Development of Northern White-Cedar (Thuja occidentalis L.) Plantations within and outside Deer Yards
Abstract
:1. Introduction
2. Materials and Methods
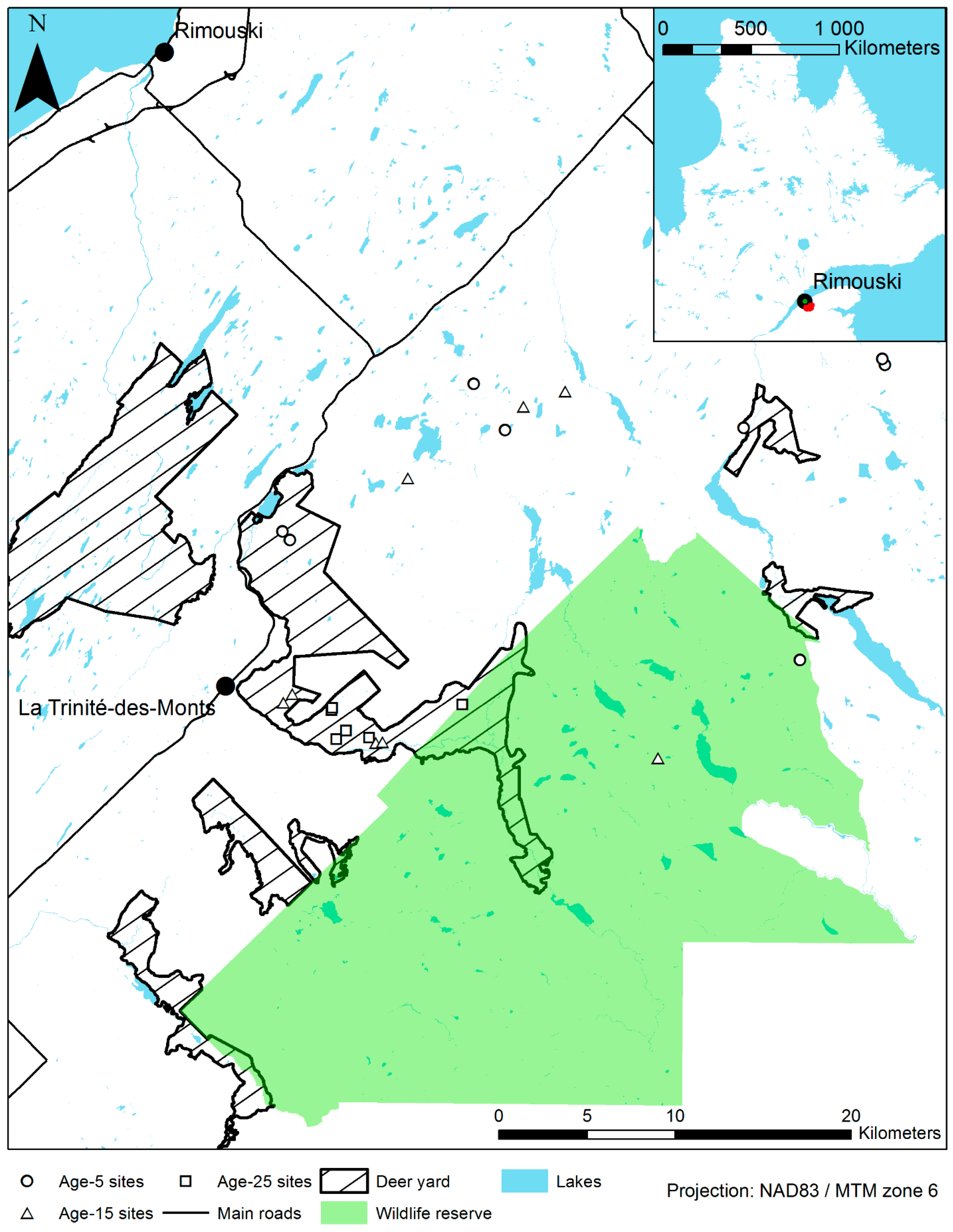
2.1. Study Area
2.2. Experimental Design
2.3. Sampling
2.4. Statistical Analyses
3. Results
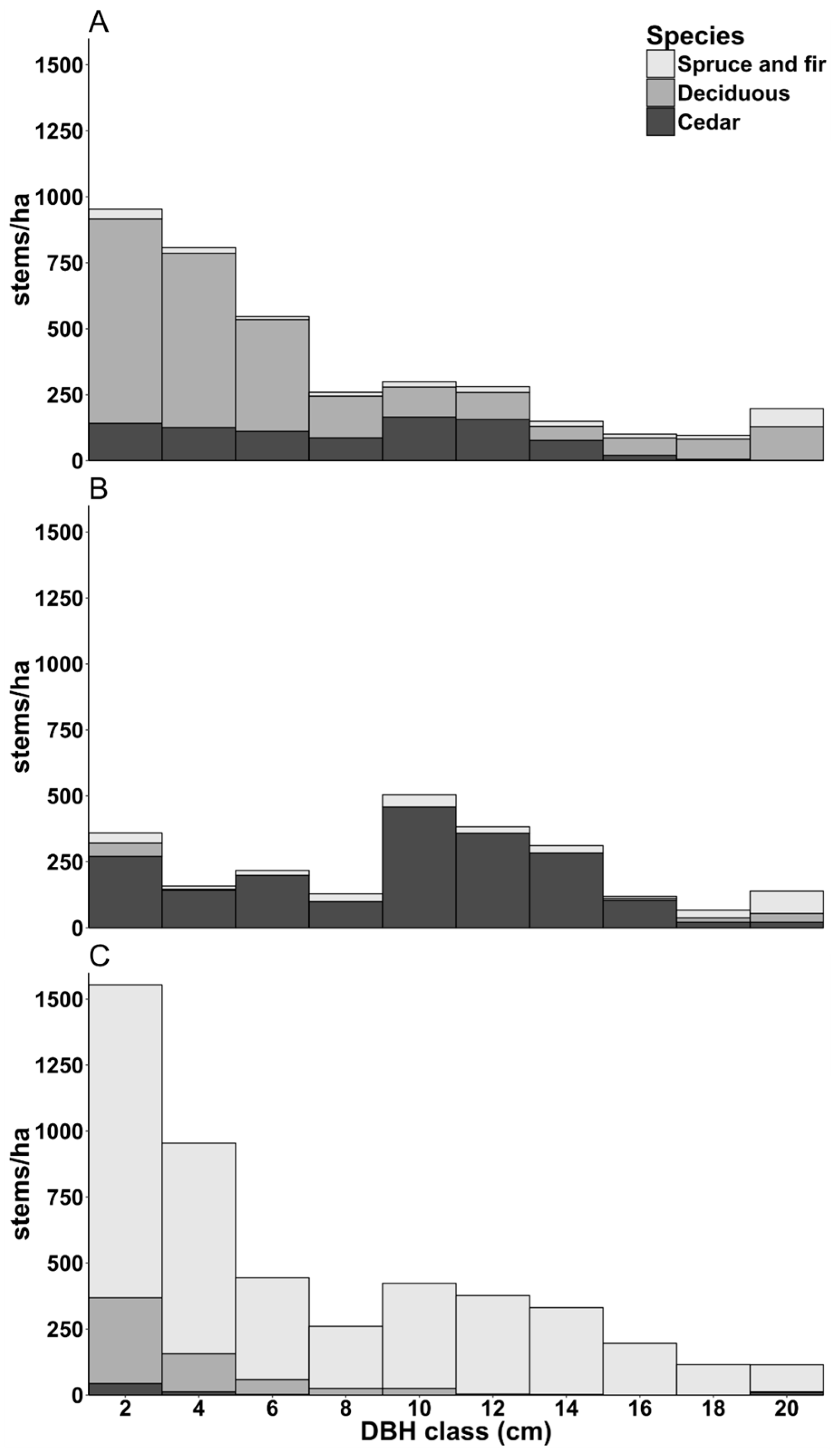
3.1. Overview of Cedar Plantations
3.2. Forking
3.3. Browsing
3.4. Competition Indices
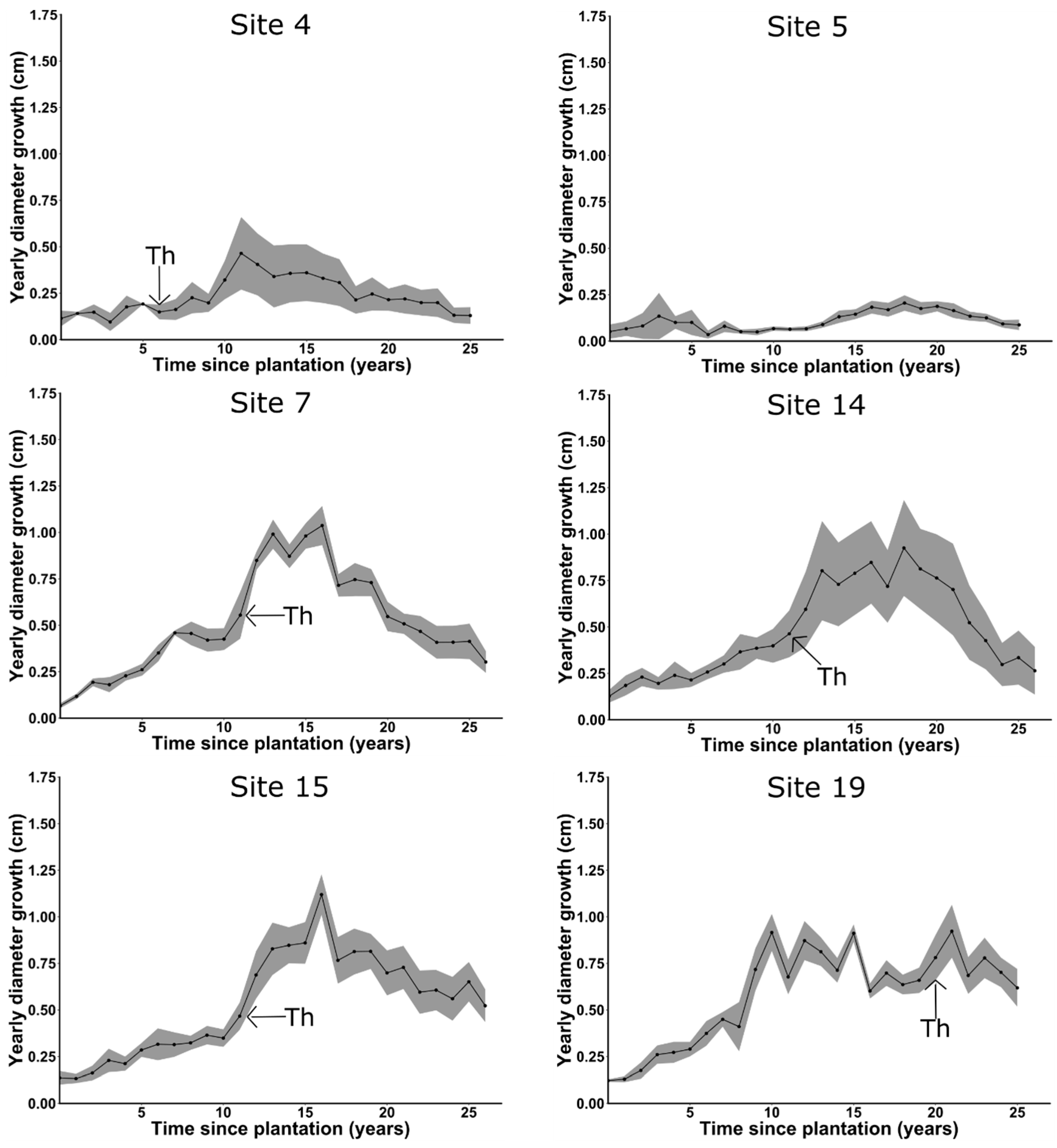
3.5. Cedar Growth
4. Discussion
4.1. Cedar Growth
4.2. Forking
4.3. Browsing
4.4. Management Implications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
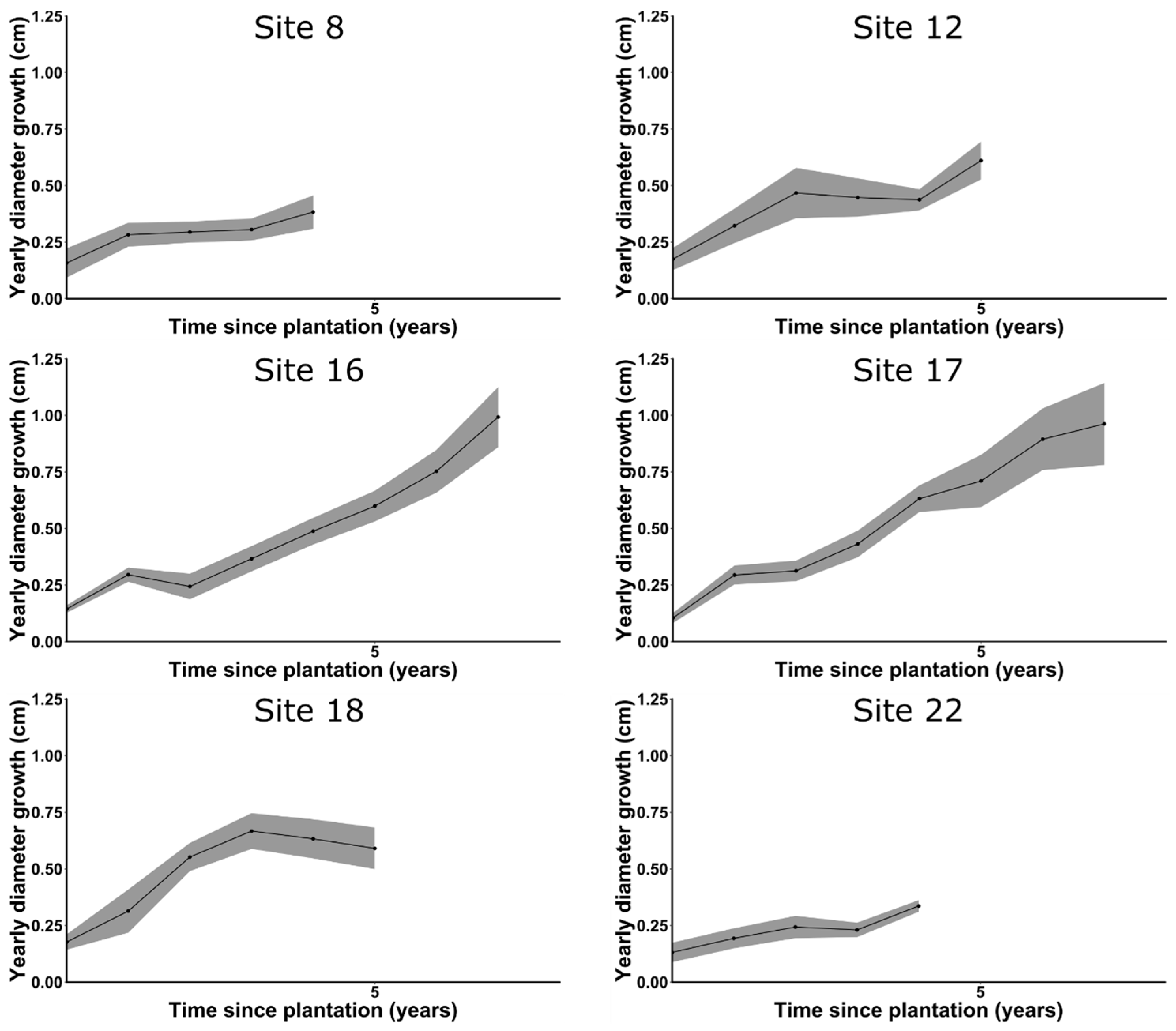

Appendix B

Appendix C
References
- Johnston, W.M. Thuja occidentalis L.—Northern white-cedar. In Silvics of North America; United States Department of Agriculture: Washington, DC, USA, 1990; pp. 580–589. [Google Scholar]
- Ullrey, D.E.; Youatt, W.G.; Johnson, H.E.; Fay, L.D.; Brent, B.E.; Kemp, K.E. Digestibility of Cedar and Balsam Fir Browse for the White-Tailed Deer. J. Wildl. Manag. 1968, 32, 162–171. [Google Scholar] [CrossRef]
- Morrison, S.F.; Forbes, G.J.; Young, S.J.; Lusk, S. Within-yard habitat use by white-tailed deer at varying winter severity. For. Ecol. Manag. 2003, 172, 173–182. [Google Scholar] [CrossRef]
- Dumont, A.; Ouellet, J.P.; Crête, M.; Huot, J. Winter foraging strategy of white-tailed deer at the northern limit of its range. Ecoscience 2005, 12, 476–484. [Google Scholar] [CrossRef]
- Hébert, F.; Hénault, M.; Lamoureux, J.; Bélanger, M.; Vachon, M.; Dumont, A. Guide d’aménagement des ravages de cerfs de Virginie, 4th ed.; Ministère des Ressources Naturelles et Ministère du Développement Durable: Ville de Québec, QC, Canada, 2013.
- Boulfroy, E.; Forget, E.; Hofmeyer, P.V.; Kenefic, L.S.; Larouche, C.; Lessard, G.; Lussier, J.M.; Pinto, F.; Ruel, J.C.; Weiskittel, A. Silvicultural Guide for Northern White-Cedar (Eastern White Cedar); U.S. Department of Agriculture: Washington, DC, USA, 2012.
- Cornett, M.W.; Frelich, L.E.; Puettmann, K.J.; Reich, P.B. Conservation implications of browsing by Odocoileus virginianus in remnant upland Thuja occidentalis forests. Biol. Conserv. 2000, 93, 359–369. [Google Scholar] [CrossRef]
- Dupuis, S.; Arseneault, D.; Sirois, L. Change from pre-settlement to present-day forest composition reconstructed from early land survey records in eastern Québec, Canada. J. Veg. Sci. 2011, 22, 564–575. [Google Scholar] [CrossRef]
- Hofmeyer, P.V.; Kenefic, L.S.; Seymour, R.S. Northern White Cedar Ecology and Silviculture in the Northeastern United States and Southeastern Canada: A Synthesis of Knowledge. North. J. Appl. For. 2009, 26, 21–27. [Google Scholar]
- Danneyrolles, V.; Dupuis, S.; Arseneault, D.; Terrail, R.; Leroyer, M.; Römer, A.; Fortin, G.; Boucher, Y.; Ruel, J.-C. Eastern white cedar long-term dynamics in eastern Canada: Implications for restoration in the context of ecosystem-based management. For. Ecol. Manag. 2017, 400, 502–510. [Google Scholar] [CrossRef]
- Cornett, M.W.; Reich, P.B.; Puettmann, K.J. Canopy feedbacks and microtopography regulate conifer seedling distribution in two Minnesota conifer-deciduous forests. Ecoscience 1997, 4, 353–364. [Google Scholar] [CrossRef]
- Simard, M.J.; Bergeron, Y.; Sirois, L. Substrate and litterfall effects on conifer seedling survivorship in southern boreal stands of Canada. Can. J. For. Res. 2003, 33, 672–681. [Google Scholar] [CrossRef]
- Heitzman, E.; Pregitzer, K.S.; Miller, R.O. Origin and early development of northern white-cedar stands in northern Michigan. Can. J. For. Res. 1997, 27, 1953–1961. [Google Scholar] [CrossRef]
- Rooney, T.P.; Solheim, S.L.; Waller, D.M. Factors affecting the regeneration of northern white cedar in lowland forests of the Upper Great Lakes region, USA. For. Ecol. Manag. 2002, 163, 119–130. [Google Scholar] [CrossRef]
- Verme, L.J.; Johnston, W.M. Regeneration of Northern White Cedar Deeryards in Upper Michigan. J. Wildl. Manag. 1986, 50, 307–313. [Google Scholar] [CrossRef]
- White, M.A. Long-term effects of deer browsing: Composition, structure and productivity in a northeastern Minnesota old-growth forest. For. Ecol. Manag. 2012, 269, 222–228. [Google Scholar] [CrossRef]
- Knox, M.W. Historical Changes in the Abundance and Distribution of Deer in Virginia. In The Science of Overabundance; Smithsonian Institution Scholarly Press: Washington, DC, USA; Chantilly, VA, USA; New York, NY, USA, 1997; pp. 27–36. [Google Scholar]
- McCabe, T.R.; McCabe, R.E. Recounting whitetails past. In The Science of Overabundance; Smithsonian Institution Scholarly Press: Washington, DC, USA; Chantilly, VA, USA; New York, NY, USA, 1997; pp. 11–26. [Google Scholar]
- Rooney, T.P.; Waller, D.M. Direct and indirect effects of white-tailed deer in forest ecosystems. For. Ecol. Manag. 2003, 181, 165–176. [Google Scholar] [CrossRef]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.P.; Dussault, C.; Waller, D.M. Ecological Impacts of deer Overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar] [CrossRef]
- Heitzman, E.; Pregitzer, K.S.; Miller, R.O.; Lanasa, M.; Zuidema, M. Establishment and development of northern white-cedar following strip clearcutting. For. Ecol. Manag. 1999, 123, 97–104. [Google Scholar] [CrossRef]
- Gauthier, I.; Bastien, H.; Lefort, S. État de Situation des Principales Espèces de petit Gibier Exploitées au Québec; Direction de l'expertise sur la faune et ses habitats, Ministère des ressources naturelles et de la faune: Ville de Québec, QC, Canada, 2008.
- Johnston, W.M. Balsam Fir Dominant Species under Rethinned Northern White-Cedar; U.S. Department of Agriculture: Washington, DC, USA, 1972.
- Peek, J.M.; Urich, D.L.; Mackie, R.J. Moose Habitat Selection and Relationships to Forest Management in Northeastern Minnesota. Wildl. Monogr. 1976, 48, 3–65. [Google Scholar]
- Danell, K.; Bergström, R.; Edenius, L. Effects of Large Mammalian Browsers on Architecture, Biomass, and Nutrients of Woody Plants. J. Mammal. 1994, 75, 833–844. [Google Scholar] [CrossRef]
- Ishii, H.T.; Ford, E.D.; Kennedy, M.C. Physiological and ecological implications of adaptive reiteration as a mechanism for crown maintenance and longevity. Tree Physiol. 2007, 27, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyer, P.V.; Seymour, R.S.; Kenefic, L.S. Influence of Soil Site Class on Growth and Decay of Northern White Cedar and Two Associates in Maine. North. J. Appl. For. 2009, 26, 68–75. [Google Scholar]
- Briand, C.H.; Posluszny, U.; Larson, W. Differential axis architecture in Thuja occidentalis (eastern white cedar). Can. J. Bot. 1992, 340–348. [Google Scholar] [CrossRef]
- Logan, K.T. Growth of Tree Seedlings as Affected by Light Intensity: Black Spruce, White Spruce, Balsam Fir, and Eastern White Cedar; Canadian Forest Service: Laurentian Hills, ON, Canada, 1969.
- Cornett, M.W.; Reich, P.B.; Puettmann, K.J.; Frelich, L.E. Seedbed and moisture availability determine safe sites for early Thuja occidentalis. Am. J. Bot. 2000, 87, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Hannah, P.R. Stand structures and height growth patterns in northern white cedar stands on wet sites in Vermont. North. J. Appl. For. 2004, 21, 173–179. [Google Scholar]
- Hofmeyer, P.V.; Kenefic, L.S.; Seymour, R.S. Historical Stem Development of Northern White Cedar (Thuja occidentalis L.) in Maine. North. J. Appl. For. 2010, 27, 92–96. [Google Scholar]
- Ruel, J.C.; Lussier, J.M.; Morissette, S.; Ricodeau, N. Growth Response of Northern White-Cedar (Thuja occidentalis) to Natural Disturbances and Partial Cuts in Mixedwood Stands of Quebec, Canada. Forests 2014, 5, 1194–1211. [Google Scholar] [CrossRef]
- Larouche, C.; Ruel, J.C. Development of Northern White-Cedar Regeneration Following Partial Cutting, with and without Deer Browsing. Forests 2015, 6, 344–359. [Google Scholar] [CrossRef]
- Huot, M.; Lebel, F. Plan de gestion du cerf de Virginie au Québec 2010–2017; Ministère des Ressources naturelles et de la Faune, Secteur Faune Québec: Ville de Québec, QC, Canada, 2012.
- Saucier, J.-P.; Grondin, P.; Robitaille, A.; Gosselin, J.; Morneau, C.; Richard, P.; Brisson, J.; Sirois, L.; Leduc, A.; Morin, H.; et al. Écologie forestière. In Manuel de foresterie, 2nd ed.; Ordre des ingénieurs forestiers du Québec: Ville de Québec, QC, Canada, 2009; pp. 165–316. [Google Scholar]
- Gagnon, L.; St-Hilaire, G.; Rioux, M. Sommaire du Plan d’aménagement forestier intégré tactique—Région du Bas-Saint-Laurent-UA 011-51; Ministère des Ressources Naturelles, Direction générale du Bas-Saint-Laurent: Rimouski, QC, Canada, 2014.
- Cyr, G. Guide des stations forestières de la région écologique 4f—Collines des moyennes Appalaches; Ministère des Ressources Naturelles, Direction des Inventaires Forestiers, Division de la Classification Écologique et Productivité des Stations: Ville de Québec, QC, Canada, 2014.
- Muiruri, E.W.; Milligan, H.T.; Morath, S.; Koricheva, J. Moose browsing alters tree diversity effects on birch growth and insect herbivory. Funct. Ecol. 2015, 29, 724–735. [Google Scholar] [CrossRef]
- Saunders, M.R.; Puettmann, K.J. Use of vegetational characteristics and browsing patterns to predict deer damage in eastern white pine (Pinus strobus) plantations. North. J. Appl. For. 1999, 16, 96–102. [Google Scholar]
- Larsson, L. CooRecorder Program of the CDendropackage Version 8.1; Cybis Elektronik: Saltsjöbaden, Sweden, 2016. [Google Scholar]
- Loffredo, N.; Sun, X.; Onda, Y. DHPT 1.0: New software for automatic analysis of canopy closure from under-exposed and over-exposed digital hemispherical photographs. Comput. Electron. Agric. 2016, 125, 39–47. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: Berlin, Germany, 2002; Volume 172. [Google Scholar]
- Mazerolle, M.J. Improving data analysis in herpetology: Using Akaike’s Information Criterion (AIC) to assess the strength of biological hypotheses. Amphibia-Reptilia 2006, 27, 169–180. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.15.6. Available online: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed online 7 January 2016).
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R Package Version 2.1-0. Available online: https://cran.r-project.org/web/packages/AICcmodavg/AICcmodavg.pdf (accessed online 20 June 2017).
- Tomé, M.; Burkhart, H.E. Distance-Dependent Competition Measures for Predicting Growth of Individual Trees. For. Sci. 1989, 35, 816–831. [Google Scholar]
- Kiernan, D.H.; Bevilacqua, E.; Nyland, R.D. Individual-tree diameter growth model for sugar maple trees in uneven-aged northern hardwood stands under selection system. For. Ecol. Manag. 2009, 256, 1579–1586. [Google Scholar] [CrossRef]
- Wykoff, W.R. A basal area increment model for individual conifers in the northern Rocky Mountains. For. Sci. 1990, 36, 1077–1104. [Google Scholar]
- Boulet, B. Synthèse des connaissances sur l’écologie des espèces concurrentes. In Le Guide sylvicole du Québec, tome 1: Les fondements biologiques de la sylviculture; Ministère des Forêts, de la Faune et des Parcs: Ville de Québec, QC, Canada, 2013; pp. 242–275. [Google Scholar]
- Forester, J.D.; Anderson, D.P.; Turner, M.G. Landscape and Local Factors Affecting Northern White Cedar (Thuja occidentalis) Recruitment in The Chequamegon-Nicolet National Forest, Wisconsin (U.S.A.). Am. Midl. Nat. 2008, 160, 438–453. [Google Scholar] [CrossRef]
- Hofmeyer, P.V.; Seymour, R.S.; Kenefic, L.S. Production ecology of Thuja occidentalis. Can. J. For. Res. 2010, 40, 1155–1164. [Google Scholar] [CrossRef]
- Bélanger, M.; Hélie, C. Plan d’aménagement du ravage du Canton Varin, plan d’intervention 2006–2010; Ministère des Ressources naturelles et de la Faune, Secteur Faune Québec et Secteur Forêt Québec, Direction Générale du Bas-Saint-Laurent: Rimouski, QC, Canada, 2006.
- Lefort, S.; Massé, S. Plan de gestion de l’orignal au Québec 2012–2019; Ministère des Forêts, de la Faune et des Parcs: Ville de Québec, QC, Canada, 2015.
- Barbosa, P.; Hines, J.; Kaplan, I.; Martinson, H.; Szczepaniec, A.; Szendrei, Z. Associational Resistance and Associational Susceptibility: Having Right or Wrong Neighbors. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 1–20. [Google Scholar] [CrossRef]
- Smit, C.; Ouden, J.D.; Müller-Schärer, H. Unpalatable Plants Facilitate Tree Sapling Survival in Wooded Pastures. J. Appl. Ecol. 2006, 43, 305–312. [Google Scholar] [CrossRef]
- Alm Bergvall, U.; Rautio, P.; Kesti, K.; Tuomi, J.; Leimar, O. Associational effects of plant defences in relation to within- and between-patch food choice by a mammalian herbivore: Neighbour contrast susceptibility and defence. Oecologia 2006, 147, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Paquette, A.; Messier, C. The role of plantations in managing the world’s forests in the Anthropocene. Front. Ecol. Environ. 2010, 8, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.J.; Paquette, A.; Cavender-Bares, J.; Messier, C.; Reich, P.B. Spatial complementarity in tree crowns explains overyielding in species mixtures. Nat. Ecol. Evol. 2017, 1, 63. [Google Scholar] [CrossRef] [PubMed]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
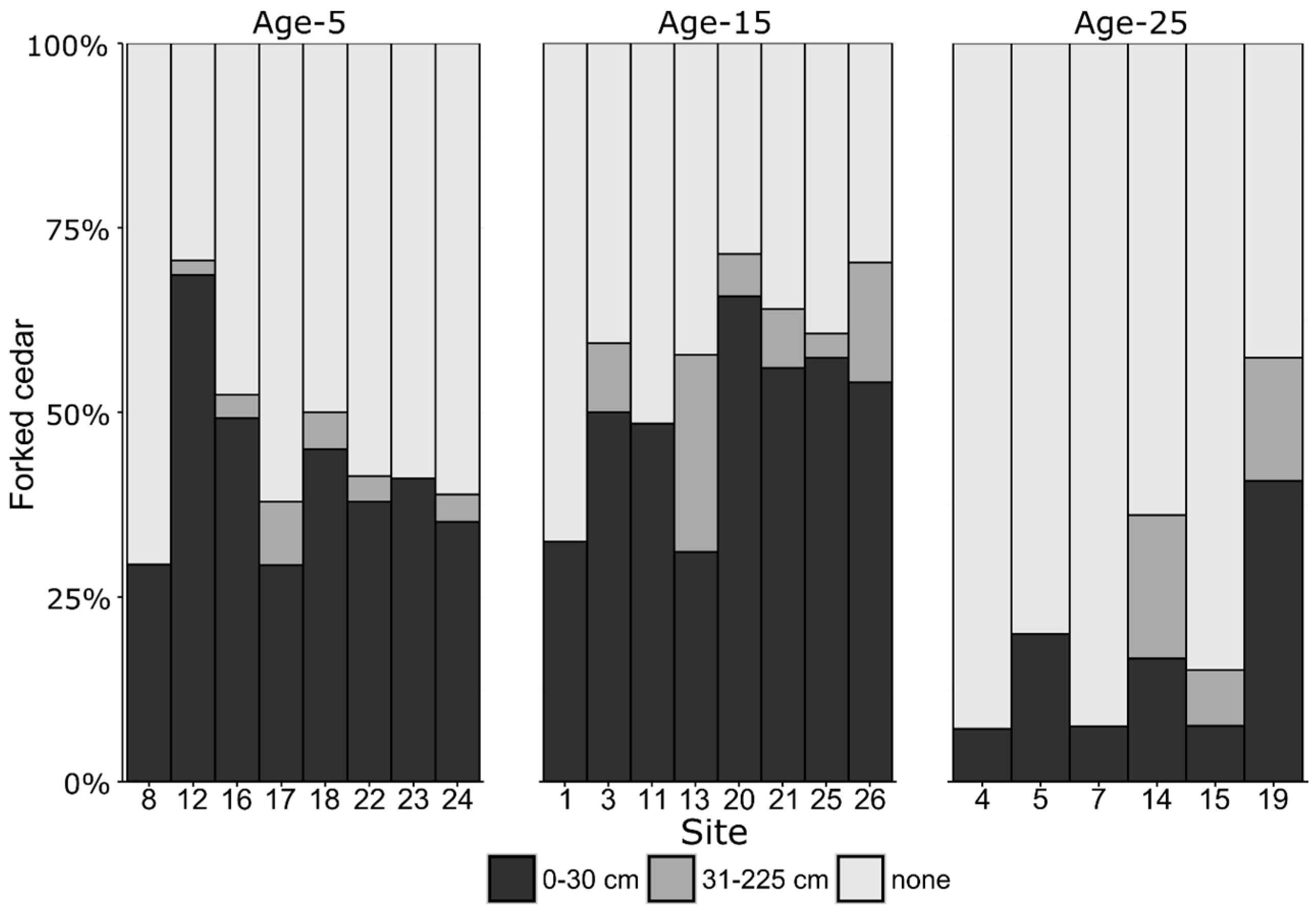
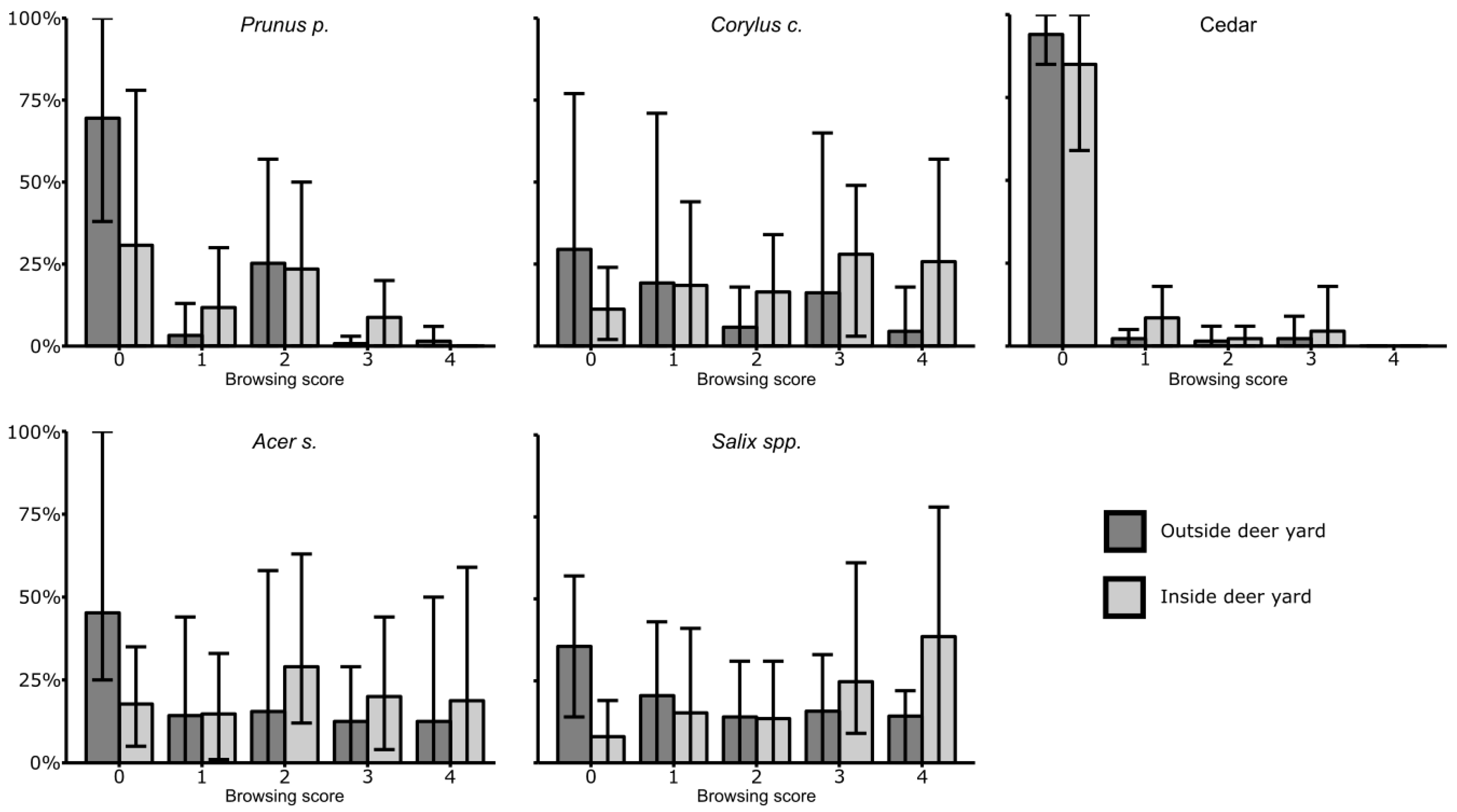
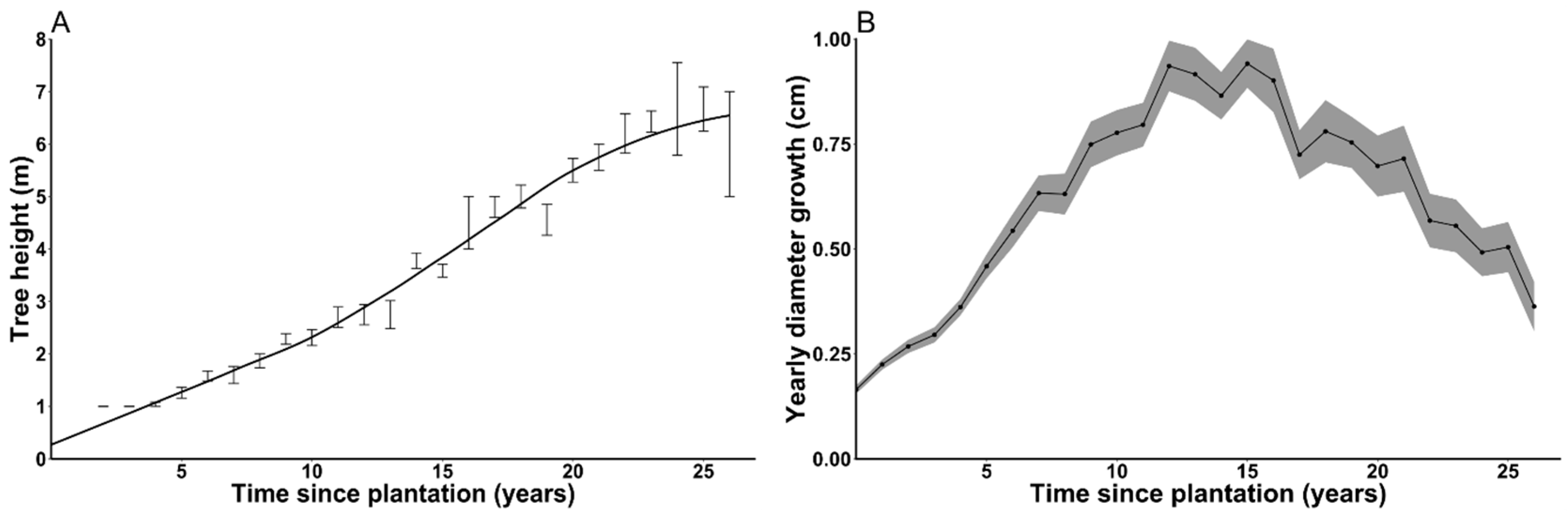
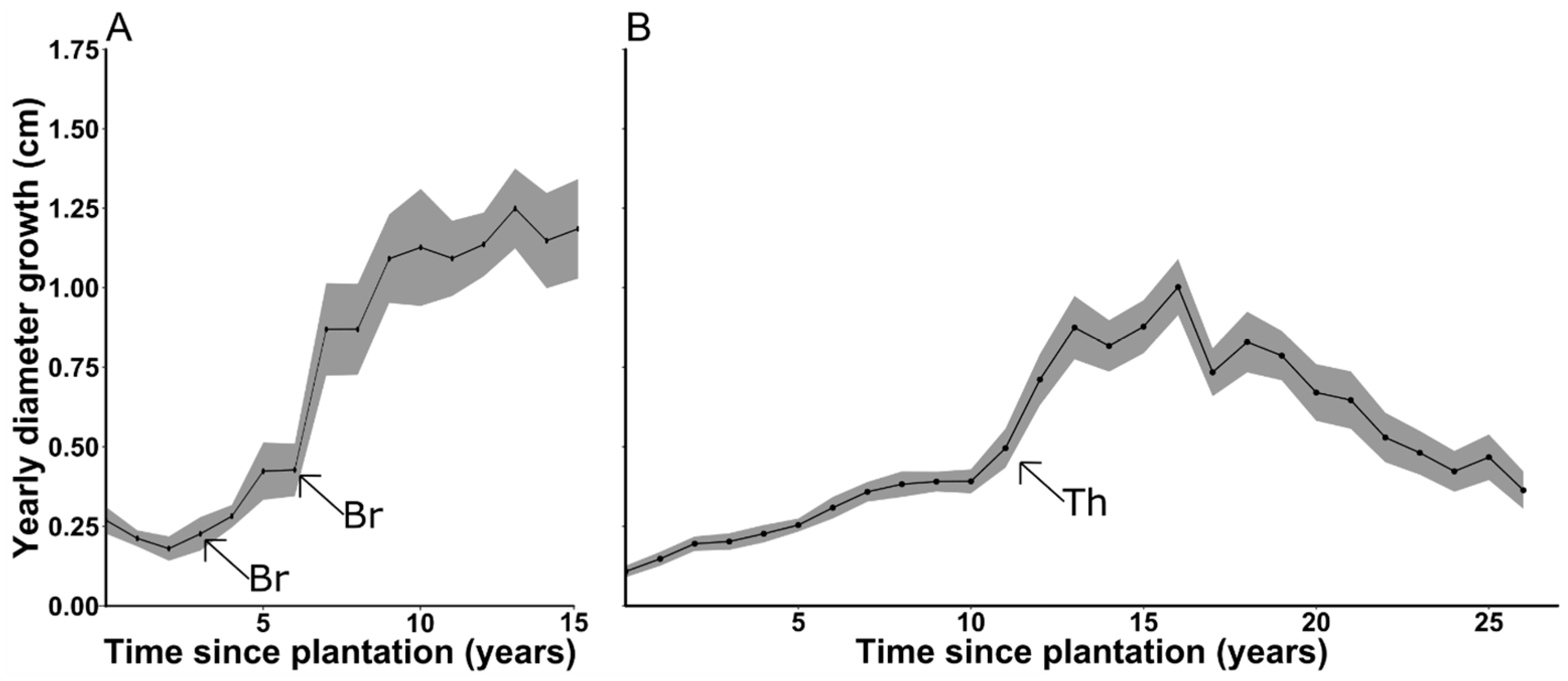
| Inside Deer Yard | Outside Deer Yard | |
|---|---|---|
| Age-5 | 3 | 5 |
| Age-15 | 4 | 4 |
| Age-25 | 6 | 0 |
| Index Name | Index Formula | Reference |
|---|---|---|
| DIq | [51] | |
| DIqdom | [51] | |
| DImax | [51] | |
| BIq | [51] | |
| BIqdom | [51] | |
| BImax | [51] | |
| BAPBA | [52] | |
| BAL | [53] | |
| BAB | [52] | |
| Canopy Closure | From hemispheric photographs |
| Site | Cedar Plantation Type | Deer Yard | Age | n | Dominant Tree Height (cm) | Root Collar Diameter (cm) | Competition Height (m) | Canopy Closure (%) |
|---|---|---|---|---|---|---|---|---|
| 7 | Age-25, deciduous cover, pure cedar planted | Inside | 27 | 30 | 661 ± 145 | 14.85 ± 4.46 | 11.7 ± 2.9 | 92 ± 5.1 |
| 15 | Inside | 27 | 30 | 652 ± 170 | 15.09 ± 4.62 | 13.2 ± 2.0 | 95 ± 0.0 | |
| 14 | Inside | 27 | 26 | 499 ± 223 | 12.05 ± 6.12 | 13.6 ± 2.5 | 95 ± 0.0 | |
| 19 | Age-25, thinned deciduous cover, pure cedar planted | Inside | 26 | 30 | 646 ± 116 | 18.39 ± 4.89 | 12.7 ± 0.9 | 77 ± 13.3 |
| 4 | Age-25, mixed cedar-spruce planted | Inside | 26 | 18 | 232 ± 116 | 4.58 ± 2.15 | 11.2 ± 2.1 | 95 ± 0.0 |
| 5 | Inside | 26 | 9 | 96 ± 63 | 2.24 ± 2.07 | 10.6 ± 2.1 | 87 ± 5.1 | |
| 13 | Age-15, pure cedar planted, no fir | Outside | 13 | 29 | 330 ± 63 | 6.36 ± 1.32 | N/A | 18 ± 5.2 |
| 1 | Outside | 13 | 26 | 285 ± 59 | 7.29 ± 2.62 | N/A | 27 ± 14.7 | |
| 21 | Age-15, pure cedar planted, with fir | Inside | 16 | 29 | 437 ± 47 | 12.01 ± 2.01 | 8.7 ± 2.6 | 36 ± 20.4 |
| 20 | Inside | 16 | 23 | 308 ± 113 | 7.82 ± 3.74 | 9.1 ± 2.3 | 53 ± 9.8 | |
| 25 | Outside | 12 | 30 | 415 ± 53 | 11.17 ± 2.48 | 5.8 ± 0.6 | 32 ± 15.1 | |
| 26 | Outside | 12 | 27 | 283 ± 63 | 9.12 ± 2.17 | 5.4 ± 0.7 | 18 ± 8.2 | |
| 11 | Age-15, mixed cedar-spruce planted | Inside | 14 | 19 | 150 ± 74 | 2.78 ± 1.49 | 6.7 ± 0.7 | 41 ± 11.4 |
| 3 | Inside | 12 | 24 | 272 ± 91 | 5.81 ± 2.61 | 6.8 ± 1.0 | 50 ± 0.0 | |
| 17 | Age-5, pure cedar planted | Outside | 8 | 29 | 299 ± 37 | 6.36 ± 1.32 | 4.2 ± 0.5 | 22 ± 20.4 |
| 16 | Outside | 8 | 30 | 279 ± 27 | 6.75 ± 1.60 | 3.3 ± 0.4 | 8 ± 5.2 | |
| 18 | Outside | 6 | 30 | 184 ± 30 | 4.59 ± 0.85 | N/A | N/A | |
| 12 | Outside | 6 | 25 | 181 ± 26 | 4.52 ± 0.87 | N/A | N/A | |
| 22 | Inside | 5 | 24 | 167 ± 22 | 3.26 ± 0.68 | N/A | N/A | |
| 24 | Inside | 5 | 25 | 122 ± 27 | 2.52 ± 0.66 | N/A | N/A | |
| 8 | Outside | 5 | 23 | 117 ± 30 | 2.74 ± 0.71 | N/A | N/A | |
| 23 | Inside | 5 | 30 | 76 ± 16 | 1.86 ± 0.42 | N/A | N/A |
| Model | AICc | ∆AICc | AICc Weight | Marginal Pseudo R2 |
|---|---|---|---|---|
| D0 (null model) | −128.81 | 19.05 | 0.00 | 0.41 |
| D0 + BAL | −147.86 | 0 | 0.99 | 0.59 |
| D0 + Canopy Closure | −133.35 | 14.51 | 0.01 | 0.46 |
| D0 + DIq | −127.14 | 20.72 | 0.00 | 0.42 |
| D0 + DIqdom | −127.11 | 20.75 | 0.00 | 0.42 |
| D0 + BIq | −127.10 | 20.76 | 0.00 | 0.42 |
| D0 + BImax | −126.83 | 21.03 | 0.00 | 0.40 |
| D0 + BIqdom | −126.81 | 21.04 | 0.00 | 0.41 |
| D0 + BAPBA | −126.65 | 21.21 | 0.00 | 0.40 |
| D0 + BAB | −126.64 | 21.22 | 0.00 | 0.40 |
| D0 + DImax | −126.64 | 21.22 | 0.00 | 0.41 |
| Model | K | AICc | ∆AICc | AICc Weight | Cumulative AICc Weight | Marginal Pseudo R2 |
|---|---|---|---|---|---|---|
| D0 + Wet + Curve + CI | 7 | −148.70 | 0.00 | 0.20 | 0.20 | 0.60 |
| D0 + Wet + CI | 6 | −148.03 | 0.67 | 0.14 | 0.34 | 0.58 |
| D0 + Wet + Curve + CI + RATIO | 8 | −147.98 | 0.72 | 0.14 | 0.47 | 0.62 |
| D0 + CI | 5 | −147.86 | 0.84 | 0.13 | 0.60 | 0.59 |
| D0 + Wet + CI + RATIO | 7 | −147.36 | 1.34 | 0.10 | 0.70 | 0.61 |
| D0 + Curve + CI | 6 | −146.81 | 1.90 | 0.08 | 0.78 | 0.60 |
| D0 + CI + RATIO | 6 | −146.70 | 2.00 | 0.07 | 0.85 | 0.61 |
| D0 + Curve + CI + RATIO | 7 | −145.50 | 3.20 | 0.04 | 0.89 | 0.62 |
| D0Hare + D0Ungu + Wet + Curve + CI | 11 | −144.30 | 4.40 | 0.02 | 0.91 | 0.64 |
| D0Hare + D0Ungu + Wet + CI | 10 | −143.64 | 5.06 | 0.02 | 0.93 | 0.63 |
| D0Hare + D0Ungu + CI | 9 | −143.50 | 5.20 | 0.01 | 0.94 | 0.63 |
| D0Hare + D0Ungu + Wet + Curve + CI + RATIO | 12 | −143.13 | 5.57 | 0.01 | 0.96 | 0.66 |
| Parameter | Estimate | Lower UCI | Upper UCI |
|---|---|---|---|
| Curve | 0.06 | −0.02 | 0.15 |
| CI | −0.08 | −0.11 | −0.06 |
| Wet | −0.12 | −0.24 | 0.01 |
| Fr | NA | NA | NA |
| RATIO | 0.06 | −0.03 | 0.15 |
| D0Hare | 0.00 | −0.01 | 0.00 |
| D0Ungu | 0.00 | −0.01 | 0.01 |
| D0 | 0.04 | 0.03 | 0.04 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villemaire-Côté, O.; Ruel, J.-C.; Sirois, L. Development of Northern White-Cedar (Thuja occidentalis L.) Plantations within and outside Deer Yards. Forests 2017, 8, 326. https://doi.org/10.3390/f8090326
Villemaire-Côté O, Ruel J-C, Sirois L. Development of Northern White-Cedar (Thuja occidentalis L.) Plantations within and outside Deer Yards. Forests. 2017; 8(9):326. https://doi.org/10.3390/f8090326
Chicago/Turabian StyleVillemaire-Côté, Olivier, Jean-Claude Ruel, and Luc Sirois. 2017. "Development of Northern White-Cedar (Thuja occidentalis L.) Plantations within and outside Deer Yards" Forests 8, no. 9: 326. https://doi.org/10.3390/f8090326





