Development of Sessile Oak and European Hornbeam Sprouts after Thinning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Analysis
- the sprouting probability model constructed using logistic regression (LR);
- the number of new sprouts model, which used a generalized linear model (GLM) with a Poisson distribution (live new sprouts) or with a zero inflated Poisson distribution (ZIP) (dead new sprouts);
- and the height model of new sprouts assembled by using multiple linear regression (MLR).
3. Results
3.1. Probability of Stump Sprouting
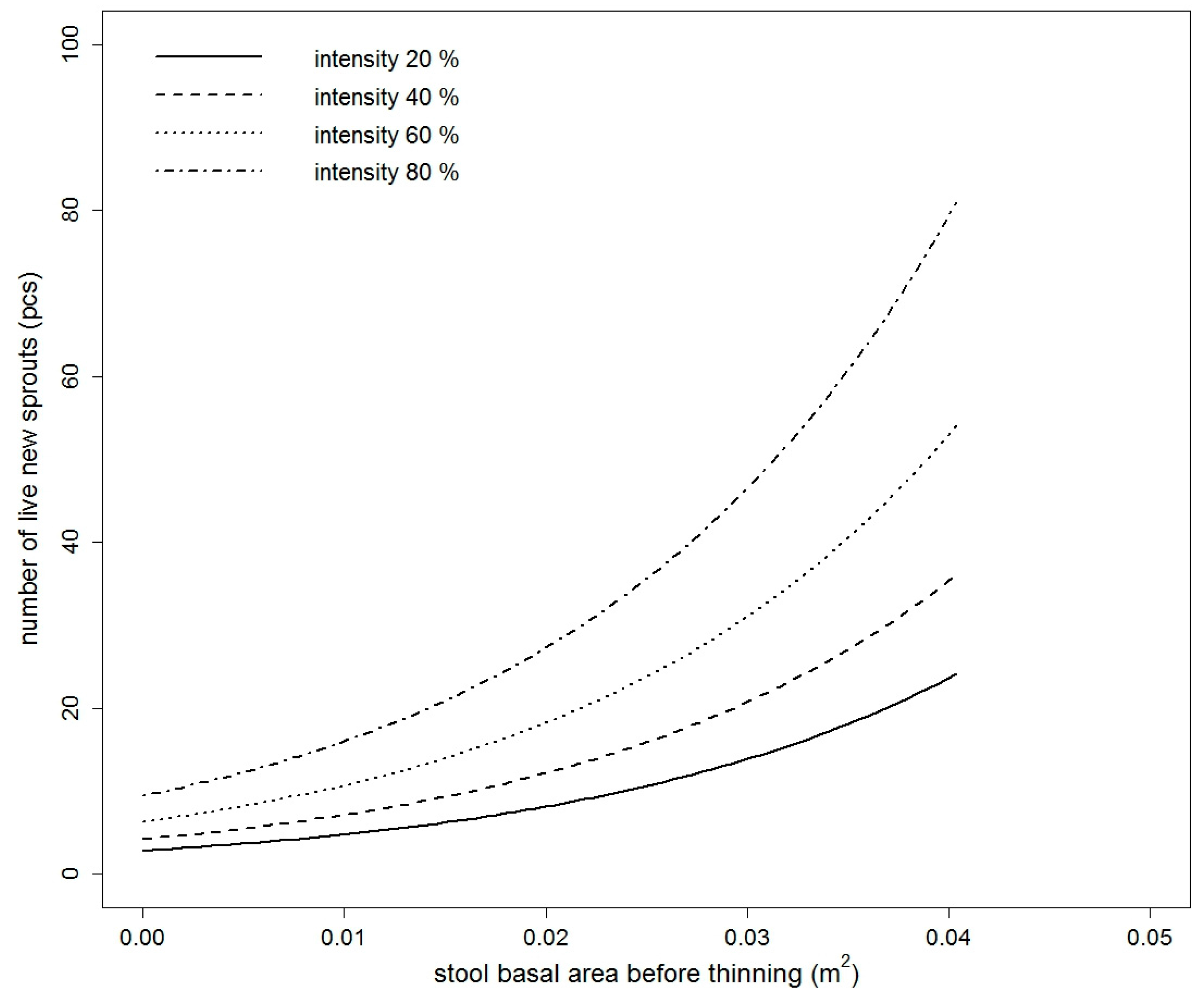
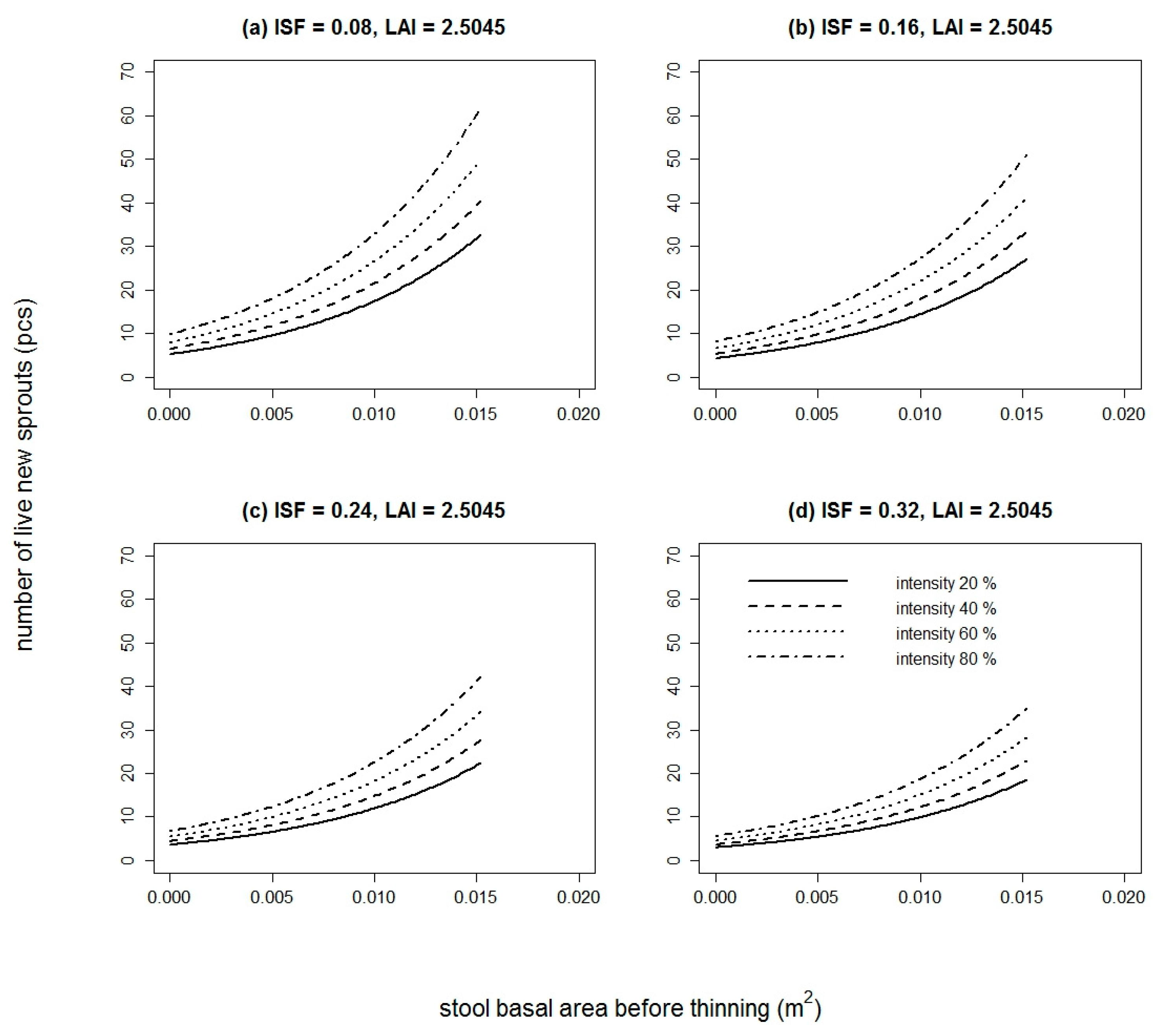
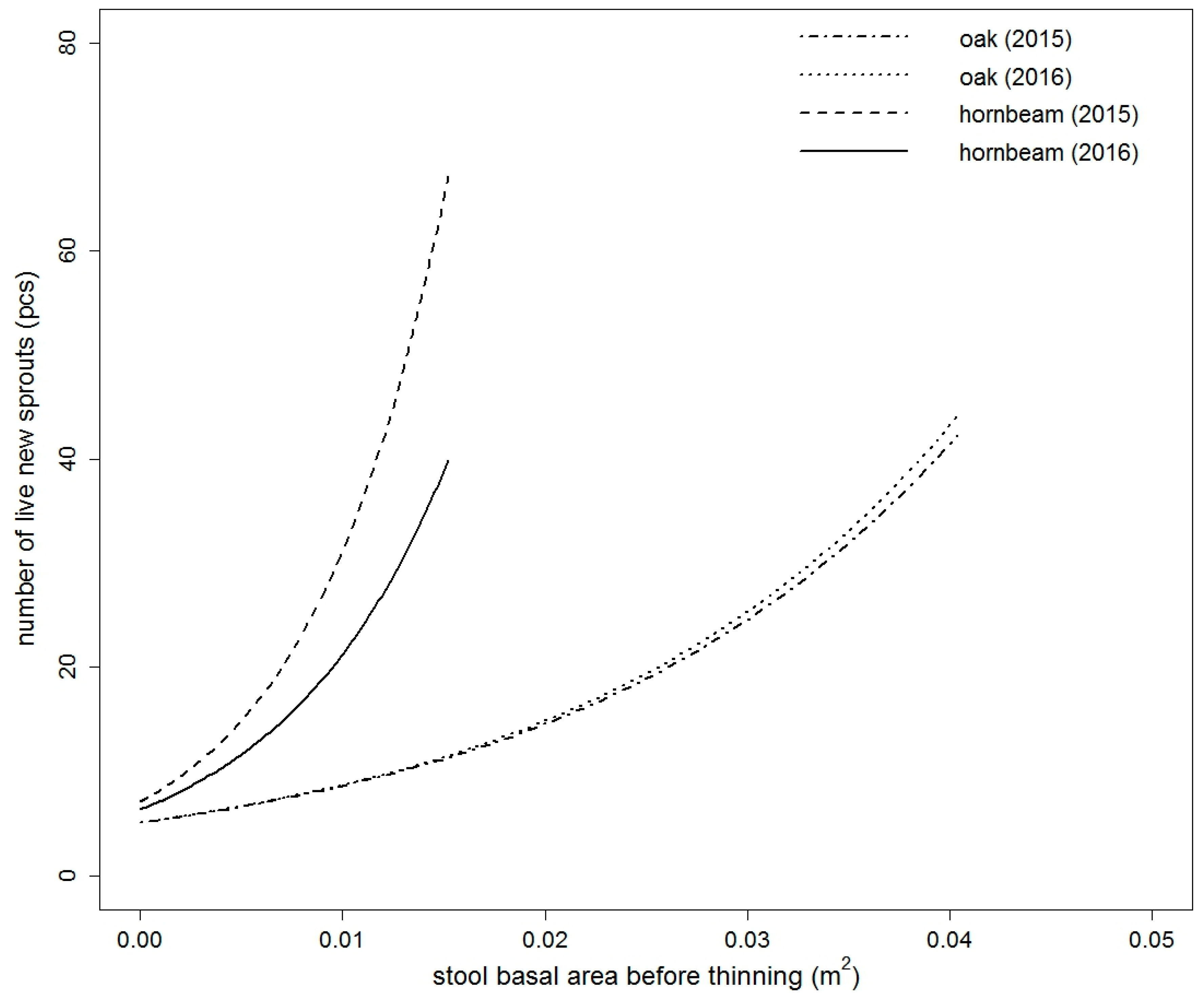
3.2. Live New Sprouts
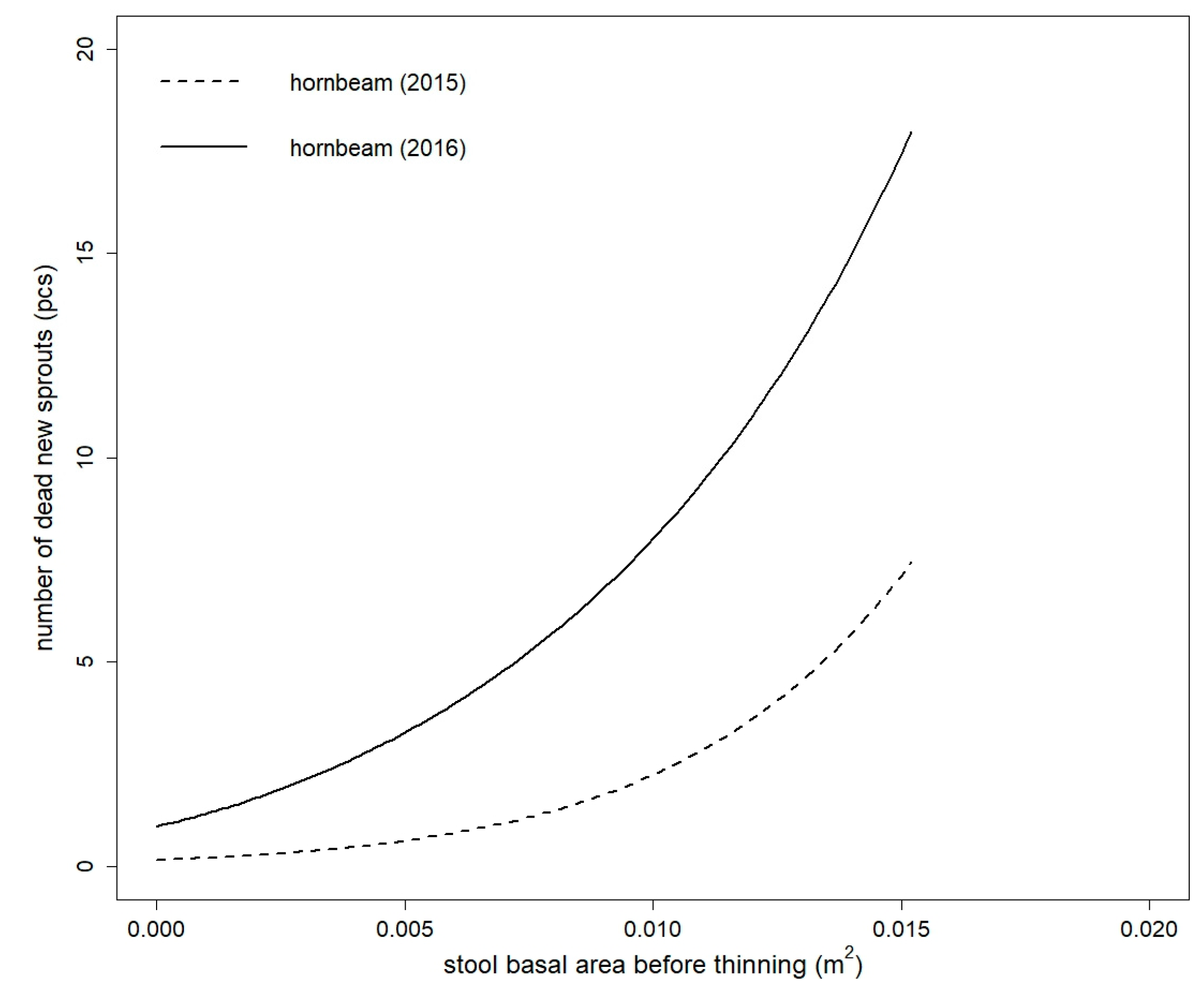
3.3. Dead New Sprouts
3.4. Height of New Sprouts
4. Discussion
4.1. Probability of Stump Sprouting
4.2. Live New Sprouts
4.3. Dead New Sprouts
4.4. Height of New Sprouts
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rackham, O. Ancient woodlands: Modern threats. New Phytol. 2008, 180, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Müllerová, J.; Hédl, R.; Szabó, P. Coppice abandonment and its implications for species diversity in forest vegetation. For. Ecol. Manag. 2015, 343, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, M.; Szatniewska, J.; Kyselová, I.; Pokorný, R.; Čater, M. Transpiration and water potential of young Quercus petraea (M.) Liebl. coppice sprouts and seedlings during favourable and drought conditions. J. For. Sci. 2017, 63, 313–323. [Google Scholar] [CrossRef]
- Nielsen, A.B.; Møller, F. Is coppice a potential for urban forestry? The social perspective. Urban For. Urban Gree. 2008, 7, 129–138. [Google Scholar] [CrossRef]
- Stajic, B.; Zlatanov, T.; Velichkov, I.; Dubravac, T.; Trajkov, P. Past and recent coppice forest management in some regions of South Eastern Europe. Silva Balc. 2009, 10, 9–19. [Google Scholar]
- Rydberg, D. Initial sprouting, growth and mortality of European aspen and birch after selective coppicing in central Sweden. For. Ecol. Manag. 2000, 130, 27–35. [Google Scholar] [CrossRef]
- Coppini, M.; Hermanin, L. Restoration of selective beech coppices: A case study in the Apennines (Italy). For. Ecol. Manag. 2007, 249, 18–27. [Google Scholar] [CrossRef]
- Quevedo, L.; Arnan, X.; Rodrigo, A. Selective thinning of Arbutus unedo coppices following fire: Effects on growth at the individual and plot level. For. Ecol. Manag. 2013, 292, 56–63. [Google Scholar] [CrossRef]
- Cotillas, M.; Sabaté, S.; Gracia, C.; Espelta, J.M. Growth response of mixed Mediterranean oak coppices to rainfall reduction. Could selective thinning have any influence on it? For. Ecol. Manag. 2009, 258, 1677–1683. [Google Scholar] [CrossRef]
- López, B.C.; Gracia, C.A.; Sabaté, S.; Keenan, T. Assessing the resilience of Mediterranean holm oaks to disturbances using selective thinning. Acta Oecol. 2009, 35, 849–854. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Pérez-Ramos, I.M.; Ourcival, J.-M.; Limousin, J.-M.; Joffre, R.; Rambal, S. Is selective thinning an adequate practice for adapting Quercus ilex coppices to climate change? Ann. For. Sci. 2011, 68, 575–585. [Google Scholar] [CrossRef]
- Kerr, G.; Haufe, J. Thinning Practice—A Silvicultural Guide, 1st ed.; Forestry Commission: Edinburgh, UK, 2011; p. 54.
- Průša, E. Silviculture Based on Forest Ecosystem Classification, 1st ed.; Lesnická Práce: Kostelec nad Černými lesy, Czech Republic, 2001; p. 590. (In Czech) [Google Scholar]
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics, 1st ed.; Wiley & Sons: New York, NY, USA, 1996; p. 544. [Google Scholar]
- Cameron, A.D. Importance of early selective thinning in the development of long-term stand stability and improved log quality: A review. Forestry 2002, 75, 25–35. [Google Scholar] [CrossRef]
- Annighöfer, P.; Beckschäfer, P.; Vor, T.; Ammer, C. Regeneration Patterns of European Oak Species (Quercus petraea (Matt.) Liebl., Quercus robur L.) in Dependence of Environment and Neighborhood. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chytrý, M. Vegetation of the Czech Republic: Diversity, ecology, history and dynamics. Preslia 2012, 84, 427–504. [Google Scholar]
- Loftsgaarden, P.O.; Andrews, P.L. Constructing and Testing Logistic Regression Models for Binary Data: Application to National Fire Danger Rating System; General technical report INT-286; USDA, Forest Service, Intermountain research station: Ogden, Utah, USA, 1992; p. 36.
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Savaliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; p. 574. [Google Scholar]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Proceedings 2nd International Symposium on Information Theory, Budapest, Hungary, 2–8 September 1973; Petrov, B.N., Csaki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 268–281. [Google Scholar]
- Nagelkerke, N.J.D. A note on a general definition of the coefficient of determination. Biometrika 1991, 78, 691–692. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 1998; p. 353. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 25 April 2017).
- Sands, B.A.; Abrams, M.D. Effects of Stump Diameter on Sprout Number and Size for Three Oak Species in a Pennsylvania Clearcut. North. J. Appl. For. 2009, 26, 122–125. [Google Scholar]
- Weigel, D.R.; Peng, C.Y.J. Predicting stump sprouting and competitive success of five oak species in southern Indiana. Can. J. For. Res. 2002, 32, 703–712. [Google Scholar] [CrossRef]
- Weigel, D.R.; Dey, D.C.; Peng, C.Y.J. Stump sprout dominance probabilities of five oak species in southern Indiana 15 years after clearcut harvesting. In Proceedings of the 13th Biennial Southern Silvicultural Research Conference, Memphis, TN, USA, 28 February–4 March 2005; Connor, K.F., Ed.; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2006; pp. 551–558. [Google Scholar]
- Weigel, D.R.; Dey, D.C.; Peng, C.Y.J. Stump sprout dominance probabilities of five oak species in southern Indiana 20 years after clearcut harvesting. In Proceedings of the 17th Central Hardwood Forest Conference, Lexington, KY, USA, 5–7 April 2010; Fei, S., Lhotka, J.M., Stringer, J.W., Gottschalk, K.W., Gary, W., Eds.; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newton Square, PA, USA, 2011; pp. 10–22. [Google Scholar]
- Šplíchalová, M.; Adamec, Z.; Kadavý, J.; Kneifl, M. Probability model of sessile oak (Quercus petraea (Matt.) Liebl.) stump sprouting in the Czech Republic. Eur. J. For. Res. 2012, 131, 1611–1618. [Google Scholar] [CrossRef]
- Atwood, C.; Fox, T.; Loftis, D. Effects of alternative silviculture on stump sprouting in the southern Appalachians. For. Ecol. Manag. 2009, 257, 1305–1313. [Google Scholar] [CrossRef]
- Matula, R.; Svátek, M.; Kůrová, J.; Úradníček, L.; Kadavý, J.; Kneifl, M. The sprouting ability of the main tree species in Central European coppices: implications for coppice restoration. Eur. J. For. Res. 2012, 131, 1501–1511. [Google Scholar] [CrossRef]
- Keyser, T.L.; Zarnoch, S.J. Stump sprout dynamics in response to reductions in stand density for nine upland hardwood species in the southern Appalachian Mountains. For. Ecol. Manag. 2014, 319, 29–35. [Google Scholar] [CrossRef]
- Keyser, T.L.; Loftis, D.L. Stump sprouting of 19 upland hardwood species 1 year following initiation of a shelterwood with reserves silvicultural system in the southern Appalachian Mountains, USA. New For. 2015, 46, 449–464. [Google Scholar] [CrossRef]
- Pyttel, P.L.; Fischer, U.F.; Suchomel, C.; Gärtner, S.M.; Bauhus, J. The effect of harvesting on stump mortality and re-sprouting in aged oak coppice forests. For. Ecol. Manag. 2013, 289, 18–27. [Google Scholar] [CrossRef]
- Giudici, F.; Zingg, A. Sprouting ability and mortality of chestnut (Castanea sativa Mill.) after coppicing. A case study. Ann. For. Sci. 2005, 62, 513–523. [Google Scholar] [CrossRef]
- Logli, F.; Joffre, R. Individual variability as related to stand structure and soil condition in a Mediterranean oak coppice. For. Ecol. Manag. 2001, 142, 53–63. [Google Scholar] [CrossRef]
- Ducrey, M.; Boisserie, M. Natural regrowth of holm oak coppice (Quercus ilex L) following partial cuts. Ann. Sci. For. 1992, 49, 91–109. [Google Scholar] [CrossRef]
- Retana, J.; Riba, M.; Castell, C.; Espelta, J.M. Regeneration by sprouting of holm-oak (Quercus ilex) stands exploited by selection thinning. Vegetatio 1992, 99, 355–364. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J. Resprouting as a key functional trait in woody plants—Challenges to developing new organizing principles. New Phytol. 2010, 188, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Borchert, R. Differences in shoot growth patterns between juvenile and adult trees and their interpretation based on systems analysis of trees. Acta Hortic. 1976, 56, 123–130. [Google Scholar] [CrossRef]
- Ford, E.D.; Newbould, P.J. Stand structure and dry weight production through the sweet chestnut (Castanea sativa Mill.) coppice cycle. J. Ecol. 1970, 58, 275–296. [Google Scholar] [CrossRef]
- Johnson, P.S. Growth and structural development of red oak sprout clumps. For. Sci. 1975, 21, 413–418. [Google Scholar]
- McDonald, I.E.; Powell, G.R. Relationships between stump sprouting and parent-tree diameter in sugar maple in the 1st year following clear-cutting. Can. J. For. Res. 1983, 13, 390–394. [Google Scholar] [CrossRef]
- Ferm, A.; Kauppi, A. Coppicing as a means for increasing hardwood biomass production. Biomass 1990, 22, 107–121. [Google Scholar] [CrossRef]
- Gardiner, E.S.; Helmig, L.M. Development of water oak stump sprouts under a partial overstory. New For. 1997, 14, 55–62. [Google Scholar] [CrossRef]
- Dey, D.C.; Jensen, R.G.; Wallendorf, M.J. Single-tree harvesting reduces survival and growth of oak stump sprouts in the Missouri Ozark Highlands. In Proceedings of the 16th Central Hardwood Forest Conference, West Lafayette, IN, USA, 8–9 April 2008; Jacobs, D.F., Michler, C.H., Eds.; U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: Newton Square, PA, USA, 2008; pp. 26–37. [Google Scholar]
- Lockhart, B.R.; Chambers, J.L. Cherry bark oak stump sprout survival and development five years following plantation thinning in the lower Mississippi alluvial valley, USA. New For. 2007, 33, 183–192. [Google Scholar] [CrossRef]
- Knapp, B.O.; Olson, M.G.; Dey, D.C. Early stump sprout development after two levels of harvest in a Midwestern Bottomland Hardwood Forest. For. Sci. 2017, 63, 377–387. [Google Scholar] [CrossRef]
- Kharitonovich, F.M. Reforestation by coppice shoots. In Field-Protecting Forest Belts, Moscow, Russia; Sorokin, I.F., Ed.; Moscow, Russia, 1937; pp. 175–239. (In Russian) [Google Scholar]
- O’Hara, K.L.; Berrill, J.-P. Dynamics of coast redwood sprout clump development in variable light environments. J. For. Res. 2010, 15, 131–139. [Google Scholar] [CrossRef]
- Finzi, A.C.; Canham, C.D. Sapling growth in response to light and nitrogen availability in a southern New England forest. For. Ecol. Manag. 2000, 131, 153–165. [Google Scholar] [CrossRef]
- Gratzer, G.; Darabant, A.; Chhetri, P.B.; Rai, P.B.; Eckmüllner, O. Interspecific variation in the response of growth, crown morphology, and survivorship to light of six tree species in the conifer belt of the Bhutan Himalayas. Can. J. For. Res. 2004, 34, 1093–1107. [Google Scholar] [CrossRef]
- Stancioiu, P.T.; O’Hara, K.L. Regeneration growth in different light environments of mixed species, multiaged, mountainous forests of Romania. Eur. J. For. Res. 2006, 125, 151–162. [Google Scholar] [CrossRef]
- Dey, D.C.; Jensen, R.G. Stump sprouting potential of oaks in Missouri Ozark forests managed by even- and uneven-aged silviculture. In Proceedings of the 2nd Missouri Ozark Forest Ecosystem Project Symposium: Post-Treatment Results of the Landscape Experiment, St. Louis, MO, USA, 17–18 October 2000; Shifley, S.R., Kabrick, J.M., Eds.; U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 2002; pp. 102–113. [Google Scholar]
- Gracia, M.; Retana, J. Effect of site quality and shading on sprouting patterns of holm oak coppices. For. Ecol. Manag. 2004, 188, 39–49. [Google Scholar] [CrossRef]
- Ducrey, M.; Turrel, M. Influence of cutting methods and dates on stump sprouting in Holm oak (Quercus ilex L.) coppice. Ann. For. Sci. 1992, 49, 449–464. [Google Scholar] [CrossRef]
- Espelta, J.M.; Retana, J.; Habrouk, A. Resprouting patterns after fire and response to stool cleaning of two coexisting Mediterranean oaks with contrasting leaf habits on two different sites. For. Ecol. Manag. 2003, 179, 401–414. [Google Scholar] [CrossRef]
| Variable | Species | Mean Value | Standard Deviation | Minimum Value | Maximum Value |
|---|---|---|---|---|---|
| BAbt (m2) | O | 0.0050 | 0.0055 | 0.0005 | 0.0404 |
| H | 0.0050 | 0.0030 | 0.0008 | 0.0152 | |
| BAt (m2) | O | 0.0024 | 0.0033 | 0.0002 | 0.0243 |
| H | 0.0025 | 0.0018 | 0.0002 | 0.0082 | |
| BAat (m2) | O | 0.0025 | 0.0025 | 0.0003 | 0.0161 |
| H | 0.0025 | 0.0015 | 0.0002 | 0.0092 | |
| It (%) | O | 46.9289 | 15.7934 | 6.1305 | 80.3030 |
| H | 48.8586 | 14.1870 | 15.0495 | 82.9964 | |
| GF (%) | O | 11.3858 | 3.4163 | 3.3400 | 20.8100 |
| H | 8.5797 | 2.8654 | 4.3900 | 18.0800 | |
| OP (%) | O | 12.2251 | 3.6223 | 3.6200 | 22.3400 |
| H | 9.3228 | 3.1226 | 4.8500 | 19.3700 | |
| LAI (m2/m2) | O | 2.2394 | 0.3539 | 1.3500 | 3.2100 |
| H | 2.5045 | 0.2652 | 1.7500 | 3.0300 | |
| DSF | O | 0.2003 | 0.1239 | 0.0345 | 0.5898 |
| H | 0.1550 | 0.0993 | 0.0494 | 0.6307 | |
| ISF | O | 0.1622 | 0.0503 | 0.0516 | 0.3014 |
| H | 0.1313 | 0.0468 | 0.0641 | 0.3192 | |
| TSF | O | 0.1953 | 0.1124 | 0.0367 | 0.5492 |
| H | 0.1519 | 0.0906 | 0.0561 | 0.5857 |
| Variable | Year | Species | Mean Value | Standard Deviation | Minimum Value | Maximum Value |
|---|---|---|---|---|---|---|
| height of new sprouts (cm) | 2016 | O | 47.44 | 25.40 | 7.00 | 140.00 |
| H | 57.44 | 21.33 | 20.00 | 138.00 | ||
| number of live new sprouts (pcs/stool) | 2015 | O | 7.17 | 8.47 | 0 | 49 |
| H | 17.26 | 16.70 | 1 | 108 | ||
| 2016 | O | 7.21 | 8.89 | 1 | 50 | |
| H | 13.36 | 11.30 | 0 | 82 | ||
| number of dead new sprouts (pcs/stool) | 2015 | O | 0.36 | 0.72 | 0 | 4 |
| H | 0.93 | 2.35 | 0 | 15 | ||
| 2016 | O | 1.66 | 1.94 | 0 | 9 | |
| H | 3.76 | 4.65 | 0 | 30 |
| Species | Year | Predictor | χ2 (DF) | p | ps. R2 | AIC |
|---|---|---|---|---|---|---|
| O | 2015 | BAbt + It | 219.20 (3) | <0.0001 | 0.9265 | 626.5 |
| 2016 | BAbt + It | 223.21 (3) | <0.0001 | 0.9299 | 657.0 | |
| H | 2015 | BAbt + It + LAI | 781.71 (4) | <0.0001 | 0.9964 | 1488.6 |
| 2016 | BAbt + It + LAI + ISF | 528.52 (5) | <0.0001 | 0.9768 | 1129.3 |
| Species (year) | Predictor | Parameter | Estimation | SE | z | p |
|---|---|---|---|---|---|---|
| sessile oak (2015) | BAbt + It | β0 | 0.5819 | 0.1583 | 3.676 | 0.0002 |
| β1 | 52.2106 | 3.8415 | 13.591 | <0.0001 | ||
| b2 | 0.0211 | 0.0029 | 7.114 | <0.0001 | ||
| sessile oak (2016) | BAbt + It | β0 | 0.6266 | 0.1569 | 3.993 | <0.0001 |
| β1 | 53.2652 | 3.7962 | 14.031 | <0.0001 | ||
| β2 | 0.0202 | 0.0029 | 6.848 | <0.0001 | ||
| European hornbeam (2015) | BAbt + It + LAI | β0 | 2.7737 | 0.2463 | 11.261 | <0.0001 |
| β1 | 147.5858 | 5.8988 | 25.020 | <0.0001 | ||
| β2 | 0.0116 | 0.0017 | 6.739 | <0.0001 | ||
| β3 | −0.5539 | 0.0834 | −6.644 | <0.0001 | ||
| European hornbeam (2016) | BAbt + It + LAI + ISF | β0 | 5.2030 | 0.5453 | 9.542 | <0.0001 |
| β1 | 120.4584 | 7.0269 | 17.142 | <0.0001 | ||
| β2 | 0.0106 | 0.0020 | 5.378 | <0.0001 | ||
| β3 | −1.4214 | 0.1748 | −8.131 | <0.0001 | ||
| β4 | −2.3678 | 0.9455 | −2.504 | 0.0123 |
| Species | Year | Predictor | χ2 (DF) | p | ps. R2 | AIC |
|---|---|---|---|---|---|---|
| European hornbeam | 2015 | BAbt + It | 53.42 (3) | <0.0001 | 0.3411 | 337.3 |
| 2016 | BAbt | 128.42 (1) | <0.0001 | 0.6045 | 717.8 |
| Species (year) | Predictor | Parameter | Estimation | SE | z | p |
|---|---|---|---|---|---|---|
| European hornbeam (2015) | BAbt + It | β0 | −1.1881 | 0.5304 | −2.240 | 0.0251 |
| β1 | 174.6932 | 27.6850 | 6.310 | <0.0001 | ||
| β2 | 0.0193 | 0.0083 | 2.336 | 0.0195 | ||
| γ0 | 1.3417 | 0.4788 | 2.802 | 0.0051 | ||
| γ1 | −134.7950 | 69.8329 | −2.041 | 0.0412 | ||
| γ2 | ----- | ----- | ----- | ----- | ||
| European hornbeam (2016) | BAbt | β0 | 0.6750 | 0.1017 | 6.637 | <0.0001 |
| β1 | 146.5458 | 12.8754 | 11.382 | <0.0001 | ||
| γ0 | ----- | ----- | ----- | ----- | ||
| γ1 | −280.8806 | 48.6000 | −5.780 | <0.0001 |
| Species | Predictor | F (DF) | p | R2 | AIC |
|---|---|---|---|---|---|
| sessile oak | ISF + BAbt | 230.862 (2, 82) | <0.0001 | 0.3166 | 514.4 |
| European hornbeam | LAI | 14.133 (1, 137) | 0.0003 | 0.0935 | 840.1 |
| Species | Predictor | Parameter | Estimation | SE | t | p |
|---|---|---|---|---|---|---|
| sessile oak | ISF + BAbt | β0 | --- | --- | --- | --- |
| β1 | 223.339 | 18.046 | 12.376 | <0.0001 | ||
| β2 | 2097.330 | 413.649 | 5.070 | <0.0001 | ||
| European hornbeam | LAI | β0 | 119.124 | 16.499 | 7.220 | <0.0001 |
| β1 | −24.600 | 6.544 | −3.759 | 0.0003 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamec, Z.; Kadavý, J.; Fedorová, B.; Knott, R.; Kneifl, M.; Drápela, K. Development of Sessile Oak and European Hornbeam Sprouts after Thinning. Forests 2017, 8, 308. https://doi.org/10.3390/f8090308
Adamec Z, Kadavý J, Fedorová B, Knott R, Kneifl M, Drápela K. Development of Sessile Oak and European Hornbeam Sprouts after Thinning. Forests. 2017; 8(9):308. https://doi.org/10.3390/f8090308
Chicago/Turabian StyleAdamec, Zdeněk, Jan Kadavý, Barbora Fedorová, Robert Knott, Michal Kneifl, and Karel Drápela. 2017. "Development of Sessile Oak and European Hornbeam Sprouts after Thinning" Forests 8, no. 9: 308. https://doi.org/10.3390/f8090308




