Functional Response Trait Analysis Improves Climate Sensitivity Estimation in Beech Forests at a Trailing Edge
Abstract
:1. Introduction
2. Materials and Methods
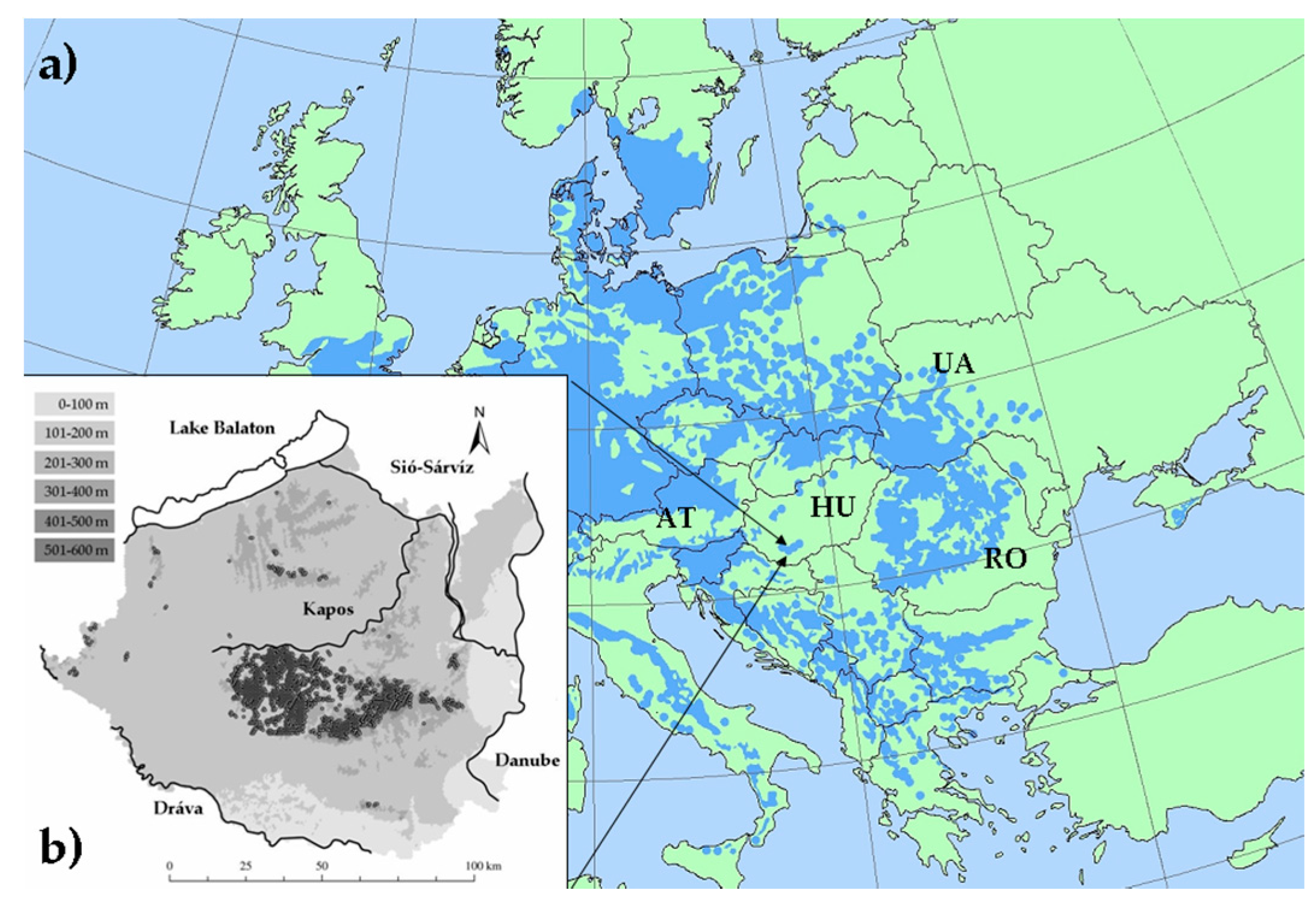
2.1. Site Description and Location
2.2. Vegetation Survey and Beech Forests
2.3. Bioclimatic Variables
2.4. Data Processing and Analyses
2.5. Climatic Traits and Distribution Response Functions
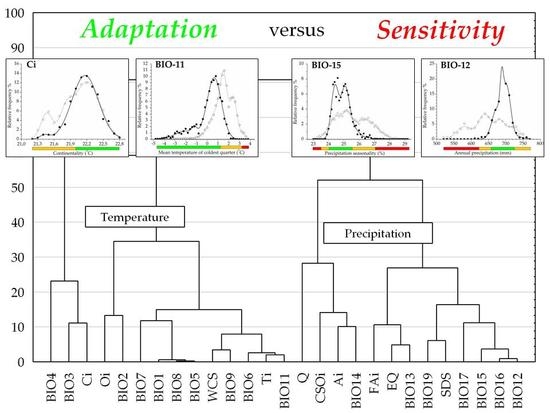
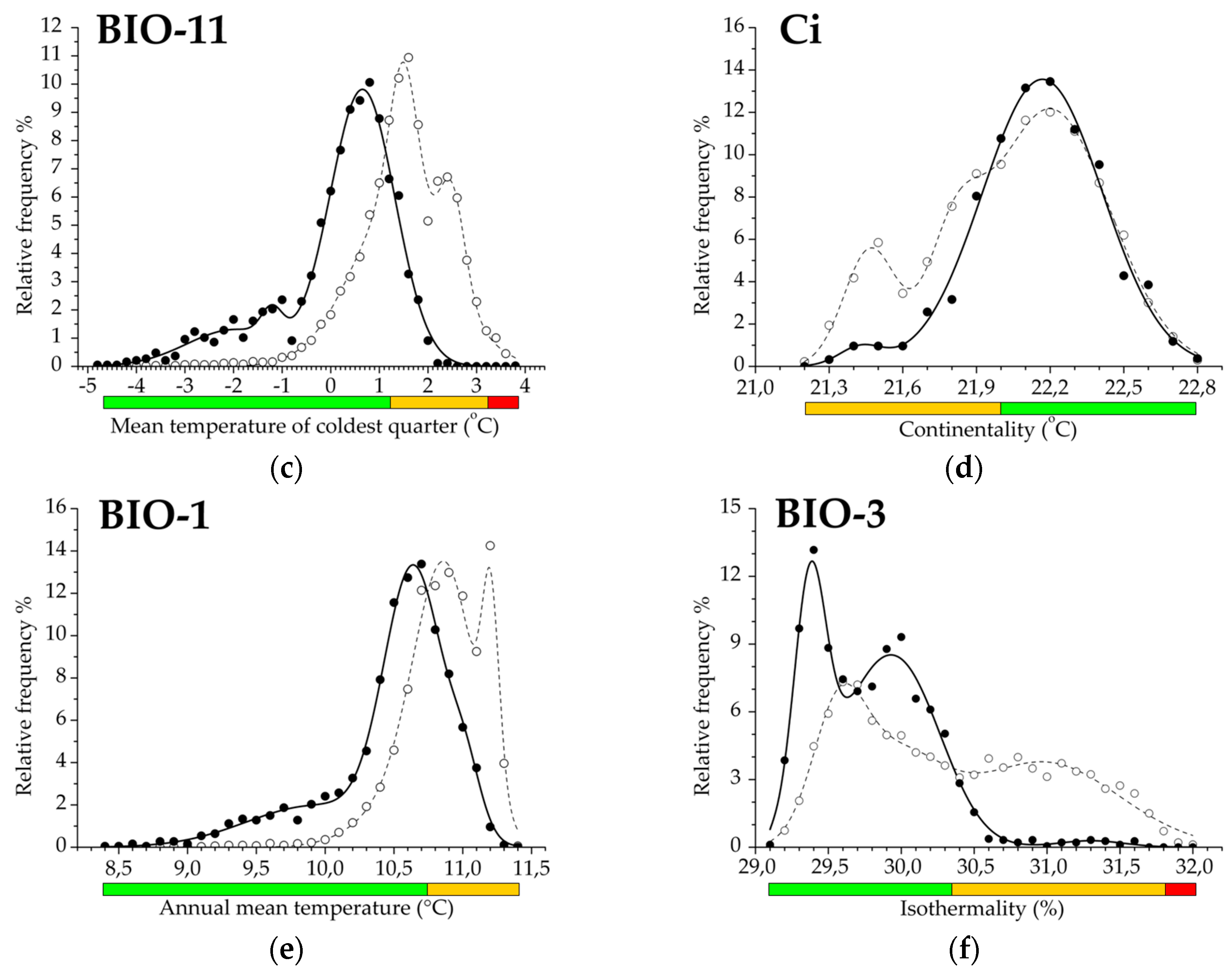
3. Results
3.1. Climatic Variability of Beech Forests Distribution
3.2. Climatic Traits and Response Functions
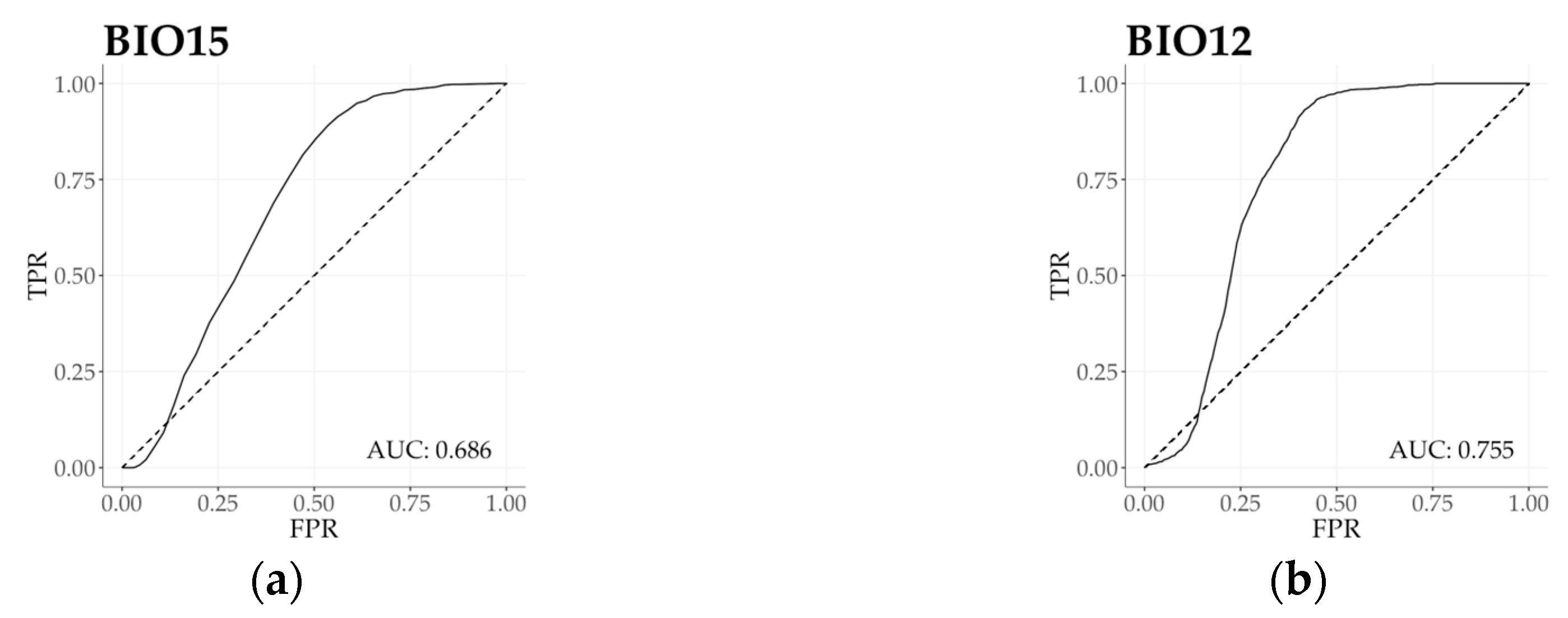
3.3. Climatic Behavior Estimation by ROC Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Violle, C.; Navas, M.L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnir, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Díaz, S.; Purvis, A.; Cornelissen, J.H.C.; Mace, M.G.; Donoghue, M.J.; Ewers, R.M.; Jordano, P.; Pearse, W.D. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecol. Evol. 2013, 3, 2958–2975. [Google Scholar] [CrossRef] [PubMed]
- Nock, C.A.; Vogt, R.J.; Beisner, B.E. Functional Traits. eLS 2016, 1–8. [Google Scholar] [CrossRef]
- Walther, G.-R. Community and ecosystem responses to recent climate change. Phil. Trans. R. Soc. B 2010, 365, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Grimm, N.B.; Chapin, F.S.; Bierwagen, B.; Gonzalez, P.; Groffman, P.M.; Luo, Y.; Melton, F.; Nadelhoffer, K.; Pairis, A.; Raymond, P.A.; et al. The impacts of climate change on ecosystem structure and function. Front. Ecol. Environ. 2013, 11, 474–482. [Google Scholar] [CrossRef]
- Adger, W.N.; Agrawala, S.; Mirza, M.M.Q.; Conde, C.; O’Brien, K.; Pulhin, J.; Pulwarty, R.; Smit, B.; Takahashi, K. Assessment of adaptation practices, options, constraints and capacity. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 717–743. [Google Scholar]
- Kolström, M.; Lindner, M.; Vilén, T.; Maroschek, M.; Seidl, R.; Lexer, M.; Netherer, S.; Kremer, A.; Delzon, S.; Barbati, A.; et al. Reviewing the Science and Implementation of Climate Change Adaptation Measures in European Forestry. Forests 2011, 2, 961–982. [Google Scholar] [CrossRef] [Green Version]
- Adaptation and Mitigation. Available online: http://know.climateofconcern.org/index.php?option=com_content&task=article&id=139 (accessed on 14 July 2017).
- Climate Change Synthesis Report: Annex B 2001. Available online: https://gridarendal-website.s3.amazonaws.com/production/documents/:s_document/293/original/annex.pdf?1488203667 (accessed on 23 August 2017).
- Casalegno, S.; Amatulli, G.; Bastrup-Birk, A.; Houston Durrant, T.; Pekkarinen, A. Modelling and mapping the suitability of European forest formations at 1-km resolution. Eur. J. For. Res. 2011, 130, 971–981. [Google Scholar] [CrossRef]
- Willner, W.; Jimenez-Alfaro, B.; Agrillo, E.; Biurrun, I.; Campos, J.A.; Carni, A.; Casella, L.; Csiky, J.; Custerevska, R.; Didukh, J.P.; et al. Classification of European beech forests: A Gordian knot ? Appl. Veg. Sci. 2017, 20, 494–512. [Google Scholar] [CrossRef]
- Ozenda, P.; Borel, J.L. An ecological map of Europe: Why and how? C. R. Acad. Sci. 2000, 983–994. [Google Scholar] [CrossRef]
- Dengler, A. Waldbau auf Ökologischer Grundlage, 3rd ed.; Springer: Berlin, Germany, 1944. [Google Scholar]
- Bolte, A.; Czajkowski, T.; Kompa, T. The north-eastern distribution range of European beech—A review. Forestry 2007, 80, 413–429. [Google Scholar] [CrossRef]
- Fang, J.; Lechowicz, M.J. Climatic limits for the present distribution of beech (Fagus L.) species in the world. J. Biogeogr. 2006, 33, 1804–1819. [Google Scholar] [CrossRef]
- Augustaitis, A.; Kliučius, A.; Marozas, V.; Pilkauskas, M.; Augustaitiene, I.; Vitas, A.; Staszewski, T.; Jansons, A.; Dreimanis, A. Sensitivity of European beech trees to unfavorable environmental factors on the edge and outside of their distribution range in north-eastern Europe. iForest 2015, 9, 259–269. [Google Scholar] [CrossRef]
- Marinšek, A.; Šilc, U.; Čarni, A. Geographical and ecological differentiation of Fagus forest vegetation in SE Europe. Appl. Veg. Sci. 2013, 131–147. [Google Scholar] [CrossRef]
- Salamon-Albert, É.; Lőrincz, P.; Pauler, G.; Bartha, D.; Horváth, F. Drought stress distribution responses of continental beech forests at their xeric edge in Central Europe. Forests 2016, 7, 298. [Google Scholar] [CrossRef]
- Mátyás, C.; Berki, I.; Czúcz, B.; Gálos, B.; Móricz, N.; Rasztovits, E. Future of beech in Southeast Europe from the perspective of evolutionary ecology. Acta Silv. Lign. Hung. 2010, 6, 91–110. [Google Scholar]
- Czúcz, B.; Gálhidy, L.; Mátyás, C. Present and forecasted xeric climatic limits of beech and sessile oak distribution at low altitudes in Central Europe. Ann. For. Sci. 2011, 68, 99–108. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Kržič, A.; Matović, A.; Orlović, S.; Duputie, A.; Djurdjević, A.; Galić, A.; Stojnić, S. Prediction of the European beech (Fagus sylvatica L.) xeric limit using a regional climate model: An example from southeast Europe. Agric. For. Met. 2013, 176, 94–103. [Google Scholar] [CrossRef]
- Budeanu, M.; Petritan, A.M.; Popescu, F.; Vasile, D.; Tudose, C. The resistance of European beech (Fagus sylvatica) from the eastern natural limit of species to climate change. Not. Bot. Horti Agrobot. 2016, 44, 625–633. [Google Scholar] [CrossRef]
- Gálos, B.; Führer, E.; Czimber, K.; Gulyás, K.; Bidló, A.; Hänsler, A.; Jacob, D.; Mátyás, C. Climatic threats determining future adaptive forest management—A case study of Zala County. Időjárás 2015, 119, 425–441. [Google Scholar]
- Hacket-Pain, A.J.; Cavin, L.; Friend, A.D.; Jump, A.S. Consistent limitation of growth by high temperature and low precipitation from range core to southern edge of European beech indicates widespread vulnerability to changing climate. Eur. J. For. Res. 2016. [Google Scholar] [CrossRef]
- Di Filippo, A.; Biondi, F.; Čufar, K.; De Luis, M.; Grabner, M.; Maugeri, M.; Saba, E.P.; Schirone, B.; Piovesan, G. Bioclimatology of beech (Fagus sylvatica L.) in the Eastern Alps: Spatial and altitudinal climatic signals identified through a tree-ring network. J. Biogeogr. 2007, 1873–1892. [Google Scholar] [CrossRef]
- Tegel, W.; Seim, A.; Hakelberg, D.; Hoffmann, S.; Panev, M.; Westphal, T.; Büntgen, U. A recent growth increase of European beech (Fagus sylvatica L.) at its Mediterranean distribution limit contradicts drought stress. Eur. J. For. Res. 2014, 133, 61–71. [Google Scholar] [CrossRef]
- Michelot, A.; Bréda, N.; Damesin, C.; Dufrêne, E. Differing growth responses to climatic variations and soil water deficits of Fagus sylvatica, Quercus petraea and Pinus sylvestris in a temperate forest. For. Ecol. Manag. 2012, 265, 161–171. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Bolte, A.; Czajkowski, T.; Cocozza, C.; Tognetti, R.; de Miguel, M.; Psidova, E.; Ditmarova, L.; Dinca, L.; Delzon, S.; Cochard, H.; et al. Desiccation and Mortality Dynamics in Seedlings of Different European Beech (Fagus sylvatica L.) populations under Extreme Drought Conditions. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nahm, M.; Radoglou, K.; Halyvopoulos, G.; Gessler, A.; Renneberg, H.; Fotelli, M.N. Physiological Performance of Beech (Fagus sylvatica L.) at its Southeastern Distribution Limit in Europe: Seasonal Changes in Nitrogen, Carbon and Water Balance. Plant Biol. 2006, 8, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Fotelli, M.N.; Nahm, M.; Radoglu, K.; Renneberg, H.; Halyvopulus, G.; Matzarakis, A. Seasonal and interannual ecophysiological responses of beech (Fagus sylvatica) at its south-eastern distribution limit in Europe. For. Ecol. Manag. 2009, 257, 1157–1164. [Google Scholar] [CrossRef]
- De Candolle, A. Origin of Cultivated Plants; Appleton: New York, NY, USA, 1855; p. 497. [Google Scholar]
- Köppen, F.T. Geographische Verbreitung der Holzgewächse des Europäischen Russlands und des Kaukasus; Kaiserlichen Akademie der Wissenschaften: St. Petersburg, Russia, 1889. [Google Scholar]
- Europe’s Biodiversity—Biogeographical Regions and Seas. Biogeographical Regions in Europe. The Pannonian Region. Available online: https://www.eea.europa.eu/publications/report_2002_0524_154909 (accessed on 13 July 2017).
- Mersich, I.; Práger, T.; Ambrózy, P.; Hunkár, M.; Dunkel, Z. Climate Atlas of Hungary (in Hungarian); Hungarian Meteorological Service: Budapest, Hungary, 2001; p. 107. [Google Scholar]
- Climate of Hungary—General Characteristics. Available online: http://www.met.hu/en/eghajlat/magyarorszag_eghajlata/altalanos_eghajlati_jellemzes/altalanos_leiras/ (accessed on 14 July 2017).
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Borhidi, A. Klimadiagramme und klimazonale Karte Ungarns. Ann. Univ. Sci. Budapest. Sec. Geogr. 1961, 4, 21–50. [Google Scholar]
- Zólyomi, B.; Kéri, M.; Horváth, F. Spatial and temporal changes in the frequency of climatic year types in the Carpathian Basin. Coenoses 1997, 12, 33–41. [Google Scholar]
- Distribution Map of Fagus sylvatica. Available online: http://www.euforgen.org/species/fagus-sylvatica/ (accessed on 7 October 2017).
- MÉTA—Methodology. Available online: http://www.novenyzetiterkep.hu/english/node/59 (accessed on 14 July 2017).
- Molnár, Z.; Bartha, S.; Seregélyes, T.; Illyés, E.; Botta-Dukát, Z.; Tímár, G.; Horváth, F.; Révész, A.; Kun, A.; Bölöni, J.; et al. A grid-based, satellite-image supported, multi-attributed vegetation mapping method (MÉTA). Folia Geobot. 2007, 42, 225–247. [Google Scholar] [CrossRef]
- EEA. European Forest Types—Categories and Types for Sustainable Forest Management Reporting and Policy. 2006. Available online: https://www.eea.europa.eu/publications/technical_report_2006_9 (accessed on 13 July 2017).
- Bölöni, J.; Molnár, Z.; Biró, M.; Horváth, F. Distribution of the (semi-)natural habitats in Hungary II. Woodlands and shrublands. Acta Bot. Hung. 2008, 50, 107–148. [Google Scholar] [CrossRef]
- Borhidi, A.; Kevey, B.; Lendvai, G. Plant Communities of Hungary, 1st ed.; Akadémiai Kiadó: Budapest, Hungary, 2012; pp. 423–426. [Google Scholar]
- Csiky, J.; Borhidi, A.; Bölöni, J.; Fekete, G.; Nagy, J.; Tímár, G.; Ódor, P.; Bartha, D.; Bodonczi, L. K5—Beech forests. In Magyarország élőhelyei; Vegetációtípusok leírása és határozója—ÁNÉR 2011 (in Hungarian); Bölöni, J., Molnár, Z., Kun, A., Eds.; MTA Ökológiai és Botanikai Kutatóintézet: Vácrátót, Hungary, 2011; pp. 268–273. [Google Scholar]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Clim. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Bioclim—Bioclimatic variables. Available online: http://www.worldclim.org/bioclim (accessed on 14 July 2017).
- Bihari, Z. Climate Atlas of Hungary (in Hungarian). In Forest and Climate (in Hungarian); Mátyás, C., Víg, P., Eds.; University of West Hungary: Sopron, Hungary, 2004; pp. 23–34. [Google Scholar]
- Benichou, P.; Le Breton, O. Prise en compte de la topographie pour la cartographie de champs pluviométriques: la méthode AURELHY. Agrométéorol. Rég. Moy. Mont. 1986, 39, 51–69. [Google Scholar]
- De Martonne, E. Regions of interior basin drainage. Geogr. Rev. 1927, 17, 397–414. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Sánchez-Mata, D.; Costa, M. North American boreal and western temperate forest vegetation. Itin. Geobot. 1999, 12, 5–316. [Google Scholar]
- Ellenberg, H. Vegetation Mitteleuropas mit den Alpen, 5th ed.; Ulmer: Stuttgart, Germany, 1996; p. 1095. [Google Scholar]
- Führer, E.; Horváth, L.; Jagodics, A.; Machon, A.; Szabados, I. Application of a new aridity index in Hungarian forestry practice. Idojaras 2011, 115, 205–216. [Google Scholar]
- Emberger, L. La végétation de la region méditérranéenne. Essai d’une classification des groupements végétaux. Rev. Bot. 1930, 503, 642–662. [Google Scholar]
- Mitrakos, K. A theory for Mediterranean plant life. Acta Oecol. 1980, 1, 245–252. [Google Scholar]
- Krzanowski, W.J.; Hand, D.J. ROC Curves for Continuous Data; Chapman & Hall/CRC: Boca Raton, UK, 2009. [Google Scholar]
- Origin. Available online: http://www.originlab.com/index.aspx?go=PRODUCTS/Origin (accessed on 14 July 2017).
- Statistica 12—Product Features. Available online: http://www.statsoft.com/Products/STATISTICA-Features/Version-12 (accessed on 14 July 2017).
- The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 10 July 2017).
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.; Hogg, E.H.T.; et al. A global overview of drought and heat-induced mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Bauwe, A.; Jurasinski, G.; Scharnweber, T.; Schröder, C.; Lennartz, B. Impact of climate change on tree-ring growth of Scots pine, common beech and pedunculate oak in northeastern Germany. iForest 2015, 9, 1–11. [Google Scholar] [CrossRef]
- Prentice, I.C.; Cramer, W.; Harrison, S.; Leemnas, R.; Monserud, A.M.; Solomon, A.M. Special Paper: A Global Biome Model Based on Plant Physiology and Dominance, Soil Properties and Climate. J. Biogeogr. 1992, 19, 117–134. [Google Scholar] [CrossRef]
- Bakkenes, M.; Alkemade, J.R.M.; Ihle, F.; Leemans, R.; Latour, J.B. Assessing effects of forecasted climate change on the diversity and distribution of European higher plants for 2050. Glob. Chang. Biol. 2002, 8, 390–407. [Google Scholar] [CrossRef]
- Bolte, A.; Ammer, C.; Löf, M.; Madsen, P.; Nabuurs, G.-J.; Schall, P.; Spathelf, P.; Rock, J. Adaptive forest management in central Europe: Climate change impacts, strategies and integrative concept. Scand. J. For. Res. 2009, 24, 473–482. [Google Scholar] [CrossRef]
- Mezősi, G.; Meyer, B.C.; Loibl, W.; Aubrecht, C.; Csorba, P.; Bata, T. Assessment of regional climate change impacts on Hungarian landscapes. Reg. Environ. Chang. 2013, 13, 797–811. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; ven der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainities, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Pommering, A.; Murphy, S.T. A review of the history, definitions and methods of continuous cover forestry with special attention to afforestation and restocking. Forestry 2004, 77, 27–44. [Google Scholar] [CrossRef]
- Brang, P.; Spathelf, P.; Larsen, B.J.; Bauhus, J.; Boncina, A.; Chauvin, C.; Drössler, L.; Garcia-Güemes, C.; Heiri, C.; Kerr, G.; et al. Suitability of close-to-nature silviculture for adapting temperate European forests to climate change. Forestry 2014, 87, 492–503. [Google Scholar] [CrossRef]



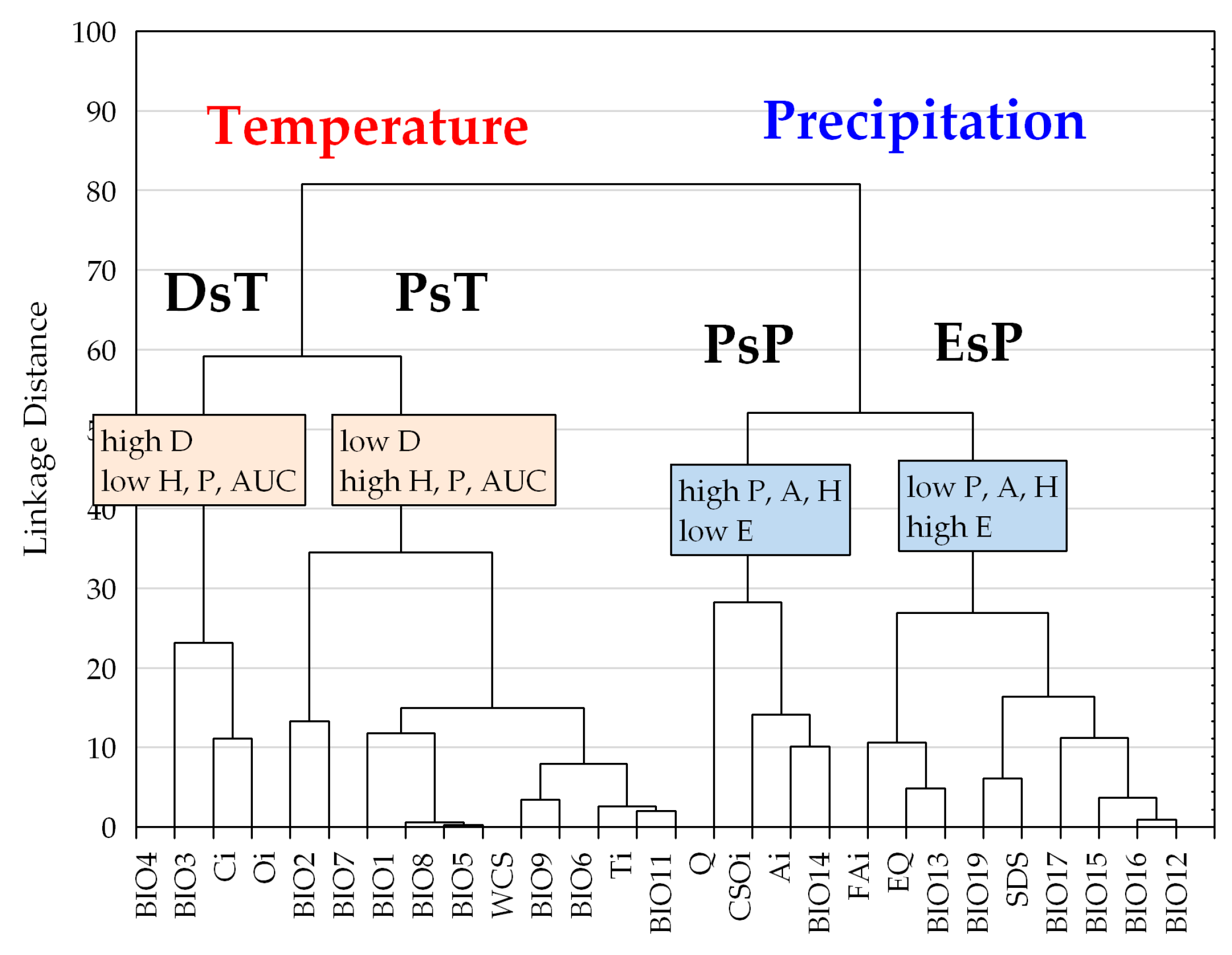
| Variable | Abbreviation | Type | PC-1 | PC-2 | PC-3 | PC-4 |
|---|---|---|---|---|---|---|
| Annual precipitation | BIO12 | P | −0.948 | |||
| Precipitation of the wettest month 1 | BIO13 | P | −0.907 | |||
| Precipitation of the wettest/warmest quarter 2 | BIO16/BIO18 | P | −0.902 | |||
| Precipitation of the driest quarter 3 | BIO17 | P | −0.901 | |||
| Summer Drought Stress | SDS | P | 0.901 | |||
| Precipitation of the coldest quarter 4 | BIO19 | P | −0.853 | |||
| Precipitation of the driest month 5 | BIO14 | P | −0.799 | |||
| Aridity index | Ai | P/T | −0.778 | |||
| Ellenberg’s Quotient | EQ | T/P | 0.774 | |||
| Forestry Aridity index | FAI | T/P | 0.733 | |||
| Compensated Summer Ombrothermic Index | CSOi | P/T | −0.707 | |||
| Mean temperature of the coldest quarter 3 | BIO11 | T | 0.972 | |||
| Mean temperature of the driest quarter 3 | BIO9 | T | 0.970 | |||
| Thermicity index | Ti | T | 0.962 | |||
| Winter Cold Stress | WCS | T | −0.954 | |||
| Minimum temperature of the coldest month 5 | BIO6 | T | 0.951 | |||
| Mean diurnal range of temperature | BIO2 | T | 0.916 | |||
| Maximum temperature of the warmest month 6 | BIO5 | T | 0.916 | |||
| Annual mean temperature | BIO1 | T | 0.907 | |||
| Mean temp. of the wettest/warmest quarter 7 | BIO8/BIO10 | T | 0.874 | |||
| Temperature annual range | BIO7 | T | 0.778 | |||
| Pluviothermic Quotient | Q | P/T | −0.753 | |||
| Ombrothermic index | Oi | P/T | −0.701 | |||
| Continentality index | Ci | T | −0.824 | |||
| Temperature seasonality | BIO4 | T | −0.800 | |||
| Isothermality | BIO3 | T | 0.757 | |||
| Precipitation seasonality | BIO15 | P | −0.962 |
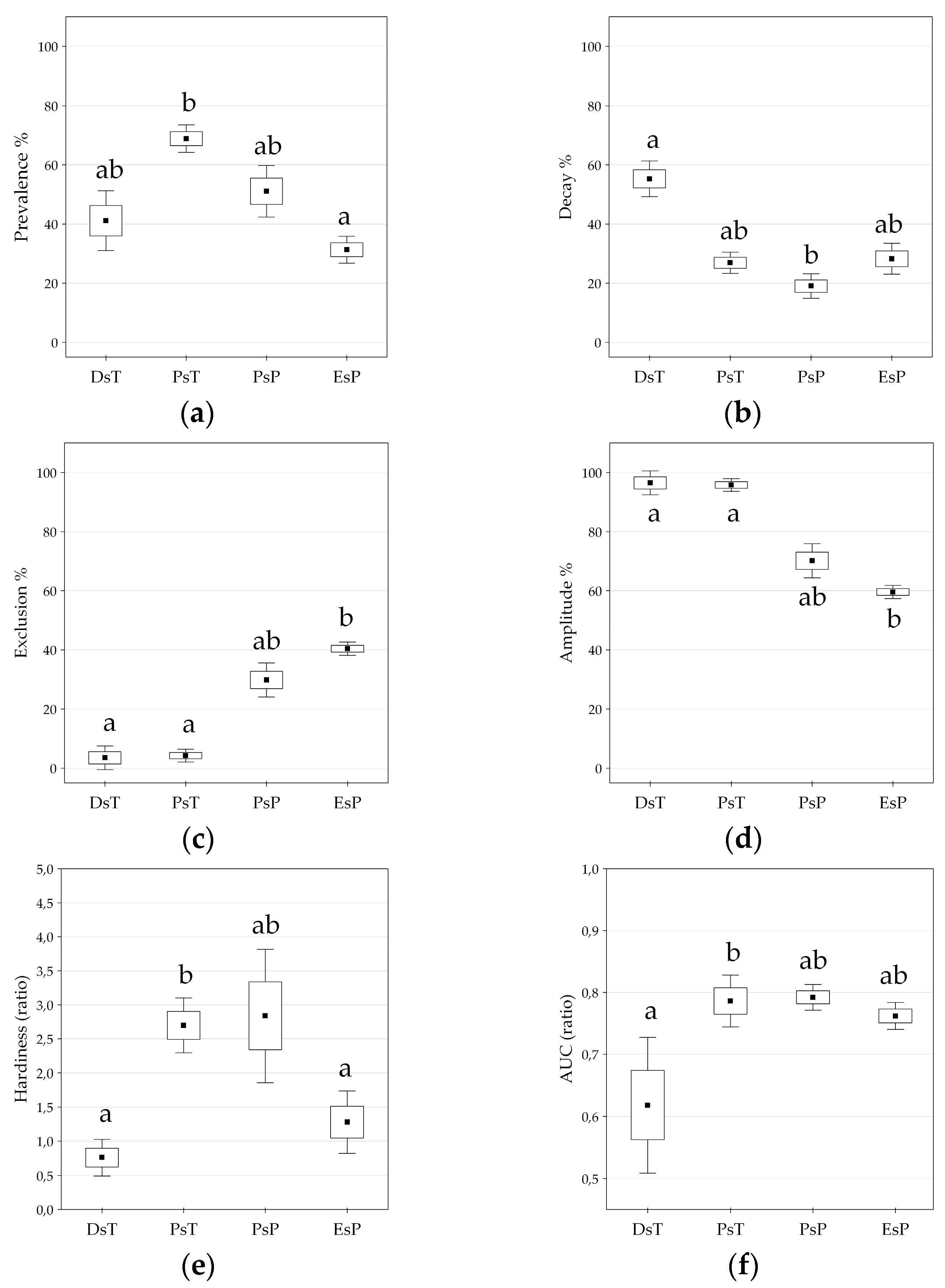
| Variable | Abbreviation | P% | D%B | E% | A% | H%%−1 | AUC |
|---|---|---|---|---|---|---|---|
| Annual precipitation | BIO12 | 25.2 | 34.0 | 40.8 | 59.2 | 0.74 | 0.755 |
| Precipitation of the wettest month | BIO13 | 42.3 | 19.2 | 38.5 | 61.5 | 2.20 | 0.809 |
| Precipitation of the warmest quarter | BIO16 | 25.6 | 34.1 | 40.2 | 59.8 | 0.75 | 0.756 |
| Precipitation of the driest quarter | BIO17 | 30.2 | 25.6 | 44.2 | 55.8 | 1.18 | 0.763 |
| Summer Drought Stress | SDS | 30.9 | 34.5 | 34.5 | 65.5 | 0.89 | 0.756 |
| Precipitation of the coldest quarter | BIO19 | 26.3 | 36.8 | 36.8 | 63.2 | 0.71 | 0.784 |
| Precipitation of the driest month | BIO14 | 41.7 | 25.0 | 33.3 | 66.7 | 1.67 | 0.781 |
| Aridity index | Ai | 47.4 | 17.2 | 35.3 | 64.7 | 2.75 | 0.805 |
| Ellenberg’s Quotient | EQ | 39.0 | 20.1 | 40.9 | 59.1 | 1.94 | 0.782 |
| Forestry Aridity index | FAi | 38.7 | 16.1 | 45.2 | 54.8 | 2.40 | 0.767 |
| Compensated Summer Ombr. Index | CSOi | 52.5 | 18.6 | 28.8 | 71.2 | 2.82 | 0.768 |
| Mean temp. of the coldest quarter | BIO11 | 69.8 | 23.3 | 7.0 | 93.0 | 3.00 | 0.832 |
| Mean temp. of the driest quarter | BIO9 | 71.0 | 25.8 | 3.2 | 96.8 | 2.75 | 0.807 |
| Thermicity index | Ti | 69.8 | 22.1 | 8.1 | 91.9 | 3.16 | 0.841 |
| Winter Cold Stress | WCS | 71.2 | 23.7 | 5.1 | 94.9 | 3.00 | 0.833 |
| Minimum temp. of the coldest month | BIO6 | 68.0 | 24.0 | 8.0 | 92.0 | 2.83 | 0.850 |
| Mean diurnal range of temperature | BIO2 | 50.0 | 41.7 | 8.3 | 91.7 | 1.20 | 0.761 |
| Max. temp. of the warmest month | BIO5 | 76.3 | 23.7 | 0.0 | 100.0 | 3.22 | 0.799 |
| Annual mean temperature | BIO1 | 76.7 | 23.3 | 0.0 | 100.0 | 3.29 | 0.760 |
| Mean temp. of the wettest quarter | BIO8 | 76.5 | 23.5 | 0.0 | 100.0 | 3.25 | 0.781 |
| Temperature annual range | BIO7 | 68.4 | 31.6 | 0.0 | 100.0 | 2.17 | 0.594 |
| Pluviothermic Quotient | Q | 62.7 | 15.3 | 22.0 | 78.0 | 4.11 | 0.814 |
| Ombrothermic index | Oi | 60.0 | 33.3 | 6.7 | 93.3 | 1.80 | 0.789 |
| Continentality index | Ci | 50.0 | 50.0 | 0.0 | 100.0 | 1.00 | 0.584 |
| Temperature seasonality | BIO4 | 32.1 | 60.7 | 7.1 | 92.9 | 0.53 | 0.543 |
| Isothermality | BIO3 | 41.4 | 55.2 | 3.4 | 96.6 | 0.75 | 0.727 |
| Precipitation seasonality | BIO15 | 23.7 | 33.9 | 42.4 | 57.6 | 0.70 | 0.686 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamon-Albert, É.; Abaligeti, G.; Ortmann-Ajkai, A. Functional Response Trait Analysis Improves Climate Sensitivity Estimation in Beech Forests at a Trailing Edge. Forests 2017, 8, 324. https://doi.org/10.3390/f8090324
Salamon-Albert É, Abaligeti G, Ortmann-Ajkai A. Functional Response Trait Analysis Improves Climate Sensitivity Estimation in Beech Forests at a Trailing Edge. Forests. 2017; 8(9):324. https://doi.org/10.3390/f8090324
Chicago/Turabian StyleSalamon-Albert, Éva, Gallusz Abaligeti, and Adrienne Ortmann-Ajkai. 2017. "Functional Response Trait Analysis Improves Climate Sensitivity Estimation in Beech Forests at a Trailing Edge" Forests 8, no. 9: 324. https://doi.org/10.3390/f8090324