Trailblazing the Carbon Cycle of Tropical Forests from Puerto Rico
Abstract
:1. Introduction
2. Foundational Research
3. Carbon Flux and Storage Studies at the Institute
- ➢
- ➢
- Large pools of carbon and nutrients in these forests tend to be belowground (mostly in soil), contrary to early beliefs that tropical forests stored most of their nutrients and mass aboveground [45].
- ➢
- Stand biomass of mature forest varies as much as five-fold depending on topographic position on the landscape [11].
- ➢
- Root biomass tends to be higher in mature forests compared to successional ones and higher in native forests compared to timber tree plantations of similar age [32].
- ➢
- Forested watershed export of carbon is proportional to runoff [29,46,47]. The export of organic matter from a floodplain forest at high elevation is high (35 g/m2·year; [29]) compared to forested tropical watersheds at lower elevations (2.2 to 15.5 g/m2·year). Forested watersheds in turn exhibit higher organic matter exports than intensively used watersheds (weighted average of 3.7 g/m2·year (range of 1.5 to 10.5 g/m2·year) for 14 intensively used watersheds) [47].
- ➢
- Secondary and novel forests accumulate aboveground biomass and nutrients at high rates, and circulate a large fraction of their net primary productivity to the forest floor [41,48,49]. Novel forests have a higher rate of litterfall and nutrient return to the forest floor than native forests in similar climates and soils [39,40,41,42,43].
- ➢
- Wood density increases with age and maturity of forest stands. For example, stand-weighted specific wood density increased by 3.9% (from 304 Kg C/m3 to 316 Kg C/m3) among dichotyledon trees in mature Cyrilla racemiflora L. forests over a 35-year period in the Luquillo Mountains [50].
- ➢
- Logging can be an atmospheric carbon source and its carbon effects can be mitigated, but not eliminated, through management such as reduced impact logging [36].
4. Estimating the Global Role of Tropical Forests in the Carbon Cycle
- ➢
- In mature tropical forests, carbon storage above and belowground was negatively correlated with the T/P ratio, (i.e., lower at drier locations (Figure 2)). Aboveground carbon storage peaked in moist tropical and subtropical forests and decreased towards the wet, rain, and dry forests, with dry forests exhibiting the lowest carbon density. Soil carbon storage was higher in wet and rain forests and declined towards dry forests.
- ➢
- While deforestation and subsequent agricultural land use caused reductions in soil organic carbon, land abandonment and plant succession accumulated soil organic carbon at rates of 0.3 to 0.5 Mg/ha·year over 40 to 100 years [71,76]. Past land use, life zone, and stage of succession influenced soil organic carbon accumulation with higher values in older and more structurally developed moist secondary forests [67].
- ➢
- ➢
- ➢
- Succession from pasture to forests was associated with reductions in pasture derived soil organic carbon (−0.4 Mg/ha·year) and increases in forest derived soil organic carbon of 0.9 Mg/ha·year with a net increase in soil carbon of 33 Mg/ha over 61 years [70].
- ➢
- Litterfall rate peaked in the moist forests and declined towards the wet, rain, and dry forests [3].
- ➢
- Rate of carbon storage in secondary forests is a function of age, peaking at about 20 years depending on the life zone [74].
- ➢
- Functional attributes related to organic matter production and circulation such as net primary productivity and leaf litterfall were up to ten times faster in secondary forests compared to mature forests [48].
- ➢
- Large trees, defined as those with a diameter at breast height equal or greater than 70 cm, contributed few stems, generally no more than 3% of stand tree density, but can account for more than 40% of the aboveground biomass. Total aboveground biomass of stands generally increased with increasing number of large trees. These results were first reported for the Brazilian Amazon [77] but were also found to be true for Southeast Asia [78], and southeastern United States [79]. Because of their longevity, big trees collectively operate as a large and slow carbon sink in forests.
- ➢
- The removal of large trees by legal or illicit felling lowers the biomass of stands and stimulates the growth of remaining trees for a limited period. This degradation process transforms mature forests into short-term successional forests with changes in rates of carbon sequestration without canopy opening. Any increase in carbon sequestration of residual trees, usually of a limited time period [80], does not make up for the loss of the felling of the large diameter trees. The assumption that closed forests are mature forests in carbon steady state is invalidated when those stands are experiencing net growth as a result of recovery from past disturbances [78].
- ➢
- As stands age and larger trees develop, their rate of carbon accumulation through succession can be larger than when they were younger [70].
- ➢
- Secondary forests accelerate the carbon cycle of tropical forests by turning over as much as 100 Mg/ha of biomass in a decade [48].
- ➢
- ➢
- Disturbances such as hurricanes accelerate the carbon cycle even more in secondary forests [83]. Hurricanes can also generate carbon sinks because initial biomass regeneration after the event can be faster than the decomposition of downed woody debris and burial of organic matter associated with landslides [84].
- ➢
- ➢
- Coarse woody debris (necromass) production in Amazon intact and logged forests can account for 14% to 19% of the forests’ annual carbon flux. The residence time of this necromass is 4.2 year. However, the amount of necromass in the carbon cycle of a forest cannot be accurately estimated from tree mortality data [38].
5. Tropical Forests: Carbon Sources or Sinks?
6. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holdridge, L.R. Determination of the world plant formations from simple climatic data. Science 1947, 105, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Holdridge, L.R. Life Zone Ecology; Tropical Science Center: San José, Costa Rica, 1967. [Google Scholar]
- Brown, S.; Lugo, A.E. The storage and production of organic matter in tropical forests and their role in the global carbon cycle. Biotropica 1982, 14, 161–187. [Google Scholar] [CrossRef]
- Wadsworth, F.H. A review of past research in the Luquillo Mountains. In A tropical Rain Forest; Odum, H.T., Pigeon, R.F., Eds.; United States Atomic Energy Commission (AEC), Division of Technical Information: Oak Ridge, TN, USA, 1970; pp. B33–B46. [Google Scholar]
- Wadsworth, F.H. A forest research institution in the West Indies: The first 50 years. In Tropical forests: Management and Ecology; Lugo, A.E., Lowe, C., Eds.; Springer: New York, NY, USA, 1995; pp. 33–56. [Google Scholar]
- Little, E.L.; Wadsworth, F.H. Common Trees of Puerto Rico and the Virgin Islands; Agriculture Handbook 249; USDA Forest Service: Washington, DC, USA, 1964.
- Little, E.L.; Woodbury, R.O.; Wadsworth, F.H. Trees of Puerto Rico and the Virgin Islands; USDA Forest Service, Agriculture Handbook 449; United States Department of Agriculture (USDA): Washington, DC, USA, 1974; Volume 2.
- Longwood, F.R. Puerto Rican Woods: Their Machining, Seasoning, and Related Characteristics; United States Department of Agriculture Forest Service, Agricultural Handbook 205; United States Department of Agriculture (USDA): Washington, DC, USA, 1961.
- Reyes, G.; Brown, S.; Chapman, J.; Lugo, A.E. Wood Densities of Tropical Tree Species; USDA Forest Service, General Technical Report SO-88; Southern Forest Experiment Station: New Orleans, LA, USA, 1992.
- Odum, H.T. Summary: An emerging view of the ecological systems at El Verde. In A Tropical Rain Forest; Odum, H.T., Pigeon, R.F., Eds.; National Technical Information Service: Springfield, VA, USA, 1970; pp. I191–I289. [Google Scholar]
- Ovington, J.D.; Olson, J.S. Biomass and chemical content of El Verde lower montane rain forest plants. In A Tropical Rain Forest. A Study of Irradiation and Ecology at El Verde, Puerto Rico; Odum, H.T., Pigeon, R.F., Eds.; National Technical Information Service: Springfield, VA, USA, 1970; pp. H53–H77. [Google Scholar]
- Weaver, P.L.; Gillespie, A.J.R. Tree biomass equations for the forests of the Luquillo Mountains, Puerto Rico. Common. For. Rev. 1992, 71, 35–39. [Google Scholar]
- Brandeis, T.J.; del Rocio, M.R.; Rozo, S. Effects of model choice and forest structure on inventory-based estimations of Puerto Rican forest biomass. Caribb. J. Sci. 2005, 41, 250–268. [Google Scholar]
- Wadsworth, F.H. The development of the forest land resources of the Luquillo Mountains of Puerto Rico. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 1949. [Google Scholar]
- Briscoe, C.B.; Wadsworth, F.H. Stand structure and yield in the tabonuco forest of Puerto Rico. In A Tropical Rain Forest: A Study of Irradiation and Ecology at El Verde, Puerto Rico; Odum, H.T., Pigeon, R.F., Eds.; National Technical Information Service: Springfield, VA, USA, 1970. [Google Scholar]
- Wadsworth, F.H. Growth in the lower montane rain forest of Puerto Rico. Caribb. For. 1947, 8, 27–43. [Google Scholar]
- Weaver, P.L. Tree Growth in Several Tropical Forests of Puerto Rico; USDA Forest Service Research Paper SO-152; Southern Forest Experiment Station: New Orleans, LA, USA, 1979.
- Wadsworth, F.H.; Parresol, B.R.; Figueroa Colón, J.C. Tree increment indicators in a subtropical wet forest. In Proceedings of Seminar on Growth and Yield in Tropical Mixed/Moist Forests; Wan Razali, W.M., Chan, H.T., Appanah, S., Eds.; Forest Research Institute: Kuala Lumpur, Malaysia, 1989; pp. 205–212. [Google Scholar]
- Brown, S.; Lugo, A.E.; Silander, S.; Liegel, L. Research History and Opportunities in the Luquillo Experimental Forest; USDA Forest Service, Southern Forest Experiment Station, General Technical Report SO-44; United States Department of Agriculture (USDA): New Orleans, LA, USA, 1983.
- Francis, J.K. Forest plantations in Puerto Rico. In Tropical Forests: Management and Ecology; Lugo, A.E., Lowe, C.A., Eds.; Springer: New York, NY, USA, 1995; pp. 210–223. [Google Scholar]
- Golley, F.B.; Odum, H.T.; Wilson, R.F. The structure and metabolism of a Puerto Rican red mangrove forest in May. Ecology 1962, 43, 9–19. [Google Scholar] [CrossRef]
- Odum, H.T. Man and the ecosystem. In Lockwood Conference on the Suburban Forest and Ecology; The Connecticut Agricultural Experiment Station: New Haven, CN, USA, 1962; pp. 57–75. [Google Scholar]
- Odum, H.T.; Copeland, B.J.; Brown, R.Z. Direct and optical assay of leaf mass of the lower montane rain forest of Puerto Rico. Proc. Natl. Acad. Sci. USA 1963, 49, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Odum, H.T.; Abbott, W.; Selander, R.K.; Golley, F.B.; Wilson, R.F. Estimates of chlorophyll and biomass of the tabonuco forest of Puerto Rico. In A Tropical Rain Forest a Study of Irradiation and Ecology at El Verde, Puerto Rico; Odum, H.T., Pigeon, R.F., Eds.; National Technical Information Service: Springfield, VA, USA, 1970; pp. I3–I19. [Google Scholar]
- Odum, H.T.; Pigeon, R.F. A Tropical Rain Forest; National Technical Information Service: Springfield, VA, USA, 1970. [Google Scholar]
- Lugo, A.E.; González Liboy, J.A.; Cintrón, B.; Dugger, K. Structure, productivity, and transpiration of a subtropical dry forest in Puerto Rico. Biotropica 1978, 10, 278–291. [Google Scholar] [CrossRef]
- Murphy, P.G.; Lugo, A.E. Structure and biomass of a subtropical dry forest in Puerto Rico. Biotropica 1986, 18, 89–96. [Google Scholar] [CrossRef]
- Murphy, P.G.; Lugo, A.E.; Murphy, A.J.; Nepstad, D.C. The dry forests of Puerto Rico’s south coast. In Tropical Forests: Management and Ecology; Lugo, A.E., Lowe, C., Eds.; Springer: New York, NY, USA, 1995; pp. 178–209. [Google Scholar]
- Frangi, J.L.; Lugo, A.E. Ecosystem dynamics of a subtropical floodplain forest. Ecol. Monogr. 1985, 55, 351–369. [Google Scholar] [CrossRef]
- Wang, D.; Bormann, F.H.; Lugo, A.E.; Bowden, R.D. Comparison of nutrient-use efficiency and biomass production in five tropical tree taxa. For. Ecol. Manag. 1991, 46, 1–21. [Google Scholar] [CrossRef]
- Lugo, A.E.; Wang, D.; Bormann, F.H. A comparative analysis of biomass production in five tropical tree species. For. Ecol. Manag. 1990, 31, 153–166. [Google Scholar] [CrossRef]
- Lugo, A.E. Comparison of tropical tree plantations with secondary forests of similar age. Ecol. Monogr. 1992, 62, 1–41. [Google Scholar] [CrossRef]
- Keller, M.; Palace, M.; Hurtt, G.E. Biomass estimation in the Tapajos National Forest, Brazil: Examination of sampling and allometric uncertainties. For. Ecol. Manag. 2001, 154, 371–382. [Google Scholar] [CrossRef]
- Lefsky, M.A.; Harding, D.J.; Keller, M.; Cohen, W.B.; Carabajal, C.C.; Del Espirito-Santo, F.B.; Hunter, M.O.; de Oliveira, R. Estimates of forest canopy height and aboveground biomass using ICESat. Geophys. Res. Lett. 2005, 33. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, D.; Keller, M.; dos-Santos, M.; Bolfe, E.; Feng, Y.; Wang, C. Modeling and mapping agroforestry aboveground biomass in the Brazilian Amazon using airborne LIDAR data. Remote Sens. 2015, 8, 21. [Google Scholar] [CrossRef]
- Keller, M.; Asner, G.P.; Silva, N.; Palace, M. Sustainability of selective logging of upland forests in the Brazilian Amazon: Carbon budgets and remote sensing as tools for evaluating logging effects. In Working Forests in the Neotropics: Conservation Through Sustainable Management? Zarin, D.J., Alavalapati, J.R.R., Putz, F.E., Schmink, M., Eds.; Columbia University Press: New York, NY, USA, 2004; pp. 41–63. [Google Scholar]
- Palace, M.; Keller, M.; Asner, G.P.; Silva, J.N.M.; Passos, C. Necromass in undisturbed and logged forests in the Brazilian Amazon. For. Ecol. Manag. 2007, 238, 309–318. [Google Scholar] [CrossRef]
- Palace, M.; Keller, M.; Silva, H. Necromass production: Studies in undisturbed and logged Amazon forests. Ecol. Appl. 2008, 18, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Lugo, A.E.; da-Silva, J.F.; Sáez-Uribe, A. Balance de carbono en un bosque de Castilla elastica: Resultados preliminares. Acta Cient. 2008, 22, 13–28. [Google Scholar]
- Lugo, A.E.; Abelleira, O.J.; Collado, A.; Viera, C.A.; Santiago, C.; Vélez, D.O.; Soto, E.; Amaro, G.; Charón, G.; Colón, H.; et al. Allometry, biomass, and chemical content of novel African tulip tree (Spathodea campanulata) forests in Puerto Rico. New For. 2011, 42, 267–283. [Google Scholar] [CrossRef]
- Lugo, A.E.; Martínez, O.A.; da Silva, J.F. Aboveground biomass, wood volume, nutrient stocks and leaf litter in novel forests compared to native forests and tree plantations in Puerto Rico. Bois For. Trop. 2012, 314, 7–16. [Google Scholar]
- Abelleira Martínez, O.J. Flooding and profuse flowering result in high litterfall in novel Spathodea campanulata forests in northern Puerto Rico. Ecosphere 2011, 2, 105. [Google Scholar]
- Da Silva, J.F. Ecophysiology and Productivity of Castilla Elastica, an Introduced Tropical Tree Species. Master’s Thesis, University of Puerto Rico, Rio Piedras, Puerto Rico, 2011. [Google Scholar]
- Del Arroyo, G.; Santiago, O. Soil Respiration of a Novel Subtropical Moist Forest: From Diel to Seasonal Patterns. Master’s Thesis, University of Puerto Rico, San Juan, Puerto Rico, 2014. [Google Scholar]
- Lugo, A.E.; Brown, S. Tropical lands: Popular mis2014 conceptions. Mazingira 1981, 5, 10–19. [Google Scholar]
- Lugo, A.E. Organic carbon export by riverine waters of Spain. In Transport of Carbon and Minerals in Major World Rivers; Degens, E.T., Kempe, S., Soliman, H., Eds.; University of Hamburg: Hamburg, Germany, 1983; pp. 267–279. [Google Scholar]
- Lugo, A.E.; Quiñones, F. Organic carbon export from intensively used watersheds in Puerto Rico. In Transport of Carbon and Minerals in Major World Rivers; Degens, E.T., Kempe, S., Soliman, H., Eds.; University of Hamburg: Hamburg, Germany, 1983; pp. 237–242. [Google Scholar]
- Brown, S.; Lugo, A.E. Tropical secondary forests. J. Trop. Ecol. 1990, 6, 1–32. [Google Scholar] [CrossRef]
- Lugo, A.E.; Domínguez Cristóbal, C.; Santos, A.; Torres Morales, E. Nutrient return and accumulation in litter of a secondary forest in the coffee region of Puerto Rico. Acta Cient. 1999, 13, 43–74. [Google Scholar]
- Weaver, P.L. The colorado and dwarf forests of Puerto Rico’s Luquillo Mountainsin. In Tropical Forests: Management and Ecology; Lugo, A.E., Lowe, C., Eds.; Springer: New York, NY, USA, 1995; pp. 109–141. [Google Scholar]
- Woodwell, G.M.; Whittaker, R.H.; Reiners, W.A.; Likens, G.E.; Delwiche, C.S.; Botkin, D.B. The biota and the world carbon budget. Science 1978, 199, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Broecker, W.S.; Takahashi, T.; Simpson, H.J.; Peng, T.P. Fate of fossil fuel carbon dioxide and the global carbon budget. Science 1979, 206, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.H.; Likens, G.E. Carbon in the biota. In Carbon and the Biosphere; Technical Information Center: Springfield, VA, USA, 1973; pp. 281–302. [Google Scholar]
- Ajtay, G.L.; Ketner, P.; Duvigneaud, P. Terrestrial primary production and phytomass. In The Global Carbon Cycle; Bolin, B., Degens, E.T., Kempe, S., Ketner, P., Eds.; John Wiley & Sons: Chichester, UK, 1979; pp. 129–181. [Google Scholar]
- Woodwell, G.M.; Hobbie, J.E.; Houghton, R.A.; Melillo, J.M.; Moore, B.; Peterson, B.J.; Shaver, G.R. Global deforestation: Contribution to atmospheric carbon dioxide. Science 1983, 222, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.T.; Rodhe, H.; Oeschger, H.; Siegenthaler, U. Greehouse gases and aerosols. In Cimate Change. The IPCC Scientific Assessment; Houghton, J.T., Jenkins, G.J., Ephraums, J.J., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 1–40. [Google Scholar]
- Brown, S.; Lugo, A.E. Biomass of tropical forests: A new estimate based on forest volumes. Science 1984, 223, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Gillespie, A.J.R.; Lugo, A.E. Biomass estimation methods for tropical forests with applications to forest inventory data. For. Sci. 1989, 35, 881–902. [Google Scholar]
- Brown, S. Estimating Biomass and Biomass Change of Tropical Forests: A Primer; FAO Forestry Paper 134; Food and Agriculture Organization of the United Nations: Rome, Italy, 1997. [Google Scholar]
- Brown, S.; Gillespie, A.J.R.; Lugo, A.E. Use of forest inventory data for biomass estimation of tropical forests. In Global Natural Resource Monitoring and Assessments: Preparing for the 21st Century; Lund, H.G., Preto, G., Eds.; American Society for Photogrammetry and Remote Sensing: Bethesda, MD, USA, 1990; pp. 1046–1055. [Google Scholar]
- Gillespie, A.J.R.; Brown, S.; Lugo, A.E. Biomass estimates for tropical forests based on existing inventory data. In State-of-the-Art Methodology of Forest Inventory: A Symposium Proceedings; Bau, V.J.L., Cunia, T., Eds.; USDA Forest Service Pacific Northwest Research Station General Technical Report PNW-GTR-263; United States Department of Agriculture (USDA): Portland, OR, USA, 1990; pp. 246–253. [Google Scholar]
- Gillespie, A.J.R.; Brown, S.; Lugo, A.E. Tropical forest biomass estimation from truncated stand tables. For. Ecol. Manag. 1992, 48, 69–87. [Google Scholar] [CrossRef]
- Cairns, M.A.; Brown, S.; Helmer, E.H.; Baumgardner, G.A. Root biomass allocation in the world’s upland forests. Oecologia 1997, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lugo, A.E.; Schmidt, R.; Brown, S. Preliminary estimates of storage and production of stemwood and organic matter in tropical tree plantations. In Wood Production in the Neotropics via Plantations; Whitmore, J.L., Ed.; IUFRO/MAB/Forest Service Symposium: Washington, DC, USA, 1981; pp. 8–17. [Google Scholar]
- Lugo, A.E.; Brown, S.; Chapman, J. An analytical review of production rates and stemwood biomass of tropical forest plantations. For. Ecol. Manag. 1988, 23, 179–200. [Google Scholar] [CrossRef]
- Weaver, P.L.; Birdsey, R.A.; Lugo, A.E. Soil organic matter in secondary forests of Puerto Rico. Biotropica 1987, 19, 17–23. [Google Scholar] [CrossRef]
- Lugo, A.E.; Cuevas, E.; Sanchez, M.J. Nutrients and mass in litter and top soil of ten tropical tree plantations. Plant Soil 1990, 125, 263–280. [Google Scholar] [CrossRef]
- Cuevas, E.; Brown, S.; Lugo, A.E. Above and belowground organic matter storage and production in a tropical pine plantation and a paired broadleaf secondary forest. Plant Soil 1991, 135, 257–268. [Google Scholar] [CrossRef]
- Silver, W.L.; Kueppers, L.M.; Lugo, A.E.; Ostertag, R.; Matzek, V. Carbon sequestration and plant community dynamics following reforestation of tropical pasture. Ecol. Appl. 2004, 14, 1115–1127. [Google Scholar] [CrossRef]
- Lugo, A.E.; Sánchez, M.J.; Brown, S. Land use and organic carbon content of some subtropical soils. Plant Soil 1986, 96, 185–196. [Google Scholar] [CrossRef]
- Beinroth, F.H.; Vázquez, M.A.; Snyder, V.A.; Reich, P.F.; Pérez Alegría, L.R. Factors Controlling Carbon Sequestration in Tropical Soils: A Case Study of Puerto Rico; University of Puerto Rico at Mayagüez and USDA Natural Resources Conservation Service: Mayagüez, Puerto Rico, 1996.
- Brown, S.; Glubczynski, A.; Lugo, A.E. Effects of land use and climate on the organic carbon content of tropical forest soils in Puerto Rico. In Proceedings of the Convention of the Society of American Foresters; Society of American Foresters: Washington, DC, USA; Portland, Oregon, OR, USA, 1984; pp. 204–209. [Google Scholar]
- Brown, S.; Lugo, A.E. Effects of forest clearing and succession on the carbon and nitrogen content of soils in Puerto Rico and US Virgin Islands. Plant Soil 1990, 124, 53–64. [Google Scholar] [CrossRef]
- Lugo, A.E.; Brown, S. Management of tropical soils as sinks or sources of atmospheric carbon. Plant Soil 1993, 149, 27–41. [Google Scholar] [CrossRef]
- Silver, W.L.; Ostertag, R.; Lugo, A.E. The potential for carbon sequestration through reforestation of abandoned tropical agricultural and pasture lands. Restor. Ecol. 2000, 8, 394–407. [Google Scholar] [CrossRef]
- Brown, S.; Lugo, A.E. Above ground biomass estimates for tropical moist forests of the Brazilian Amazon. Interciencia 1992, 17, 8–18. [Google Scholar]
- Brown, S.; Gillespie, A.J.R.; Lugo, A.E. Biomass of tropical forests of southeast Asia. Can. J. For. Res. 1991, 21, 111–117. [Google Scholar] [CrossRef]
- Brown, S.; Schroeder, P.; Birdsey, R. Aboveground biomass distribution of US Eastern hardwood forests and the use of large trees as an indicator of forest development. For. Ecol. Manag. 1997, 96, 37–47. [Google Scholar] [CrossRef]
- Pearson, T.R.H.; Brown, S.; Casarim, F.M. Carbon emissions from tropical forest degradation caused by logging. Environ. Res. Lett. 2014, 9, 034017. [Google Scholar] [CrossRef]
- Brown, S.; Lugo, A.E.; Chapman, J. Biomass of tropical tree plantations and its implications for the global carbon budget. Can. J. For. Res. 1986, 16, 390–394. [Google Scholar] [CrossRef]
- Keenan, R.J.; Reams, G.A.; Achard, F.; de-Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Lugo, A.E.; Domínguez Cristóbal, C.; Méndez, N. Hurricane Georges accelerated litterfall fluxes of a 26-year-old novel secondary forest in Puerto Rico. In Recent Hurricane Research: Climate, Dynamics, and Societal Impacts; Lupo, A.R., Ed.; InTech: Rijeka, Croatia, 2011; pp. 535–554. [Google Scholar]
- Lugo, A.E. Visible and invisible effects of hurricanes on forest ecosystems: an international review. Austral Ecol. 2008, 33, 368–398. [Google Scholar] [CrossRef]
- Scatena, F.N.; Moya, S.; Estrada, C.; Chinea, J.D. The first five years in the reorganization of aboveground biomass and nutrient use following Hurricane Hugo in the Bisley Experimental Watersheds, Luquillo Experimental Forest, Puerto Rico. Biotropica 1996, 28, 424–440. [Google Scholar] [CrossRef]
- Molina Colón, S.; Lugo, A.E. Recovery of a subtropical dry forest after abandonment of different land uses. Biotropica 2006, 38, 354–364. [Google Scholar] [CrossRef]
- Lugo, A.E.; Heartsill Scalley, T. Research in the Luquillo Experimental Forest has advanced understanding of tropical forests and resolved management issues. In USDA Forest Service Experimental Forests and Ranges: Research for the Long-Term; Hayes, D.C., Stout, S.L., Crawford, R.H., Hoover, A.P., Eds.; Springer: New York, NY, USA, 2014; pp. 435–461. [Google Scholar]
- Aide, T.M.; Zimmerman, J.K.; Pascarella, J.B.; Rivera, L.; Marcano-Vega, H. Forest regeneration in a chronosequence of tropical abandoned pastures: Implications for restoration. Restor. Ecol. 2000, 8, 328–338. [Google Scholar] [CrossRef]
- Zon, R.; Sparhawk, W.N. Forest Resources of the World; McGraw-Hill Book Co.: New York, NY, USA, 1923. [Google Scholar]
- Food and Agriculture Organization (FAO). Forest Resources Assessment 1990: Tropical Countries; Food and Agriculture Organization Forestry Paper 112; Food and Agriculture Organization (FAO): Rome, Italy, 1993. [Google Scholar]
- Food and Agriculture Organization (FAO). Global Forest Resources Assessment 2015: How Are the World’s Forests Changing? Food and Agriculture Organization: Rome, Italy, 2016. [Google Scholar]
- Lugo, A.E.; Brown, S. Steady state ecosystems and the global carbon cycle. Vegetatio 1986, 68, 83–90. [Google Scholar]
- Lugo, A.E. Are tropical forest ecosystems sources or sinks of carbon? In The Role of Tropical Forests on the world Carbon Cycle; Brown, S., Lugo, A.E., Liegel, B., Eds.; CONF-800350 UC-11; U.S. Department of Energy, National Technical Information Service: Springfield, VA, USA, 1980; pp. 1–18. [Google Scholar]
- Lugo, A.E.; Brown, S. Tropical forest ecosystems: Sources or sinks of atmospheric carbon? Unasylva 1980, 32, 8–13. [Google Scholar]
- Lugo, A.E.; Brown, S. Ecological issues associated with the interpretation of atmospheric CO2 data. In The Role of Tropical Forests on the World Carbon Cycle; Brown, S., Lugo, A.E., Liegel, B., Eds.; CONF-800350 UC-11; U.S. Department of Energy, National Technical Information Service: Springfield, VA, USA, 1980; pp. 30–43. [Google Scholar]
- Brown, S.; Lugo, A.E. The Role of Terrestrial Biota in the Global CO2 Cycle; American Chemical Society (ACS) Division of Petroleum Inc. Preprints: San Diego, CA, USA, 1981; Volume 26, pp. 1019–1025. [Google Scholar]
- Brown, S.; Gertner, G.Z.; Lugo, A.E.; Novak, J.M. Carbon dioxide dynamics of the biosphere. In Energy and Ecological Modelling; Mitsch, W.J., Bosserman, R.W., Klopatek, J.M., Eds.; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1981; pp. 19–28. [Google Scholar]
- Lugo, A.E. Influence of green plants on the world carbon budget. In Alternative Energy Sources V. Part E: Nuclear/Conservation/Environment; Veziroglu, T.N., Ed.; Elsevier Science Publishers B.V. Hemisphere Publishing Corporation: Amsterdam, The Netherlands, 1983; pp. 391–398. [Google Scholar]
- Lugo, A.E.; Brown, S. Tropical forests as sinks of atmospheric carbon. For. Ecol. Manag. 1992, 54, 239–255. [Google Scholar] [CrossRef]
- Brown, S.; Lugo, A.E.; Wisniewski, J. Missing carbon dioxide. Science 1992, 257, 11. [Google Scholar]
- Wisniewski, J.; Lugo, A.E. Natural sinks of CO2. Water Air Soil Pollut. 1992, 64, 1–463. [Google Scholar]
- Wisniewski, J.; Sampson, R.N. Terrestrial biospheric carbon fluxes: Quantification of sinks and sources of CO2. Water Air Soil Pollut. 1993, 70, 1–696. [Google Scholar]
- Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; The University of Chicago Press: Chicago, IL, USA, 2014. [Google Scholar]
- Luyssaert, S.; Schulze, E.-D.; Börner, A.; Knohl, A.; Hessesmöler, D.; Law, B.E.; Ciais, P.; Grace, J. Old-growth forests as global carbon sinks. Nature 2008, 455, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Lopez Gonzalez, G.; Sonké, B.; Affum-Baffoe, K.; Baker, T.R.; Ojo, L.O.; Phillips, O.L.; Reitsma, J.M.; White, L.; Comiskey, J.A.; et al. Increasing carbon storage in intact African tropical forests. Nat. Lett. 2009, 457, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Espírito Santo, F.D.B.; Gloor, M.; Keller, M.; Malhi, Y.; Saatchi, S.; Nelson, B.; Junior, R.C.; Pereira, C.; Lloyd, J.; Frolking, S.; Palace, M.; et al. Size and frequency of natural forest disturbances and the Amazon forest carbon balance. Nat. Commun. 2014, 5, 3434. [Google Scholar] [PubMed]
- Phillips, O.L.; Brienen, R.J.W. Carbon uptake by mature Amazon forests has mitigated Amazon nation’s carbon emissions. Carbon Balance Manag. 2017, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A. Are tropical forests an important carbon sink? Reanalysis of the long-term plot data. Ecol. Appl. 2002, 12, 3–7. [Google Scholar] [CrossRef]
- Phillips, O.L.; Mahli, Y.; Higuchi, N.; Laurance, W.F.; Nunez, P.V.; Vazquez, R.M.; Laurance, S.G.; Ferreira, L.V.; Stern, M.; Brown, S.; et al. Changes in the carbon balance of tropical forests: Evidence from long-term plots. Science 1998, 282, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Brienen, R.J.W.; Phillips, O.L.; Feldpausch, T.R.; Gloor, E.; Baker, T.R.; Lloyd, J.; Lopez-Gonzalez, G.; Monteagudo-Mendoza, A.; Malhi, Y.; Lewis, S.L.; et al. Long-term decline of the Amazon carbon sink. Nature 2015, 519, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A. Sources or sinks? The responses of tropical forests to current and future climate and atmospheric composition. Philos. Trans. R. Soc. 2004, 359, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A. Tropical forests and global warming: Slowing it down or speeding it up? Front. Ecol. Environ. 2004, 2, 73–80. [Google Scholar] [CrossRef]
- Cramer, W.; Bondeau, A.; Schaohoff, S.; Lucht, W.; Smith, B.; Sitch, S. Tropical forests and the global carbon cycle: Impacts of atmospheric carbon dioxide, climate change and rate of deforestation. Philos. Trans. R. Soc. B 2004, 359, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Dutra Aguilar, A.P.; Guimarães Vieira, I.C.; Oliveira Assis, T.; Dalla-Nora, E.L.; Toledo, P.M.; Santos-Junior, R.A.O.; Batistella, M.; Coelho, A.S.; Savaget, E.K.; Nobre, C.A.; et al. Land use change emission scenarios: Anticipating a forest transition process in the Brazilian Amazon. Glob. Chang. Biol. 2016, 22, 1821–1840. [Google Scholar]
- Gloor, M.; Phillips, O.L.; Lloyd, J.J.; Lewis, S.L.; Malhi, Y.; Baker, T.R.; Lopez-Gonzalez, G.; Peacock, J.; Almeida, S.; Alves de Oliveiraet, A.C.; et al. Does the disturbance hypothesis explain the biomass increase in basin-wide Amazon forest plot data? Glob. Chang. Biol. 2009, 15, 2418–2430. [Google Scholar] [CrossRef]
- Phillips, O.L.; Lewis, S.L.; Baker, T.R.; Chao, K.J.; Higuchi, N. The changing Amazon forest. Philos. Trans. R. Soc. B 2008, 363, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Willcock, S.; Phillips, O.L.; Platts, P.J.; Swetnam, R.D.; Balmford, A.; Burgess, N.D.; Ahrends, A.; Bayliss, J.; Doggart, N.; Doody, K.; et al. Land cover change and carbon emissions over 100 years in an African biodiversity hotspot. Glob. Chang. Biol. 2016, 22, 2787–2800. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.R.H.; Brown, S.; Murray, L.; Sidman, G. Greenhouse gas emissions from tropical forest degradation: An underestimated source. Carbon Balance Manag. 2017, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Phillips, O.L.; Martínez, R.V.; Arroyo, L.; Baker, T.R.; Killeen, T.; Lewis, S.L.; Malhi, Y.; Monteagudo Mendoza, A.; Neill, D.; Núñez Vargas, P.; et al. Increasing dominance of large lianas in Amazonian forests. Nature 2002, 418, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, D.; Malhi, Y.; Affum-Baffoe, K.; Castanho, A.D.A.; Doughty, C.E.; Fisher, R.A.; Lewis, S.L.; Peh, K.S.-H.; Phillips, O.L.; Quesada, C.A.; et al. Residence times of woody biomass in tropical forests. Plant Ecol. Divers. 2013, 6, 139–157. [Google Scholar] [CrossRef]
- Johnson, M.O.; Galbraith, D.; Gloor, M.; De Deurwaerder, H.; Guimberteau, M.; Rammig, A.; Thonicke, K.; Verbeeck, H.; von Randow, C.; Monteagudo, A.; et al. Variation in stem mortality rates determines patterns of above-ground biomass in Amazonian forests: Implications for dynamic global vegetation models. Glob. Chang. Biol. 2016, 22, 3996–4013. [Google Scholar] [CrossRef] [PubMed]
- Blanc, L.; Echard, M.; Herault, B.; Bonal, D.; Marcon, E.; Chave, J.; Baraloto, C. Dynamics of aboveground carbon stocks in a selectively logged tropical forest. Ecol. Appl. 2009, 19, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A. Detecting tropical forests responses to global climatic and atmospheric change: Current challenges and a way forward. Biotropica 2007, 39, 4–19. [Google Scholar] [CrossRef]
- Clark, D.B.; Clark, D.A.; Oberbauer, S.F. Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. Glob. Chang Biol. 2010, 16, 747–759. [Google Scholar] [CrossRef]
- Clark, D.A.; Clark, D.B.; Oberbauer, S.F. Field-quantified responses of tropical rainforest aboveground productivity to increasing CO2 and climate stress, 1997–2009. J. Geophys. Res. Biogeosci. 2013, 118, 783–794. [Google Scholar] [CrossRef]
- Clark, D.A.; Piper, S.C.; Keeling, C.D.; Clark, D.B. Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. Proc. Natl. Acad. Sci. USA 2003, 100, 5852–5857. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Doughty, C.E.; Goldsmith, G.R.; Metcalfe, D.B.; Girardin, C.A.; Marthews, T.R.; del Aguila-Pasquel, J.; Aragão, L.E.; Araujo-Murakami, A.; Brando, P.; et al. The linkages between photosynthesis, productivity, growth and biomass in lowland Amazonian forests. Glob. Chang Biol. 2015, 21, 2283–2295. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.H.; Hérault, B.; Bonal, D.; Stahl, C.; Anderson, L.O.; Baker, T.R.; Becker, G.S.; Beeckman, H.; Boanerges Souza, D.; Botosso, P.C.; et al. Climate seasonality limits leaf carbon assimilation and wood productivity in tropical forests. Biogeosciences 2016, 13, 2537–2562. [Google Scholar] [CrossRef]
- Lugo, A.E. Novel tropical forests: Nature’s response to global change. Trop. Conserv. Sci. 2013, 6, 325–337. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Higgs, E.S.; Hall, C.M. Novel Ecosystems: Intervening in the New Ecological World Order; Willey-Blackwell: West Sussex, UK, 2013. [Google Scholar]
- Ellis, E.C. Ecology in an anthropogenic biosphere. Ecol. Monogr. 2015, 85, 287–331. [Google Scholar] [CrossRef]
- Chave, J.; Andalo, C.; Brown, S.; Cairns, M.A.; Chambers, J.Q.; Eamus, D.; Fölster, H.; Fromard, F.; Higuchi, N.; Kira, T.; et al. Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 2005, 145, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 2015, 20, 3177–3190. [Google Scholar] [CrossRef] [PubMed]
- Feldpausch, T.R.; Banin, L.; Phillips, O.L.; Baker, T.R.; Lewis, S.L.; Quesada, C.A.; Affum-Baffoe, K.; Arets, E.G.M.M.; Berry, N.G.; Bird, M.; et al. Height-diameter allometry of tropical forest trees. Biogeosciences 2011, 8, 1081–1106. [Google Scholar] [CrossRef]
- Goodman, R.C.; Phillips, O.L.; Baker, T.R. The importance of crown dimensions to improve tropical tree biomass estimates. Ecol. Appl. 2014, 24, 680–698. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Brown, S.; Kicklighter, D.W.; Chambers, J.Q.; Thomlinson, J.R.; Ni, J. Measuring net primary production in forests: Concepts and field methods. Ecol. Appl. 2001, 11, 356–370. [Google Scholar] [CrossRef]
- Clark, D.A.; Brown, S.; Kicklighter, D.W.; Chambers, J.Q.; Thomlinson, J.R.; Ni, J.; Holland, E.A. Net primary production in tropical forests: An evaluation and synthesis of existing field data. Ecol. Appl. 2001, 11, 371–384. [Google Scholar] [CrossRef]
- Raich, J.W.; Clark, D.A.; Schwendenmann, L.; Wood, T.E. Aboveground tree growth varies with belowground carbon allocation in a tropical rainforest environment. PLoS ONE 2014, 9, e100275. [Google Scholar] [CrossRef] [PubMed]
- Fauset, S.; Johnson, M.O.; Gloor, M.; Baker, T.R.; Monteagudo, M.A.; Brienen, R.J.W.; Feldpausch, T.R.; Lopez-Gonzalez, G.; Malhi, Y.; ter Steege, H.; et al. Hyperdominance in Amazonian forest carbon cycling. Nat. Commun. 2015, 6, 6857. [Google Scholar] [CrossRef] [PubMed]
- Báez, S.; Malizia, A.; Carilla, J.; Blundo, C.; Aguilar, M.; Aguirre, N.; Aquirre, Z.; Álvarez, E.; Cuesta, F.; Duque, Á.; et al. Large-scale patterns of turnover and basal area change in Andean forests. PLoS ONE 2015, 10, e0126594. [Google Scholar] [CrossRef] [PubMed]
- Chave, J.; Condit, R.; Lao, Z.; Caspersen, J.P.; Foster, R.B.; Hubbell, S.P. Spatial and temporal variation of biomass in a tropical forest: Results from a large census plot in Panama. J. Ecol. 2003, 91, 240–252. [Google Scholar] [CrossRef]
- Lewis, S.L.; Phillips, O.L.; Baker, T.R.; Lloyd, J.; Malhi, Y.; Almeida, S.; Higuchi, N.; Laurance, W.F.; Neill, D.A.; Silva, J.N.; et al. Concerted changes in tropical forest structure and dynamics: Evidence from 50 South American long-term plots. Philos. Trans. R. Soc. 2004, 359, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Duque, A.; Scott, C.; Wayson, C.; Galindo, G.; Cabrera, E.; Chave, J.; Peña, M.; Álvarez, E.; Cárdenas, D.; et al. Live aboveground carbon stocks in natural forests of Colombia. For. Ecol. Manag. 2016, 374, 119–128. [Google Scholar] [CrossRef]
- Vieira, S.A.; Alves, L.F.; Aidar, M.; Araújo, L.S.; Baker, T.; Batista, J.L.F.; Campos, M.C.; Camargo, P.B.; Chave, J.; Delitti, W.B.C.; et al. Estimation of biomass and carbon stocks: The case of the Atlantic Forest. Biota Neotrop 2008, 8, 21–29. [Google Scholar] [CrossRef]
- Yepes, A.; Herrera, J.; Phillips, J.; Cabrera, E.; Galindo, G.; Granados, E.; Duque, A.; Barbosa, A.; Olarte, C.; Cardona, M. Contribución de los bosques tropicales de montaña en el almacenamiento de carbono en Colombia. Rev. Biol. Trop. 2015, 63, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.B.; Clark, D.A. Landscape-scale variation in forest structure and biomass in a tropical rain forest. For. Ecol. Manag. 2000, 137, 185–198. [Google Scholar] [CrossRef]
- Laurance, W.F.; Fearnside, P.M.; Laurance, S.G.; Delamonica, P.; Lovejoy, T.E.; Rankin-de-Merona, J.M.; Chambers, J.Q.; Gascon, C. Relationship between soils and Amazon forest biomass: A landscape-scale study. For. Ecol. Manag. 1999, 118, 127–138. [Google Scholar] [CrossRef]
- Longo, M.; Keller, M.; Dos-Santos, M.N.; Leitold, V.; Pinagé, E.R.; Baccini, A.; Saatchi, S.; Nogueira, E.M.; Batistella, M.; Morton, D.C. Aboveground biomass variability across intact and degraded forests in the Brazilian Amazon. Glob. Biogeochem. Cycles 2016, 30, 1639–1660. [Google Scholar] [CrossRef]
- Sullivan, M.J.P.; Talbot, J.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Begne, S.K.; Chave, J.; Cuni-Sanchez, A.; Hubau, W.; Lopez-Gonzalez, G.; et al. Diversity and carbon storage across the tropical forest biome. Sci. Rep. 2017, 7, 39102. [Google Scholar] [CrossRef] [PubMed]
- Fernández Martínez, M.; Vicca, S.; Janssens, I.A.; Luyssaert, S.; Campioli, M.; Sardans, J.; Estiarte, M.; Peñuelas, J. Spatial variability and controls over biomass stocks, carbon fluxes, and resourse-use efficiencies across forest ecosystems. Trees 2014, 28, 597–611. [Google Scholar] [CrossRef]
- Luyssaert, S.; Inglima, I.; Jung, M.; Richardson, A.D.; Reichstein, M.; Papale, D.; Piao, S.L.; Schulze, E.D.; Wingate, L.; Matteucci, G.; et al. CO2 balance of boreal, temperate, and tropical forests derived from a global database. Glob. Change Biol. 2007, 13, 2509–2537. [Google Scholar] [CrossRef]
- Brown, S.; Iverson, L.R.; Prasad, A.; Liu, D. Geographic distribution of carbon in biomass and soils of tropical Asian forests. Geocarto Int. 1993, 8, 45–59. [Google Scholar] [CrossRef]
- Brown, S.; Iverson, L.R.; Lugo, A.E. Land-use and biomass changes of forests in Peninsular Malaysia from 1972 to 1982: A GIS approach. In Effects of Land Use Change on Atmospheric CO2 Concentrations. Southeast Asia as a Case Study; Dale, V.H., Ed.; Springer: New York, NY, USA, 1994; pp. 117–143. [Google Scholar]
- Iverson, L.R.; Brown, S.; Prasad, A.; Mitasova, H.; Gillespie, A.J.R.; Lugo, A.E. Use of GIS for estimating potential and actual forest biomass for continental south and southeast Asia. In Effects of Land Use Change on Atmospheric CO2 Concentrations. Southeast Asia as a Case Study; Dale, V.H., Ed.; Springer: New York, NY, USA, 1994; pp. 67–116. [Google Scholar]
- Brown, S.; Pearson, T.; Slaymaker, D.; Ambagis, S.; Moore, N.; Novelo, D.; Sabido, W. Creating a virtual tropical forest from three-dimensional aerial imagery: Application for estimating carbon stocks. Ecol. Appl. 2005, 15, 1083–1095. [Google Scholar] [CrossRef]
- Chave, J.; Condit, R.; Aguilar, S.; Hernandez, A.; Lao, S.; Perez, R. Error propagation and scaling for tropical forest biomass estimates. Philos. Trans. R. Soc. 2004, 359, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Campioli, M.; Malhi, Y.; Vicca, S.; Luyssaert, S.; Papale, D.; Peñuelas, J.; Reichstein, M.; Migliavacca, M.; Arain, M.A.; Janssens, I.A. Evaluating the convergence between eddy-covariance and biometric methods for assessing carbon budgets of forests. Nat. Commun. 2016, 7, 13717. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.C.; Fisher, J.B.; Malhi, Y. Evaluating the potential to monitor aboveground biomass in forest and oil palm in Sabah, Malaysia, for 2000–2008 with Landsat ETM+ and ALOS-PALSAR. Int. J. Remote Sens. 2012, 33, 3614–3639. [Google Scholar] [CrossRef]
- Réjou-Méchain, M.; Tymen, B.; Blanc, L.; Fauset, S.; Feldpausch, T.R.; Monteagudo, A.; Phillips, O.L.; Richard, H.; Chave, J. Using repeated small-footprint LIDAR acquisitions to infer spatial and temporal variations of a high-biomass Neotropical forest. Remote Sens. Environ. 2015, 169, 93–101. [Google Scholar] [CrossRef]
- Tong Minh, D.H.; Toan, T.L.; Rocca, F.; Tebaldini, S.; Villard, L.; Réjou-Méchain, M.; Phillips, O.L.; Feldpausch, T.R.; Dubois-Fernandez, P.; Scipal, K.; et al. SAR tomography for the retrieval of forest biomass and height: Cross-validation at two tropical forest sites in French Guiana. Remote Sens. Environ. 2016, 175, 138–147. [Google Scholar] [CrossRef]
- Espírito Santo, F.D.B.; Keller, M.M.; Linder, E.; Junior, R.C.O.; Pereira, C.; Oliveira, C.G. Gap formation and carbon cycling in the Brazilian Amazon: Measurement using high-resolution optical remote sensing and studies of large forest plots. Plant Ecol. Divers. 2013, 7, 305–318. [Google Scholar] [CrossRef]
- Harris, N.L.; Brown, S.; Hagen, S.C.; Saatchi, S.S.; Petrova, S.; Salas, W.; Hansen, M.C.; Potapov, P.V.; Lotsch, A. Baseline map of carbon emissions from deforestation in tropical regions. Science 2012, 336, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Gaston, G.; Brown, S.; Lorenzini, M.; Singh, K.D. State and change in carbon pools in the forests of tropical Africa. Glob. Change Biol. 1998, 4, 97–114. [Google Scholar] [CrossRef]
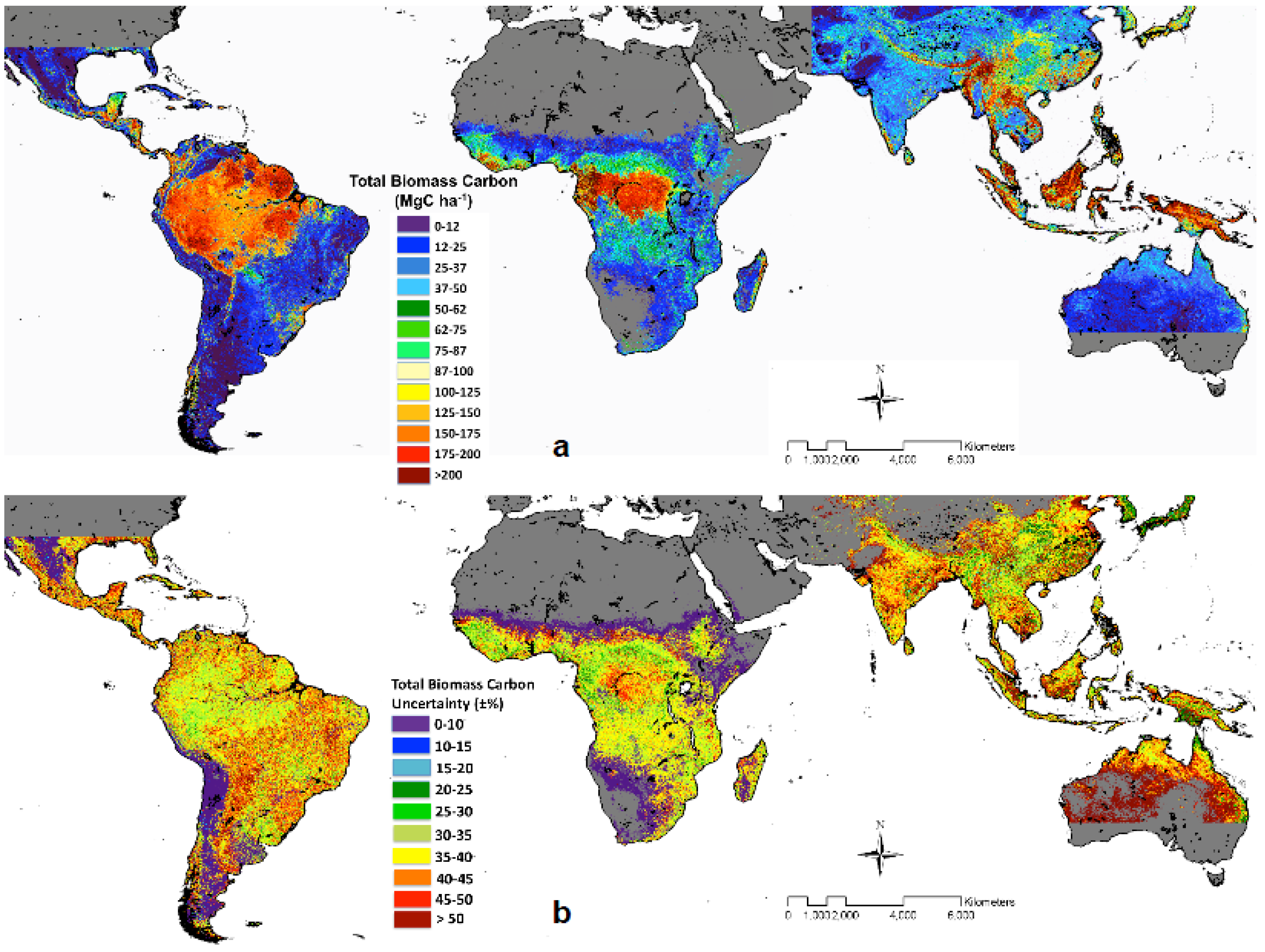
- Saatchi, S.S.; Harris, N.L.; Brown, S.; Lefsky, M.; Mitchard, E.T.A.; Salas, W.; Zutta, B.R.; Buermann, W.; Lewis, S.L.; Hagen, S.; et al. Benchmark map of forest carbon stocks in tropical regions across three continents. Proc. Natl. Acad. Sci. USA 2011, 108, 9899–9904. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, V.; Herold, M.; Heuvelink, G.B.M.; Lewis, S.L.; Phillips, O.L.; Asner, G.P.; Armston, J.; Ashton, P.S.; Banin, L.; Bayol, N.; et al. An integrated pan-tropical biomass map using multiple reference datasets. Glob. Chang Biol. 2016, 22, 1406–1420. [Google Scholar] [CrossRef] [PubMed]
- Mitchard, E.T.A.; Feldpausch, T.R.; Brienen, R.J.; Lopez Gonzalez, G.; Monteagudo, A.; Baker, T.R.; Lewis, S.L.; Lloyd, J.; Quesada, C.A.; Gloor, M.; et al. Markedly divergent estimates of Amazon forest carbon density from ground plots and satellites. Glob. Ecol. Biogeogr. 2014, 23, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Asner, G. Carnegie Airborne Observatory. Available online: https://cao.carnegiescience.edu (accessed on 27 March 2017).
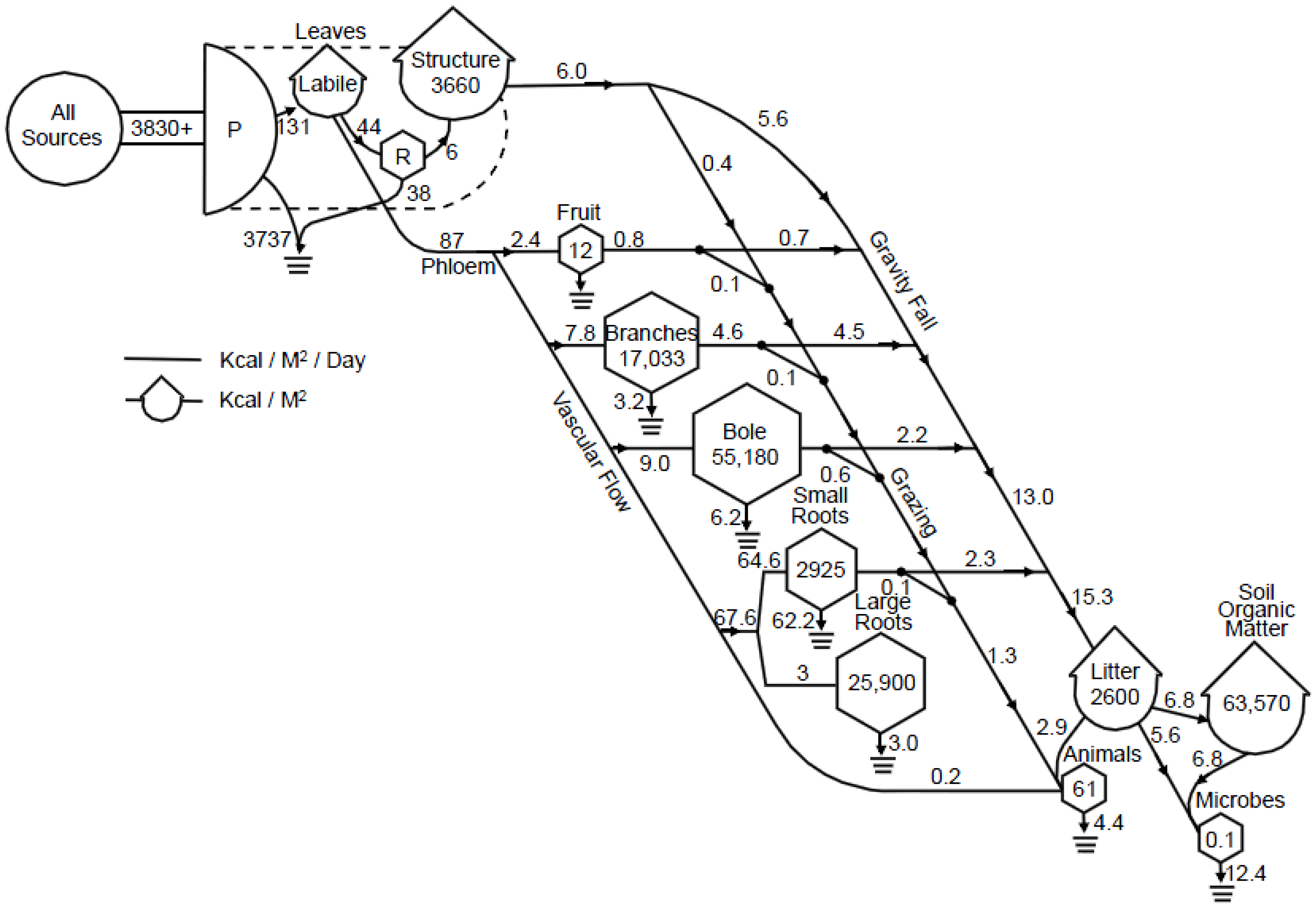
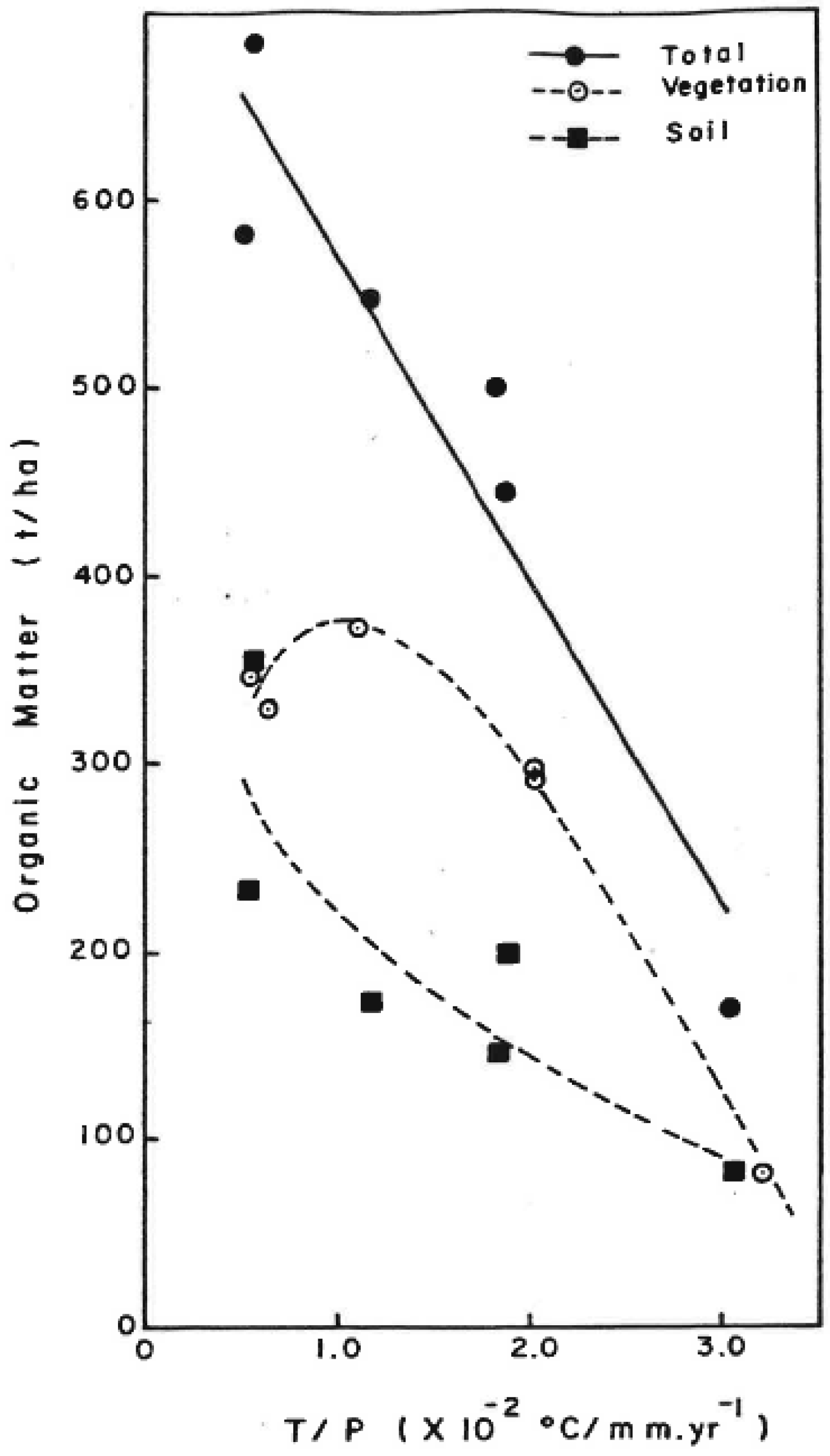
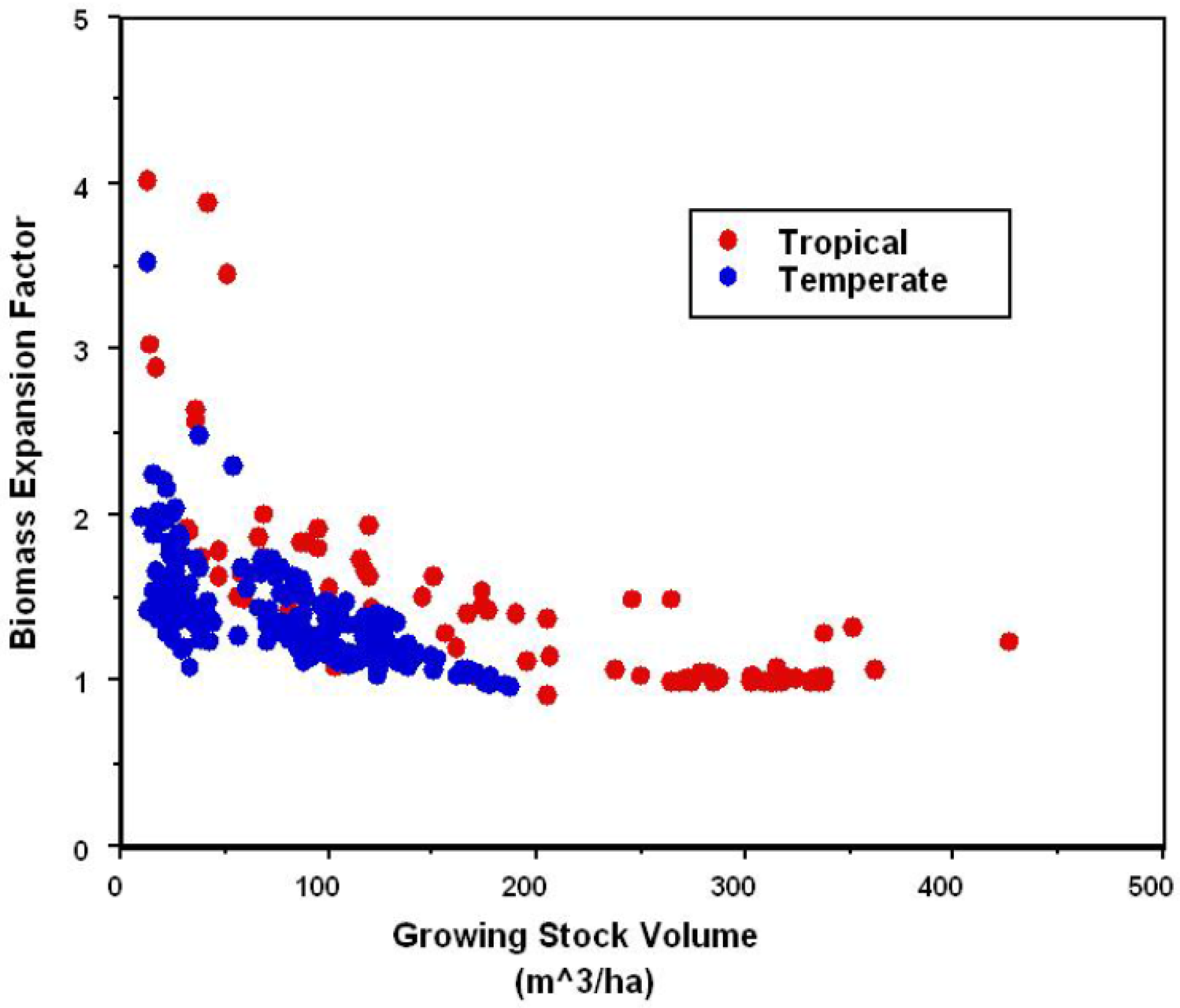
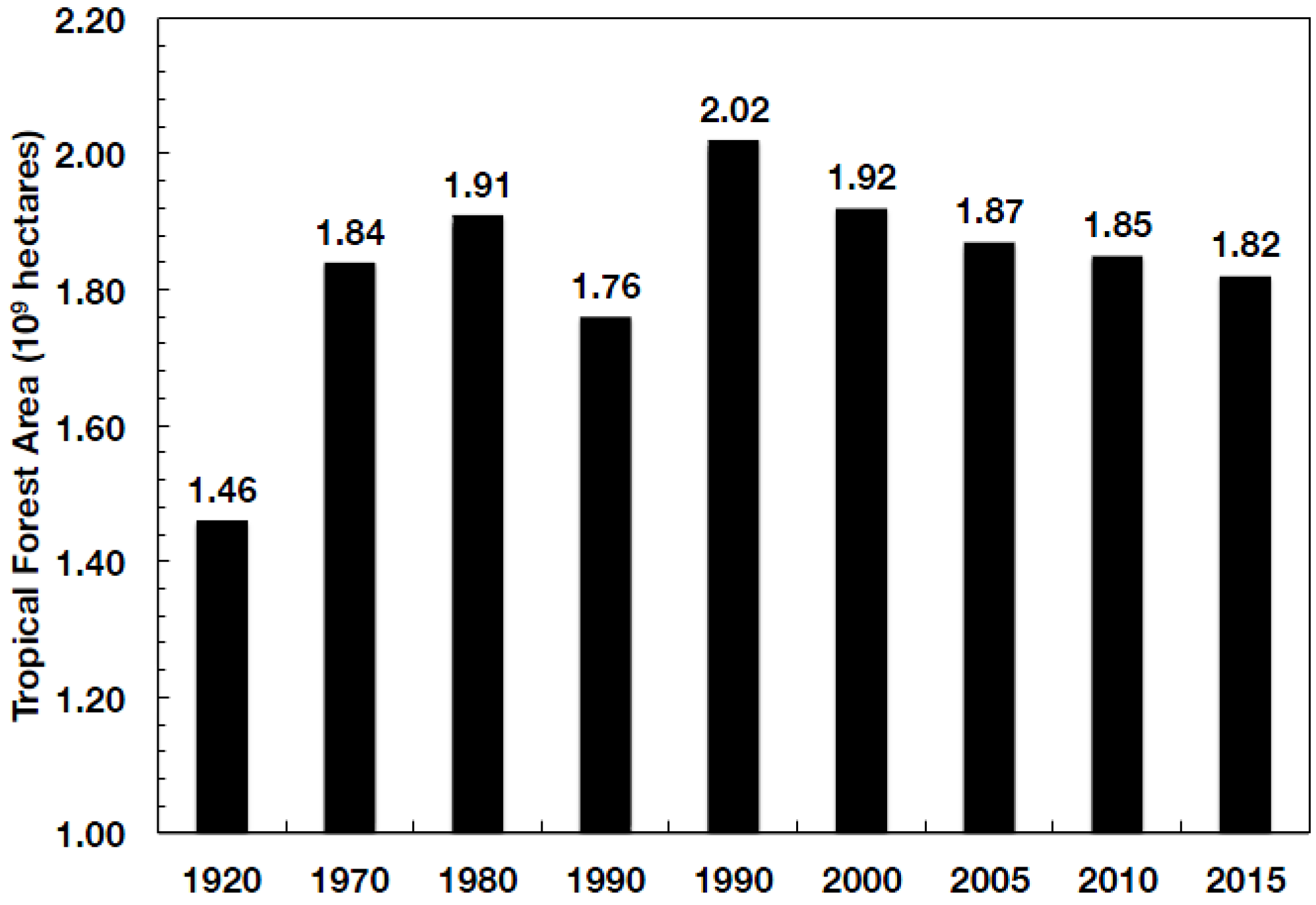
| Global Process and Sinks | 1980 | 1980–1989 | 1990–1999 | 2000–2007 |
|---|---|---|---|---|
| Sources | ||||
| Fossil fuel burning and cement manufacture | 4.5–5.9 | 5.4 ± 0.5 | 6.5 ± 0.4 | 7.6 ± 0.4 |
| Change in land use * | 1.8–3.3 | 1.6 ± 1.0 | 1.5 ± 0.7 | 1.1 ± 0.7 |
| Total | 6.3–9.2 | 7.0 | 8.0 ± 0.8 | 8.7 ± 0.8 |
| Sinks | ||||
| Atmosphere | 2.3–2.7 | 3.4 ± 0.2 | 3.2 ± 0.1 | 4.1 ± 0.1 |
| Oceans | 1.5–2.5 | 2.0 ± 0.8 | 2.2 ± 0.4 | 2.3 ± 0.4 |
| Terrestrial vegetation | 2.5 ± 0.4 | 2.3 ± 0.5 | ||
| Total | 3.8–5.2 | 5.4 | 7.9 ± 0.6 | 8.7 ± 0.7 |
| Residual (uncertainty) | 1.1–6.8 | 1.6 ± 1.4 | 0.1 ± 1.0 | 0.0 ± 1.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, S.; Lugo, A.E. Trailblazing the Carbon Cycle of Tropical Forests from Puerto Rico. Forests 2017, 8, 101. https://doi.org/10.3390/f8040101
Brown S, Lugo AE. Trailblazing the Carbon Cycle of Tropical Forests from Puerto Rico. Forests. 2017; 8(4):101. https://doi.org/10.3390/f8040101
Chicago/Turabian StyleBrown, Sandra, and Ariel E. Lugo. 2017. "Trailblazing the Carbon Cycle of Tropical Forests from Puerto Rico" Forests 8, no. 4: 101. https://doi.org/10.3390/f8040101





