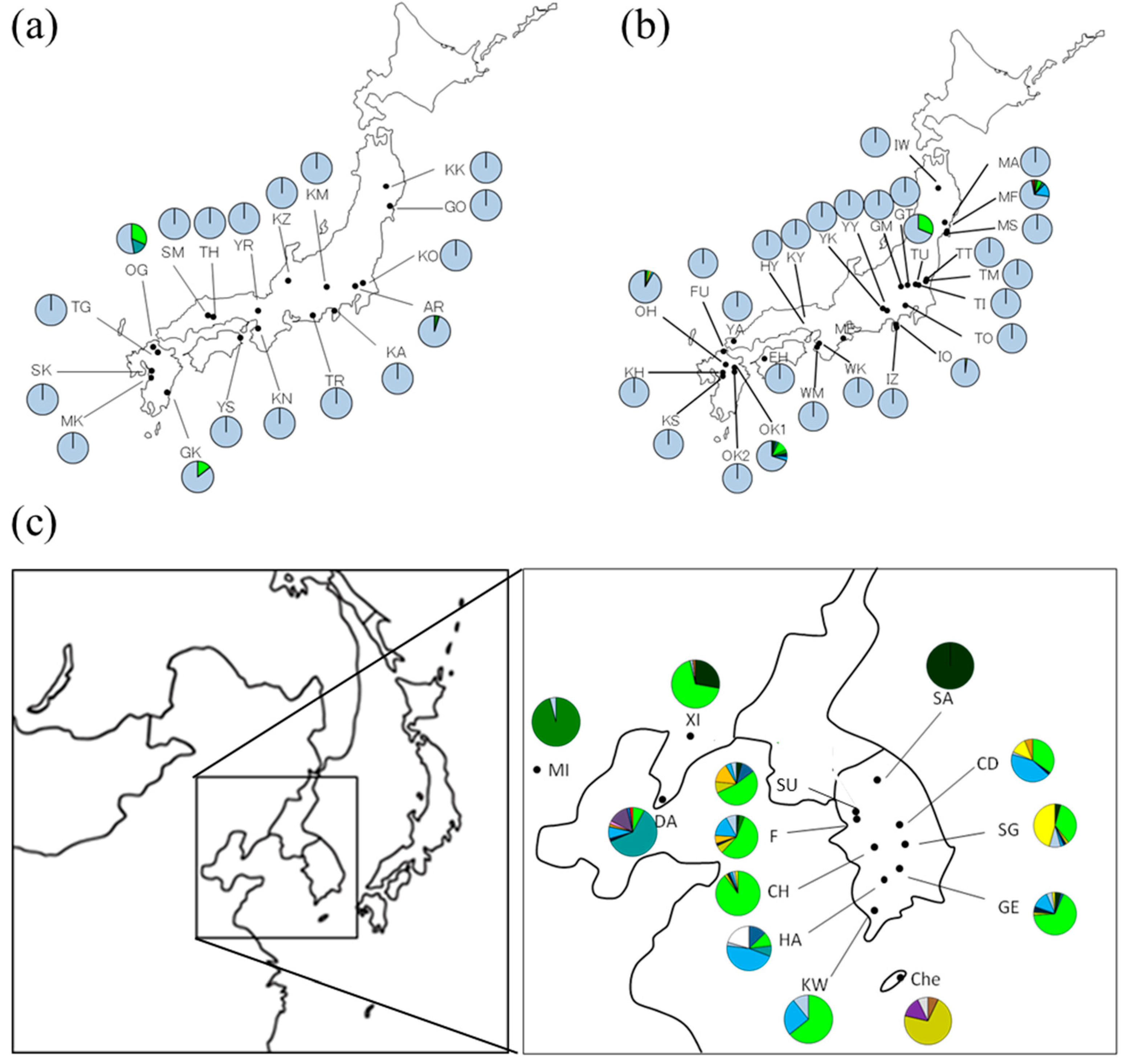
| Region | Forest Type | Code | Latitude (N) | Longitude (E) | Sample Size | Detected Haplotype |
|---|---|---|---|---|---|---|
| Japan | Natural | KK | 39°16′ | 141°07′ | 53 | 20 |
| GO | 38°45′ | 141°23′ | 16 | 20 | ||
| ARD | 36°07′ | 139°19′ | 41 | 6, 20 | ||
| KO | 36°02′ | 140°01′ | 32 | 20 | ||
| KM | 35°39′ | 138°30′ | 13 | 20 | ||
| KA | 35°10′ | 138°90′ | 4 | 20 | ||
| TR | 34°48′ | 137°49′ | 14 | 20 | ||
| KN | 34°14′ | 135°09′ | 15 | 20 | ||
| YR | 35°17′ | 135°19′ | 13 | 20 | ||
| KZ | 35°54′ | 136°38′ | 15 | 20 | ||
| YS | 34°06′ | 134°30′ | 13 | 20 | ||
| TH | 34°52′ | 133°33′ | 6 | 20 | ||
| SM | 34°53′ | 133°25′ | 12 | 20 | ||
| OG | 33°35′ | 130°39′ | 19 | 7, 9, 20 | ||
| TG | 33°20′ | 130°50′ | 8 | 20 | ||
| SK | 32°46′ | 130°36′ | 14 | 20 | ||
| MK | 32°42′ | 130°36′ | 13 | 20 | ||
| GK | 32°39′ | 131°25′ | 21 | 7,20 | ||
| Artifical | IW | 39°46′ | 141°08′ | 37 | 20 | |
| MF | 38°20′ | 140°59′ | 33 | 6, 7, 19, 20, 38 | ||
| MA | 38°38′ | 140°59′ | 49 | 20 | ||
| MS | 38°21′ | 141°00′ | 22 | 20 | ||
| TT | 36°31′ | 140°13′ | 46 | 20 | ||
| TM | 36°30′ | 140°12′ | 46 | 20 | ||
| TI | 36°29′ | 139°59′ | 48 | 20 | ||
| TU | 36°29′ | 139°55′ | 48 | 7, 20 | ||
| GT | 36°17′ | 139°04′ | 40 | 20 | ||
| GM | 36°16′ | 138°45′ | 48 | 20 | ||
| TO | 35°38′ | 139°18′ | 50 | 20 | ||
| IZ | 34°53′ | 139°06′ | 40 | 20 | ||
| IO | 35°01′ | 138°59′ | 50 | 4, 20 | ||
| YY | 35°30′ | 138°28′ | 50 | 20 | ||
| YK | 35°28′ | 138°30′ | 50 | 20 | ||
| ME | 34°21′ | 136°29′ | 50 | 20 | ||
| KY | 34°57′ | 135°32′ | 50 | 20 | ||
| HY | 34°55′ | 135°27′ | 46 | 20 | ||
| WK | 34°11′ | 135°17′ | 50 | 20 | ||
| WM | 34°02′ | 135°13′ | 31 | 20 | ||
| YA | 34°16′ | 131°18′ | 50 | 20 | ||
| EH | 33°37′ | 132°49′ | 50 | 20 | ||
| FU | 33°47′ | 130°53′ | 42 | 20 | ||
| OH | 33°19′ | 130°57′ | 48 | 3, 7, 16, 19, 20 | ||
| OK1 | 33°11′ | 131°16′ | 50 | 3, 5, 7, 10, 15, 20 | ||
| OK2 | 33°08′ | 131°16′ | 50 | 20 | ||
| KS | 33°02′ | 130°50′ | 48 | 20 | ||
| KH | 33°04′ | 130°53′ | 47 | 20 | ||
| Continent | Natural | SA | 37°36′ | 128°01′ | 41 | 3 |
| SU | 37°19′ | 127°01′ | 47 | 3, 5, 7, 12, 16, 19, 20 | ||
| F | 37°12′ | 126°59′ | 50 | 3, 6, 7, 12, 15, 16, 19, 20 | ||
| CD | 36°50′ | 127°57′ | 49 | 7, 15, 19, 20, 21, 26 | ||
| CH | 36°24′ | 127°15′ | 49 | 7, 12, 15, 19, 20, 21 | ||
| SG | 36°22′ | 128°08′ | 46 | 3, 7, 12, 15, 19, 20, 21 | ||
| GE | 35°44′ | 127°47′ | 46 | 3, 5, 7, 12, 15, 19, 20, 21 | ||
| HA | 35°38′ | 127°17′ | 39 | 5, 7, 9, 19, 20, 32 | ||
| KW | 35°09′ | 126°56′ | 28 | 7, 19, 20 | ||
| Che | 33°31′ | 126°32′ | 14 | 1, 26, 39, 41 | ||
| XI | 40°23′ | 123°18′ | 54 | 3, 7, 20, 26 | ||
| MI | 39°55′ | 116°24′ | 47 | 6, 20 | ||
| DA | 38°55′ | 121°21′ | 51 | 7, 9, 15, 19, 20, 26, 34, 36, 37, 40 |
| Haplotype | Maker | Haplotype Frequency * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| μcd4 (VIC) | μcd5 (FAM) | μdt1 (PET) | μdt3 (FAM) | μdt4 (PET) | μkk4 (NED) | Japan | Continent | Total | ||
| Natural | Artificial | |||||||||
| 20 | 101 | 81 | 90 | 129 | 139 | 115 | 0.957 | 0.965 | 0.037 | 0.7217 |
| 7 | 101 | 81 | 90 | 128 | 136 | 115 | 0.028 | 0.018 | 0.399 | 0.1190 |
| 19 | 101 | 81 | 90 | 129 | 138 | 115 | - | 0.006 | 0.121 | 0.0353 |
| 3 | 101 | 81 | 90 | 126 | 137 | 115 | - | 0.002 | 0.112 | 0.0307 |
| 6 | 101 | 81 | 90 | 127 | 137 | 115 | 0.006 | 0.002 | 0.084 | 0.0237 |
| 9 | 101 | 81 | 90 | 127 | 138 | 115 | 0.009 | 0.001 | 0.061 | 0.0177 |
| 21 | 102 | 82 | 91 | 128 | 137 | 115 | - | - | 0.052 | 0.0135 |
| 26 | 101 | 81 | 89 | 128 | 136 | 115 | - | - | 0.027 | 0.0070 |
| 5 | 100 | 81 | 90 | 127 | 138 | 115 | - | 0.001 | 0.020 | 0.0056 |
| 16 | 102 | 81 | 90 | 129 | 136 | 115 | - | 0.001 | 0.018 | 0.0051 |
| 12 | 102 | 81 | 90 | 128 | 136 | 115 | - | - | 0.018 | 0.0046 |
| 15 | 102 | 81 | 90 | 127 | 138 | 115 | - | 0.002 | 0.012 | 0.0042 |
| 32 | 102 | 81 | 90 | 129 | 138 | 115 | - | - | 0.014 | 0.0037 |
| 36 | 101 | 81 | 89 | 127 | 138 | 115 | - | - | 0.012 | 0.0033 |
| 10 | 101 | 81 | 90 | 128 | 137 | 115 | - | 0.002 | - | 0.0009 |
| 39 | 101 | 81 | 89 | 129 | 138 | 115 | - | - | 0.004 | 0.0009 |
| 4 | 101 | 81 | 90 | 127 | 136 | 115 | - | 0.001 | - | 0.0005 |
| 38 | 101 | 81 | 89 | 129 | 135 | 115 | - | 0.001 | - | 0.0005 |
| 1 | 101 | 81 | 89 | 128 | 136 | 113 | - | - | 0.002 | 0.0005 |
| 34 | 101 | 82 | 88 | 129 | 138 | 115 | - | - | 0.002 | 0.0005 |
| 37 | 101 | 81 | 89 | 128 | 139 | 115 | - | - | 0.002 | 0.0005 |
| 40 | 101 | 82 | 88 | 130 | 139 | 116 | - | - | 0.002 | 0.0005 |
| 41 | 101 | 81 | 90 | 130 | 139 | 116 | - | - | 0.002 | 0.0005 |
| Total number of allells | 3 | 2 | 4 | 5 | 5 | 3 | ||||
| Region | Forest Type | No. of Populations | Sample Size | As | hs | vs | ht | vt | GST | G‘ST | RST | NST |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Japan | Natural | 18 | 322 | 1.111 | 0.055 | 0.053 | 0.073 | 0.073 | 0.246 | 0.261 | 0.238 | 0.238 |
| (0.076) | (0.037) | (0.037) | (0.051) | (0.050) | ||||||||
| Japan | Artificial | 28 | 1269 | 1.122 | 0.059 | 0.057 | 0.072 | 0.072 | 0.180 | 0.191 | 0.201 | 0.179 |
| (0.061) | (0.029) | (0.028) | (0.035) | (0.035) | ||||||||
| Continent | Natural | 13 | 561 | 2.088 | 0.488 | 0.482 | 0.843 | 0.844 | 0.421 | 0.856 | 0.429 | 0.480 * |
| (0.159) | (0.067) | (0.063) | (0.054) | (0.099) |
| Species | Dispersal Type | No. of Populations | No. of Individuals | DNA | GST | G’ST | Reference |
|---|---|---|---|---|---|---|---|
| Quercus acutissima | G and A | 18 | 322 | C | 0.246 | 0.261 | This study |
| Quercus gilva | G and A | 25 | 135 | C | 0.668 | 0.754 | [55] |
| Quercus mongoloca | G and A | 33 | 501 | C | 0.857 | 0.979 * | [32] |
| Fagus crenata | G and A | 17 | 409 | M | 0.963 | 0.996 * | [57] |
| Fagus crenata | G and A | 21 | 351 | C | 0.95 | - | [58] |
| Betula maximowicziana | W | 25 | 400 | C | 0.950 | 0.977 | [47] |
| Picea jezoensis | W | 33 | 264 | M | 0.901 | 0.974 * | [59] |
| Prunus avium | G and A | 23 | 211 | C | 0.29 | 0.44 * | [5] |
| Castanea sativa | G and A | 38 | 181 | C | 0.43 | 0.70 * | [2] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, Y.; Tsuda, Y.; Uchiyama, K.; Fukuda, T.; Seto, Y.; Kim, P.-G.; Shen, H.-L.; Ide, Y. Genetic Variation in Quercus acutissima Carruth., in Traditional Japanese Rural Forests and Agricultural Landscapes, Revealed by Chloroplast Microsatellite Markers. Forests 2017, 8, 451. https://doi.org/10.3390/f8110451
Saito Y, Tsuda Y, Uchiyama K, Fukuda T, Seto Y, Kim P-G, Shen H-L, Ide Y. Genetic Variation in Quercus acutissima Carruth., in Traditional Japanese Rural Forests and Agricultural Landscapes, Revealed by Chloroplast Microsatellite Markers. Forests. 2017; 8(11):451. https://doi.org/10.3390/f8110451
Chicago/Turabian StyleSaito, Yoko, Yoshiaki Tsuda, Kentaro Uchiyama, Tomohide Fukuda, Yasuhiro Seto, Pan-Gi Kim, Hai-Long Shen, and Yuji Ide. 2017. "Genetic Variation in Quercus acutissima Carruth., in Traditional Japanese Rural Forests and Agricultural Landscapes, Revealed by Chloroplast Microsatellite Markers" Forests 8, no. 11: 451. https://doi.org/10.3390/f8110451





