Differentiating Structural and Compositional Attributes across Successional Stages in Chilean Temperate Rainforests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Measurements
2.3. Data Analyses
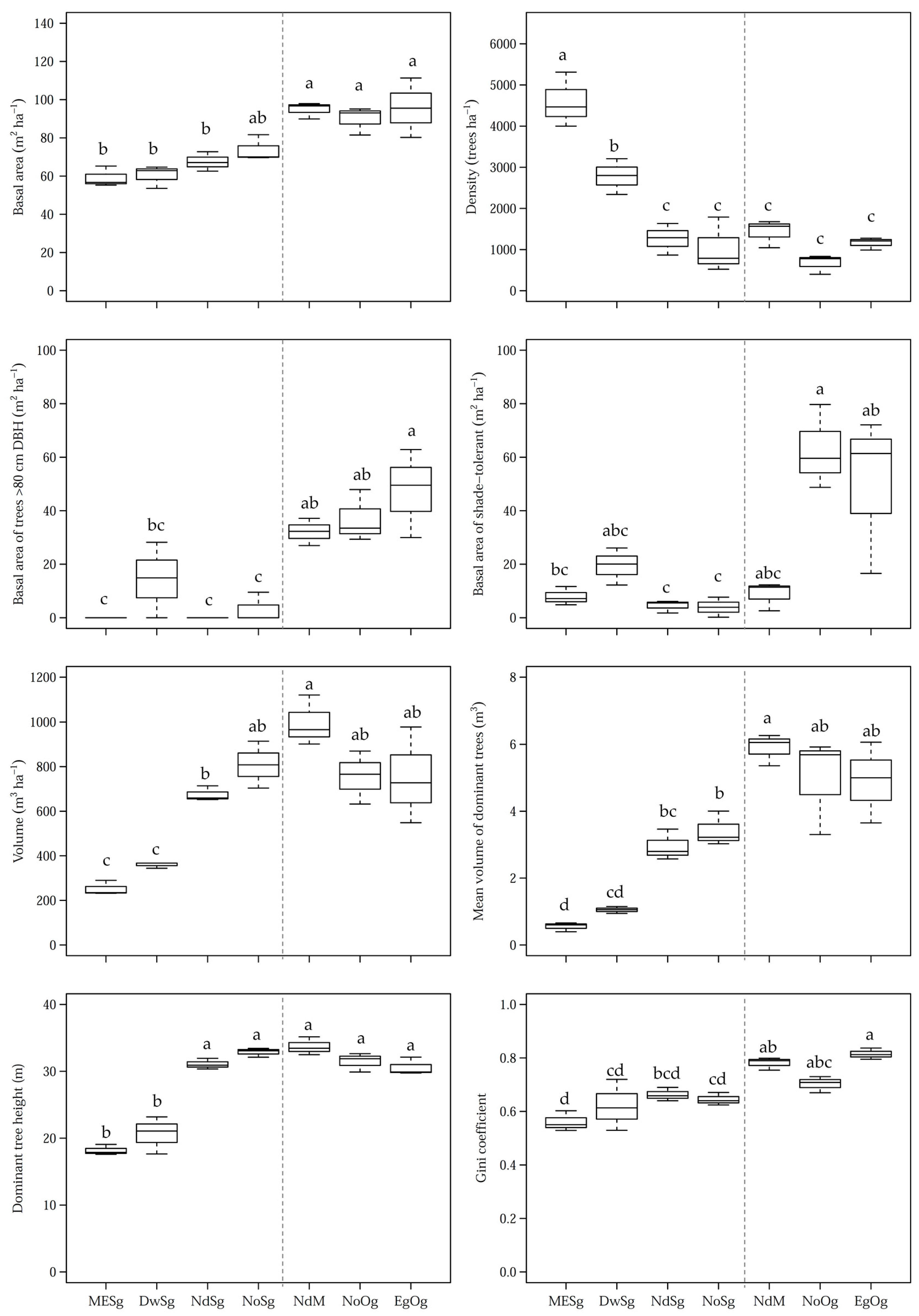
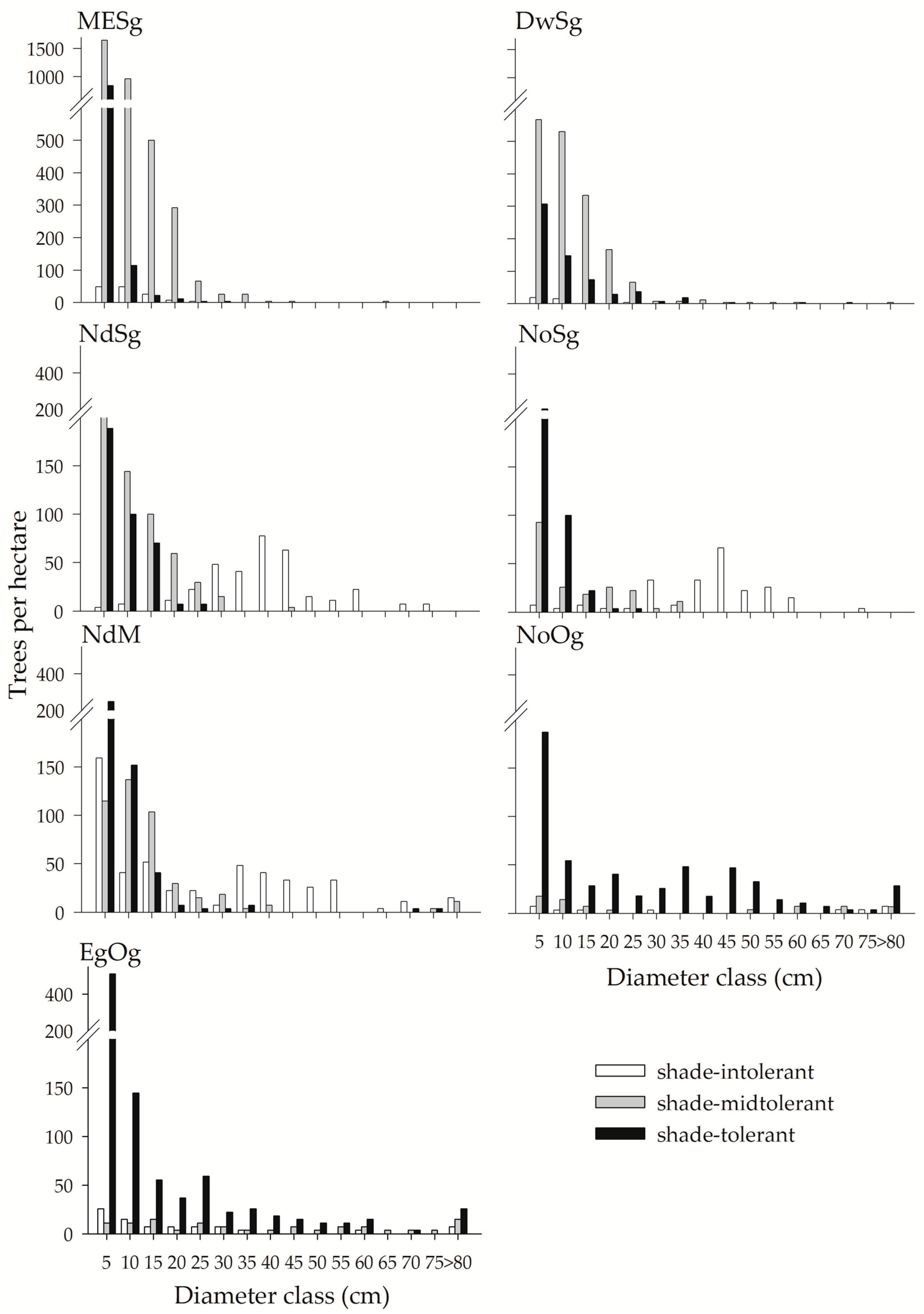
3. Results
4. Discussion and Conclusions
4.1. Causes and Patterns in the Differences between Second and Old-Growth Forests
4.2. Implications for Management and Old-Growth Restoration
Supplementary Materials
Supplementary File 1Acknowledgments
Conflicts of Interest
Appendix A. Fitting Results of the Weibull Probability Density Function for Tree Diameters by Type of Forest
| Type of Forest | Parameter | |
|---|---|---|
| MESg | 1.8923 | 12.6048 |
| DwSg | 1.7698 | 15.7581 |
| NdSg | 1.4174 | 22.9813 |
| NoSg | 1.3453 | 26.2232 |
| NdM | 1.1846 | 21.5971 |
| NoOg | 1.1889 | 32.5968 |
| EgOg | 1.0794 | 22.1172 |

References
- Armesto, J.J.; Smith-Ramírez, C.; Carmona, M.; Celis-Díez, J.L.; Díaz, I.; Gaxiola, A.; Gutierrez, A.; Nuñez-Avila, M.; Pérez, C.; Rozzi, R. Old-growth temperate Rainforests of South America: Conservation, Plant–Animal Interactions, and Baseline Biogeochemical Processes. In Old-Growth Forest: Function, Fate and Value; Wirth, C., Gleixner, G., Heimann, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 367–390. ISBN 978-3-540-92706-8. [Google Scholar]
- Wirth, C.; Gleixner, G.; Heimann, M. (Eds.) Old-Growth Forests: funtion, fate and value. In Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2009; Volume 207, ISBN 978-3-540-92705-1. [Google Scholar]
- Food and Agriculture Organization (FAO). State of the World´s Forests 2016; FAO: Rome, Italy, 2016; ISBN 978-92-5-109208-8. [Google Scholar]
- Chazdon, R.L. Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation; University of Chicago Press: Chicago, IL, USA, 2014; ISBN 9780226118079. [Google Scholar]
- Gutiérrez, A.G.; Armesto, J.J.; Aravena, J.C.; Carmona, M.; Carrasco, N.V.; Christie, D.A.; Peña, M.P.; Pérez, C.; Huth, A. Structural and environmental characterization of old-growth temperate rainforests of northern Chiloé Island, Chile: Regional and global relevance. For. Ecol. Manag. 2009, 258, 376–388. [Google Scholar] [CrossRef]
- Spies, T.A.; Franklin, J.F. The structure of natural young, mature, and old-growth Douglas-Fir forests in Oregon and Washington. In Wildlife and Vegetation of Unmanaged Douglas-Fir Forests; Ruggiero, L.F., Aubry, K.B., Carey, A.B., Huff, M.H., Eds.; U.S. Department of Agriculture, Forest Service: Portland, OR, USA, 1991; pp. 91–109. ISBN PNW-GTR-285. [Google Scholar]
- Aiba, S.I.; Hill, D.A.; Agetsuma, N. Comparison between old-growth stands and secondary stands regenerating after clear-felling in warm-temperate forests of Yakushima, southern Japan. For. Ecol. Manag. 2001, 140, 163–175. [Google Scholar] [CrossRef]
- D’Amato, A.W.; Orwig, D.A.; Foster, D.R. The influence of successional processes and disturbance on the structure of Tsuga Canadensis forests. Ecol. Appl. 2008, 18, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Lutz, J.A.; Larson, A.J.; Swanson, M.E.; Freund, J.A. Ecological importance of large-diameter trees in a temperate mixed-conifer forest. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, B.C.; Donoso, P.J. Effects of forest type and stand structure on coarse woody debris in old-growth rainforests in the Valdivian Andes, south-central Chile. For. Ecol. Manag. 2008, 255, 1906–1914. [Google Scholar] [CrossRef]
- Donoso, P.J.; Lusk, C.H. Differential effects of emergent Nothofagus dombeyi on growth and basal area of canopy species in an old-growth temperate rainforest. J. Veg. Sci. 2007, 18, 675. [Google Scholar] [CrossRef]
- Carmona, M.R.; Armesto, J.J.; Aravena, J.C.; Pérez, C.A. Coarse woody debris biomass in successional and primary temperate forests in Chiloé Island, Chile. For. Ecol. Manag. 2002, 164, 265–275. [Google Scholar] [CrossRef]
- Veblen, T.T.; Donoso, C.; Schlegel, F.M.; Escobar, B. Forest Dynamics in South-Central Chile. J. Biogeogr. 1981, 8, 211–247. [Google Scholar] [CrossRef]
- Donoso, C. Bosques Templados de Chile y Argentina: Variación, Estructura y Dinámica; Editorial Universitaria: Santiago, Chile, 1993; 484p. [Google Scholar]
- Donoso, P.J.; Cabezas, C.A.; Lavanderos, A.; Donoso, C. Desarrollo de renovales de coihue común (Nothofagus dombeyi (Mirb.) Oerst.) en la Cordillera de la Costa y de los Andes de la provincia de Valdivia en sus primeros 25 años. Bosque 1999, 20, 9–23. [Google Scholar] [CrossRef]
- Lusk, C.H.; Ortega, A. Vertical structure and basal area development in second-growth Nothofagus stands in Chile. J. Appl. Ecol. 2003, 40, 639–645. [Google Scholar] [CrossRef]
- Moorman, M.; Donoso, P.J.; Moore, S.E.; Sink, S.; Frederick, D. Sustainable Protected Area Management: The Case of Llancahue, a Highly Valued Periurban Forest in Chile. J. Sustain. For. 2013, 32, 783–805. [Google Scholar] [CrossRef]
- CONAF Sistema de Información Territorial. Available online: http://sit.conaf.cl/ (accessed on 22 March 2017).
- Veblen, T.T.; Alaback, P.B. A comparative review of forest dynamics and disturbance in the temperate rainforests of North and South America. In High-Latitude Rainforests and Associated Ecosystems of the West Coast of the Americas. Climate, Hydrology, Ecology, and Conservation; Lawford, R.G., Alaback, P., Fuentes, E., Eds.; Springer: New York, NY, USA, 1995; pp. 173–215. [Google Scholar]
- Donoso, P.J.; Frêne, C.; Flores, M.; Moorman, M.C.; Oyarzún, C.E.; Zavaleta, J.C. Balancing water supply and old-growth forest conservation in the lowlands of south-central Chile through adaptive co-management. Landsc. Ecol. 2014, 29, 245–260. [Google Scholar] [CrossRef]
- Tecklin, D.; Dellasala, D.A.; Luebert, F.; Pliscoff, P. Valdivian temperate rainforests of Chile and Argentina. In Temperate and Boreal Rainforests of the World: Ecology and Conservation; Dellasala, D.A., Ed.; Island Press: Washington, DC, USA, 2010; pp. 132–153. ISBN 9781610910088. [Google Scholar]
- Franklin, J.F.; Lindenmayer, D.; Thornburgh, D.; Van Pelt, R.; Chen, J.; Spies, T.A.; Carey, A.B.; Shaw, D.C.; Berg, D.R.; Harmon, M.E.; et al. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manag. 2002, 155, 399–423. [Google Scholar] [CrossRef]
- Donoso, P.J. Crown index: A canopy balance indicator to assess growth and regeneration in uneven-aged forest stands of the Coastal Range of Chile. Forestry 2005, 78, 337–351. [Google Scholar] [CrossRef]
- Donoso, P.J.; Nyland, R.D. Seedling density according to structure, dominance and understory cover in old-growth forest stands of the evergreen forest type in the coastal range of Chile. Rev. Chil. Hist. Nat. 2005, 78, 51–63. [Google Scholar] [CrossRef]
- Salas, C.; García, O. Modelling height development of mature Nothofagus obliqua. For. Ecol. Manag. 2006, 229, 1–6. [Google Scholar] [CrossRef]
- Hajek, E.R.; di Castri, F. Bioclimatografía de Chile; Dirección de Investigación Vice-Rectoría Académica Universidad Católica de Chile: Santiago, Chile, 1975; p. 225. [Google Scholar]
- Centro de Informaciόn de Recursos Naturales (CIREN). Estudio Agrológico de la Provincia de Valdivia—X Región; CIREN: Santiago, Chile, 1999. [Google Scholar]
- Centro de Informaciόn de Recursos Naturales (CIREN). Estudio Agrológico de la IX Región; CIREN: Santiago, Chile, 2002. [Google Scholar]
- Donoso, P.J. Establishment of the Ecological Bases for the Application of Uneven-Aged Silviculture in Chilean Evergreen Forests; University of New York: New York, NY, USA, 2002. [Google Scholar]
- Soto, D.P.; Salas, C.; Donoso, P.J.; Uteau, D. Heterogeneidad estructural y espacial de un bosque mixto dominado por Nothofagus dombeyi después de un disturbio parcial. Rev. Chil. Hist. Nat. 2010, 83, 335–347. [Google Scholar] [CrossRef]
- González, M.E.; Szejner, P.; Donoso, P.J.; Salas, C. Fire, logging and establishment patterns of second-growth forests in south-central Chile: Implications for their management and restoration. Cienc. Investig. Agrar. 2015, 42, 415–425. [Google Scholar] [CrossRef]
- Thomas, J.W.; Anderson, R.G.; Maser, C.; Bull, E.L. Snags. In Wildlife Habitatsas in Managed Forests, the Blue Montains of Oregon and Washington; USDA: Washington, DC, USA, 1979; pp. 60–77. [Google Scholar]
- Van der Maarel, E. Transformation of Cover-Abundance Values in Phytosociology and Its Effects on Community Similarity. Vegetatio 1979, 39, 97–114. [Google Scholar]
- Pabst, R.J. Methodology for developing the old-growth index. In Definition and Inventory of Old-Growth Forests on DNR-Managed State Lands; Franklin, J.F., Spies, T.A., Van Pelt, R., Eds.; Washington State Department of Natural Resources: Washington, DC, USA, 2005; p. 74. [Google Scholar]
- Mosseler, A.; Lynds, J.A.; Major, J.E. Old-growth forests of the Acadian Forest Region. Environ. Rev. 2003, 11, S47–S77. [Google Scholar] [CrossRef]
- Lexerød, N.L.; Eid, T. An evaluation of different diameter diversity indices based on criteria related to forest management planning. For. Ecol. Manag. 2006, 222, 17–28. [Google Scholar] [CrossRef]
- Steen, O.A.; Dawson, R.J.; Armleder, H.M. An old-growth index for Douglas-fir stands in portions of the Interior Douglas-fir zone, central British Columbia. BC J. Ecosyst. Manag. 2008, 9, 31–47. [Google Scholar]
- D’Amato, A.W.; Orwig, D.A.; Foster, D.R. Understory vegetation in old-growth and second-growth Tsuga canadensis forests in western Massachusetts. For. Ecol. Manag. 2009, 257, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Salas, C.; Real, P. Biometría de los bosques naturales de Chile: estado del arte. In Silvicultura en los Bosques Nativos: Avances en la Investigación en Chile, Argentina y Nueva Zelanda; Donoso, P.J., Promis, A., Eds.; Editorial María Cuneo: Valdivia, Chile, 2013; pp. 109–151. [Google Scholar]
- Salas, C. Ajuste y validación de ecuaciones de volumen para un relicto del bosque de Roble-Laurel-Lingue. Bosque 2002, 23, 81–92. [Google Scholar]
- Husch, B.; Beers, T.W.; Kershaw, J.A. Forest Mensuration, 5th ed.; Wiley: Hoboken, NJ, USA, 2002; ISBN 978-0-471-01850-6. [Google Scholar]
- Garcia, O.; Batho, A. Top height estimation in lodgepole pine sample plots. West. J. Appl. For. 2005, 20, 64–68. [Google Scholar]
- Stefańska-Krzaczek, E. Species diversity across the successional gradient of managed Scots pine stands in oligotrophic sites (SW Poland). J. For. Sci. 2012, 58, 345–356. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2016; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Goff, F.G.; West, D. Canopy-understory interaction effects on forest population structure. For. Sci. 1975, 21, 98–108. [Google Scholar]
- Bormann, F.H.; Likens, G.E. Pattern and Process in a Forested Ecosystem; Springer: New York, NY, USA, 1979; ISBN 978-0-387-94344-2. [Google Scholar]
- Oliver, C.; Larson, B. Forest Stand Dynamics, 1st ed.; Wiley: New York, NY, USA, 1996; ISBN 978-0471138334. [Google Scholar]
- Navarro, C.; Donoso, C.; Sandoval, V.; Gonzalez, C. Evaluación de raleos en un renoval de canelo (Drimys winteri (Forst.)) en la Cordillera de la Costa de Valdivia, Chile. Bosque 1997, 18, 51–65. [Google Scholar] [CrossRef]
- Donoso, P.J.; Soto, D.P.; Schlatter, J.E.; Büchner, C.A. Effects of early fertilization on the performance of Nothofagus dombeyi planted in the Coastal Range of south-central Chile. Cienc. Investig. Agrar. 2009, 36, 475–486. [Google Scholar] [CrossRef]
- Spies, T.A.; Franklin, J.F. Old growth and forest dynamics in the douglas-fir region of western oregon and washington usa. Nat. Areas J. 1988, 8, 190–201. [Google Scholar]
- Caviedes, J.; Ibarra, J.T. Influence of Anthropogenic Disturbances on Stand Structural Complexity in Andean Temperate Forests: Implications for Managing Key Habitat for Biodiversity. PLoS ONE 2017, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.F.; Van Pelt, R. Spatial aspects of structural complexity in Old-Growth Forests. J. For. 2004, 102, 22–28. [Google Scholar]
- Spies, T. Forest stand structure, composition, and function. In Creating a Forestry for the 21st Century: The Science of Ecosystem Management; Kohm, K., Franklin, J.F., Eds.; Island Press: New York, NY, USA, 1997; pp. 11–30. [Google Scholar]
- Lindenmayer, D.B.; Franklin, J.F. Towards Forest Sustainability; CSIRO Publishing: Clayton, Australia, 2003; ISBN 9780643100053. [Google Scholar]
- Veblen, T.T.; Ashton, D.H.; Schlegel, F.M.; Veblen, T.T.; Ashton, D.H.; Schlegel, M. Tree regeneration strategies in a lowland Nothofagus- dominated forest in south-central Chile. J. Biogeogr. 1979, 6, 329–340. [Google Scholar] [CrossRef]
- Keeton, W.S. Managing for late-successional/old-growth characteristics in northern hardwood-conifer forests. For. Ecol. Manag. 2006, 235, 129–142. [Google Scholar] [CrossRef]
- Harrington, C.A.; Roberts, S.D.; Brodie, L.C. Tree and Understory Responses to Variable- Density Thinning in Western Washington. In Balancing Ecosystem Values: Innovative Experiments for Sustainable Forestry; Peterson, C.E., Maguire, D.A., Eds.; USDA Forest Service General Technical Report PNWGTR; USDA Forest Service: Portland, OR, USA, 2010; pp. 97–106. [Google Scholar]
- Guldin, J.M. The silviculture of restoration: a historical perspective with contemporary application. In Integrated Restoration of Forested Ecosystems to Achieve Multiresource Benefits, Proceedings of the 2007 National Silviculture Workshop, Portland, OR, USA, 7–10 May 2007; DeaL, R.L., Ed.; Gen. Tech. Rep. PNW-GTR-733; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2008; pp. 23–35. [Google Scholar]
- Puettmann, K.J.; Coates, K.D.; Messier, C. A Critique of Silviculture: Managing for Complexity, 1st ed.; Island Press: Washington, DC, USA, 2008; ISBN 1597261467. [Google Scholar]

| Site | Area (ha) | Location | Forest Type | Code | Main Canopy Tree Species * | Total Area Per Site (ha) |
|---|---|---|---|---|---|---|
| Llancahue | 1270 | 39°84′ S 73°14′ W | Mixed evergreen second-growth | MESg | Ec, Lp, Dw | 287.4 |
| Drimys winteri second-growth | DwSg | Dw, Lp, Ec | 4.5 | |||
| Nothofagus dombeyi second-growth | NdSg | Nd, Ec, Dw | 110.4 | |||
| Nothofagus dombeyi mature | NdM | Nd, Ec, Dw | 162.7 | |||
| Evergreen old-growth | EgOg | Ec, Lp, Ap | 564.9 | |||
| Rucamanque | 435 | 38°66′ S 72°59′ W | Nothofagus obliqua second-growth | NoSg | No, Lp, Ap | 70.4 |
| Nothofagus obliqua old-growth | NoOg | No, Lp, Ap | 229.6 |
| Variables | |
|---|---|
| Density (trees ha−1) | Coarse woody debris (CWD; Mg ha−1) |
| Basal area (m2 ha−1) | Density of snags (n ha−1) |
| Basal area of trees >80 cm d (m2 ha−1) | Basal area of snags (m2 ha−1) |
| Basal area of shade-intolerant species (m2 ha−1) | Total richness of tree species |
| Basal area of shade mid-tolerant (m2 ha−1) | Richness of shade-intolerant tree species |
| Basal area of shade-tolerant species (m2 ha−1) | Richness of shade mid-tolerant tree species |
| Volume (m3 ha−1) | Richness of shade-tolerant tree species |
| Mean volume of dominant trees (m3) | Richness of vascular species in the understory |
| Dominant tree height (m) | Shannon diversity index |
| Gini coefficient | |
| Variable | Type of Forest | ||||||
|---|---|---|---|---|---|---|---|
| MESg | DwSg | NdSg | NoSg | NdM | NoOg | EgOg | |
| Density (trees ha−1) | 4593 ± 543 a | 2783 ± 355 b | 1263 ± 314 c | 1033 ± 545 c | 1430 ± 276 c | 670 ± 193 c | 1159 ± 124 c |
| Basal area (m2 ha−1) | 59.1 ± 4.4 b | 60.4 ± 4.9 b | 67.5 ± 4.2 b | 73.8 ± 5.6 ab | 94.9 ± 3.5 a | 89.9 ± 6 a | 95.7 ± 12.7 a |
| Basal area of trees >80 cm d (m2 ha−1) | 0 ± 0 c | 14.4 ± 11.5 bc | 0 ± 0 c | 3.2 ± 4.5 c | 32.1 ± 4.2 ab | 36.9 ± 8 ab | 47.4 ± 13.5 a |
| Basal area of shade-intolerant species (m2 ha−1) | 2.4 ± 0.8 b | 0.8 ± 1 b | 52.3 ± 4.5 a | 63.2 ± 8.7 a | 58.6 ± 17.2 a | 11.7 ± 13 b | 6.7 ± 9.5 b |
| Basal area of mid-tolerant species (m2 ha−1) | 48.8 ± 3.1 a | 40.1 ± 11.2 ab | 10.7 ± 2.9 bc | 6.7 ± 2.5 c | 27.5 ± 9.9 abc | 15.5 ± 12.5 abc | 39 ± 25.3 abc |
| Basal area of shade-tolerant species (m2 ha−1) | 7.9 ± 2.8 bc | 19.4 ± 5.7 abc | 4.5 ± 1.9 c | 3.9 ± 3.1 c | 8.8 ± 4.4 abc | 62.7 ± 12.8 a | 50 ± 24.1 ab |
| Volume (m3 ha−1) | 252.2 ± 26.8 c | 359.6 ± 10.9 c | 675.1 ± 27.7 b | 808.8 ± 85.8 ab | 996.2 ± 92.2 a | 756 ± 97.3 ab | 751.3 ± 176.2 ab |
| Mean volume of dominant trees (m3) | 0.6 ± 0.1 d | 1 ± 0.1 cd | 2.9 ± 0.4 bc | 3.4 ± 0.4 b | 5.9 ± 0.4 a | 5 ± 1.2 ab | 4.9 ± 1 ab |
| Dominant tree height (m) | 18.2 ± 0.6 b | 20.6 ± 2.3 b | 31.1 ± 0.7 a | 32.9 ± 0.6 a | 33.7 ± 1.1 a | 31.5 ± 1.2 a | 30.6 ± 1.1 a |
| Gini coefficient | 0.56 ± 0.03 d | 0.62 ± 0.08 cd | 0.66 ± 0.02 bcd | 0.65 ± 0.02 cd | 0.78 ± 0.02 ab | 0.7 ± 0.02 abc | 0.81 ± 0.02 a |
| Coarse woody debris (Mg ha−1) | 23 ± 21.8 | 57.2 ± 21.5 | 11 ± 5.4 | 12.3 ± 2.2 | 23.6 ± 9.4 | 32 ± 3.8 | 54.4 ± 15 |
| Density of snags (trees ha−1) | 0 ± 0 | 12 ± 13 | 8 ± 13 | 7 ± 9 | 26 ± 23 | 3 ± 6 | 9 ± 8 |
| Basal area of snags (m2 ha−1) | 0 ± 0 | 6.3 ± 7.1 | 1 ± 0.7 | 4.6 ± 2.4 | 3.2 ± 2.3 | 8.8 ± 5.1 | 13.8 ± 11.4 |
| Total richness of tree species | 16 ± 1 | 10 ± 0 | 11 ± 1 | 9 ± 2 | 9 ± 0 | 8 ± 2 | 9 ± 3 |
| Richness of shade-intolerant tree species | 2 ± 0 | 1 ± 0 | 1 ± 0 | 2 ± 0 | 2 ± 0 | 1 ± 1 | 1 ± 1 |
| Richness of mid-tolerant tree species | 9 ± 0 | 5 ± 0 | 5 ± 0 | 4 ± 1 | 5 ± 0 | 4 ± 1 | 5 ± 2 |
| Richness of shade-tolerant tree species | 6 ± 1 a | 4 ± 0 abc | 4 ± 0 ab | 3 ± 0 bc | 3 ± 0 c | 3 ± 0 c | 4 ± 0 abc |
| Richness of vascular species in the understory | 18 ± 3 abc | 24 ± 5 a | 21 ± 2 ab | 10 ± 1 c | 22 ± 6 a | 11 ± 1 bc | 20 ± 2 abc |
| Shannon diversity index | 1.08 ± 0.65 b | 2.09 ± 0.48 ab | 1.77 ± 0.19 ab | 1.58 ± 0.16 ab | 2.73 ± 0.39 a | 1.73 ± 0.14 ab | 2.49 ± 0.14 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponce, D.B.; Donoso, P.J.; Salas-Eljatib, C. Differentiating Structural and Compositional Attributes across Successional Stages in Chilean Temperate Rainforests. Forests 2017, 8, 329. https://doi.org/10.3390/f8090329
Ponce DB, Donoso PJ, Salas-Eljatib C. Differentiating Structural and Compositional Attributes across Successional Stages in Chilean Temperate Rainforests. Forests. 2017; 8(9):329. https://doi.org/10.3390/f8090329
Chicago/Turabian StylePonce, Diego B., Pablo J. Donoso, and Christian Salas-Eljatib. 2017. "Differentiating Structural and Compositional Attributes across Successional Stages in Chilean Temperate Rainforests" Forests 8, no. 9: 329. https://doi.org/10.3390/f8090329




