Ten Years of Monitoring Illustrates a Cascade of Effects of White Pine Blister Rust and Focuses Whitebark Pine Restoration in the Canadian Rocky and Columbia Mountains
Abstract
:1. Introduction
2. Study Area and Methods
3. Results
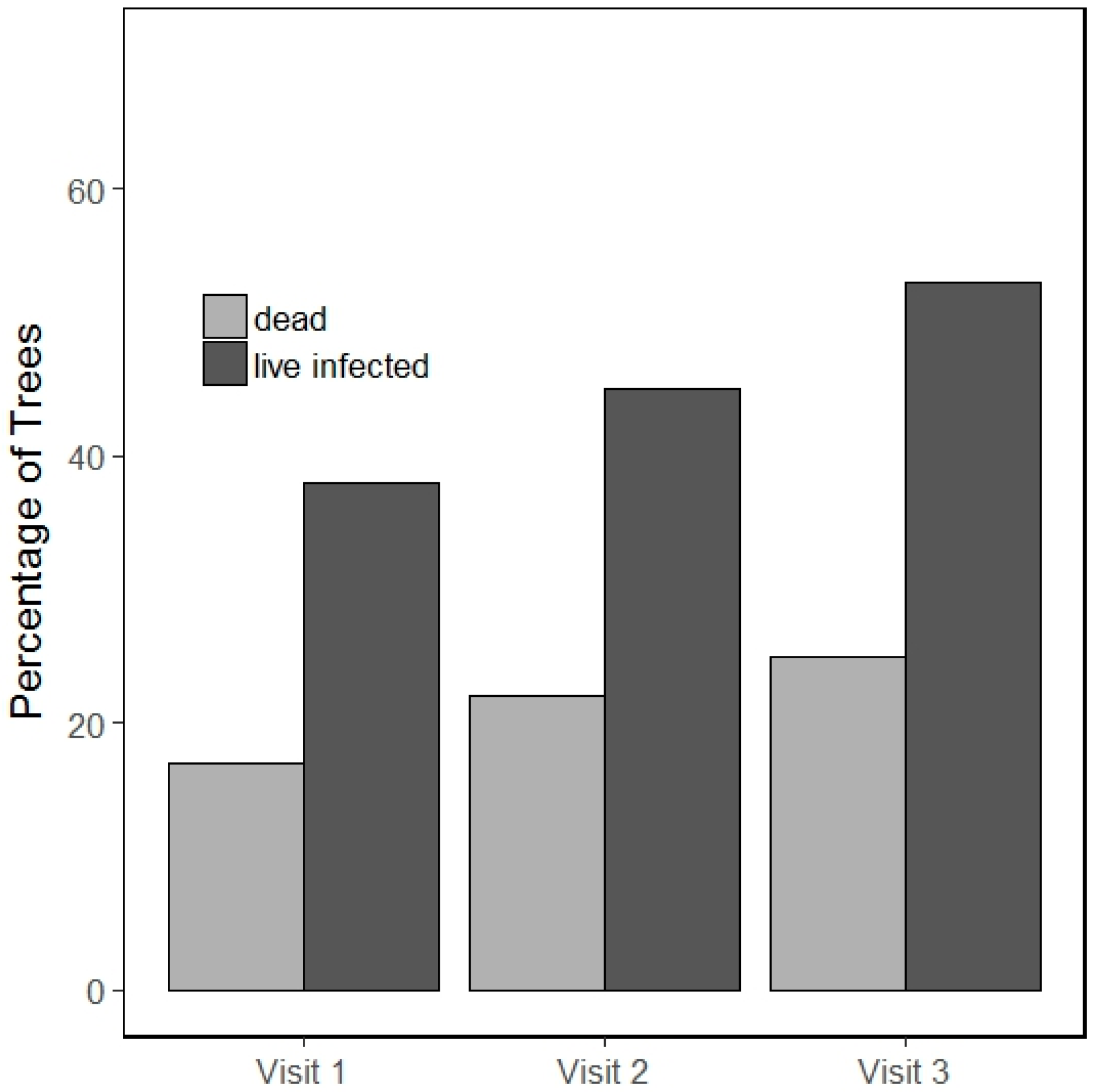
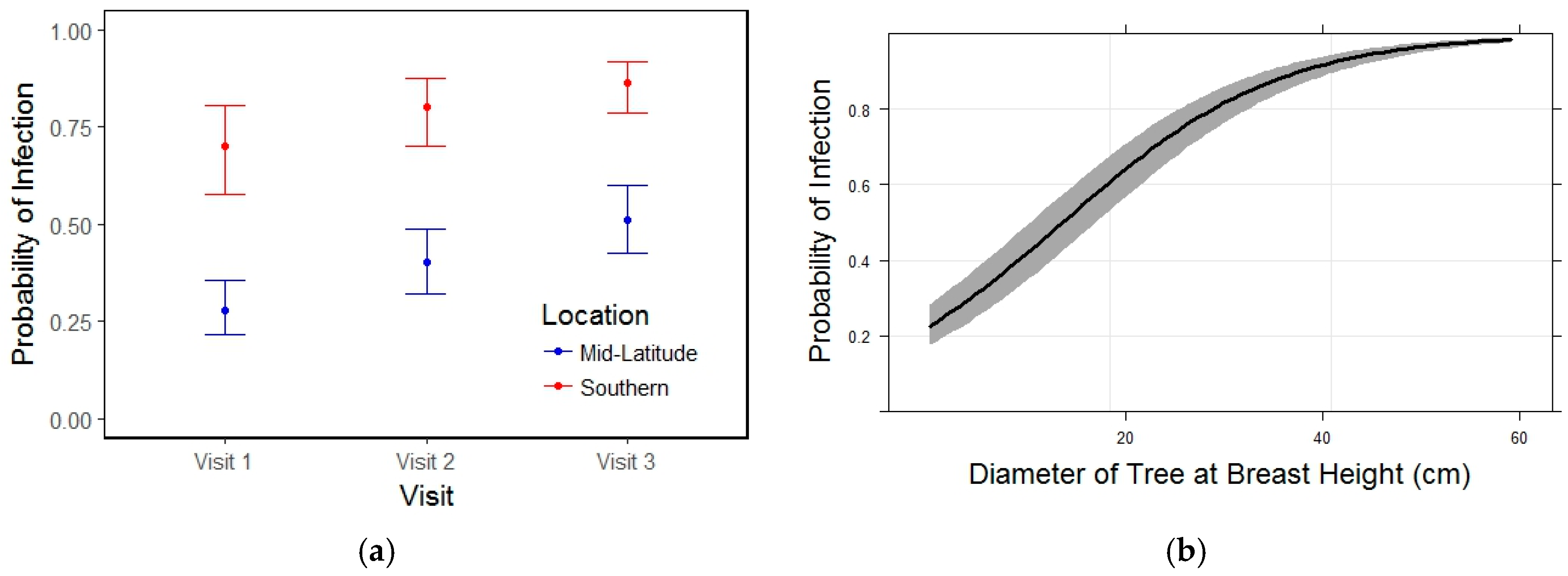
3.1. Incidence of Infection and Mortality
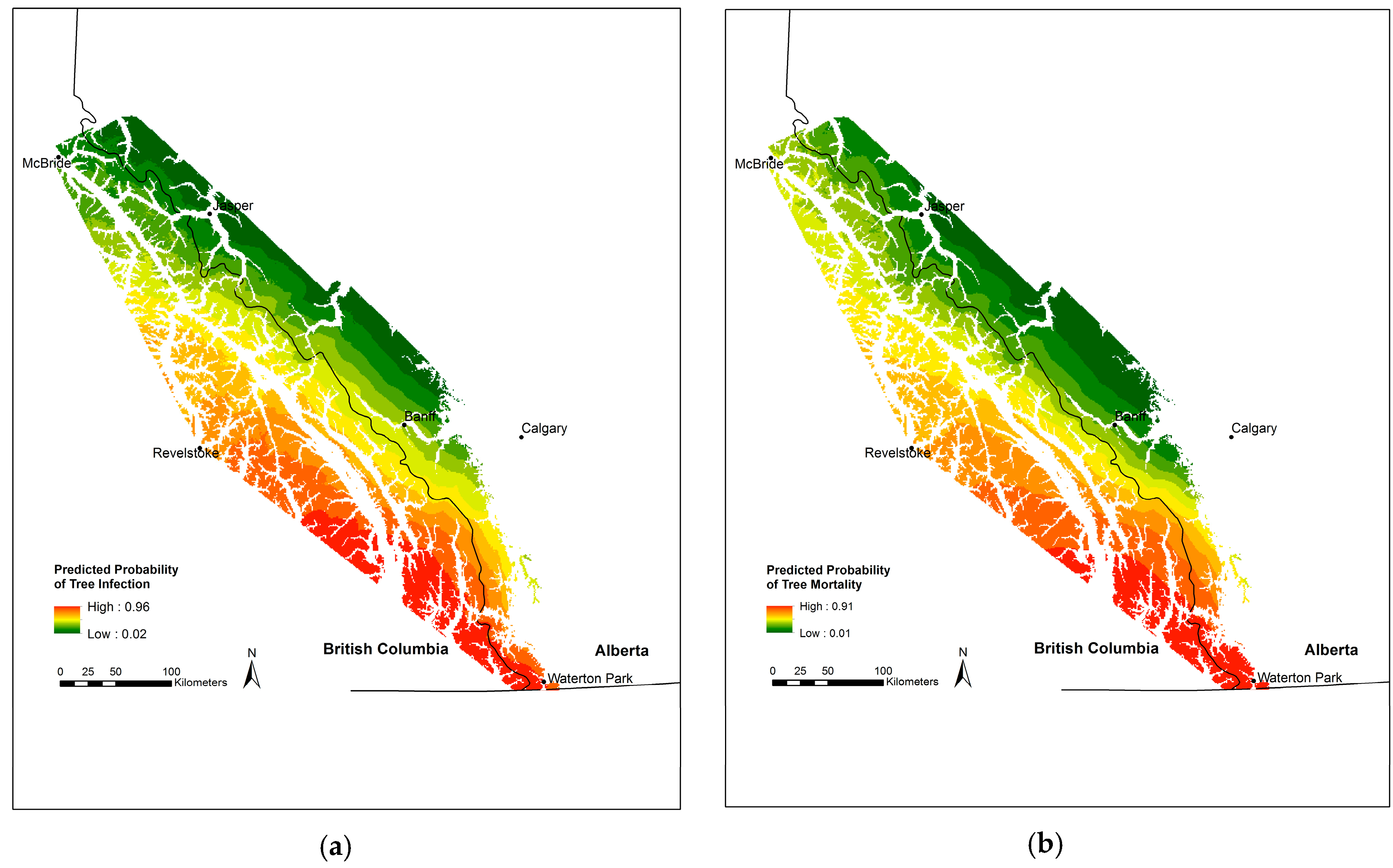
3.2. Blister Rust Infection in Live Trees
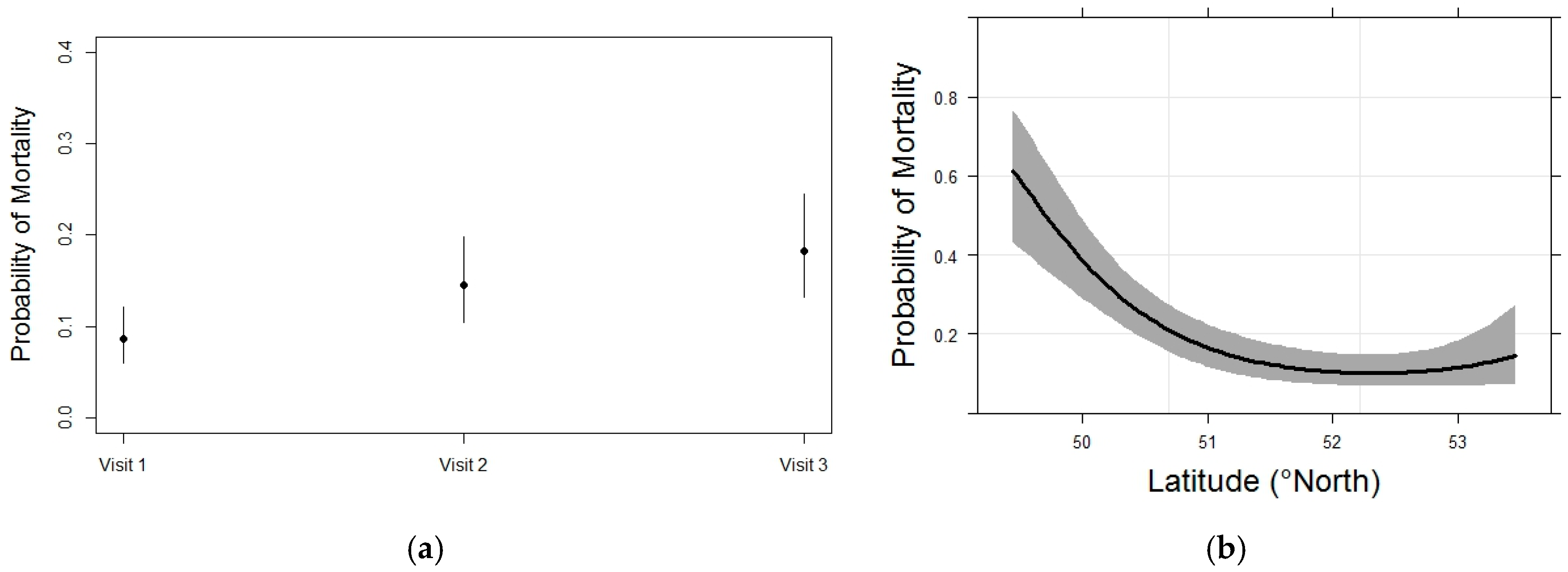
3.3. Whitebark Pine Mortality
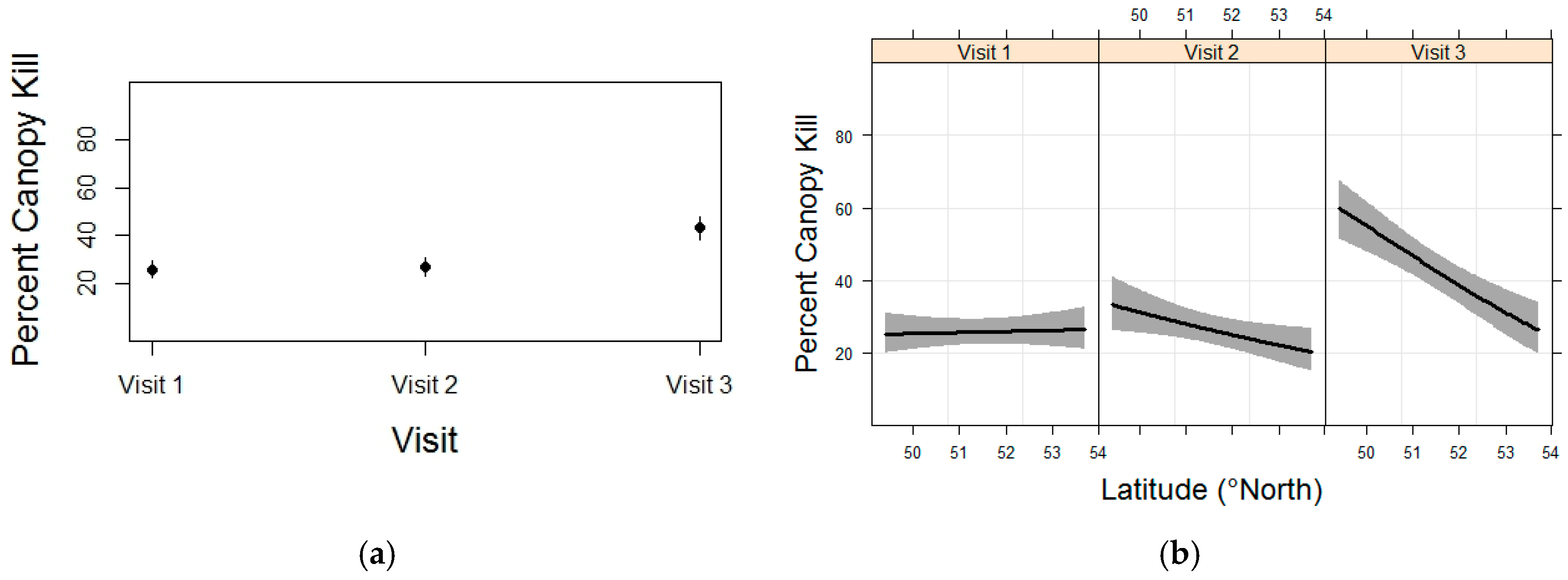
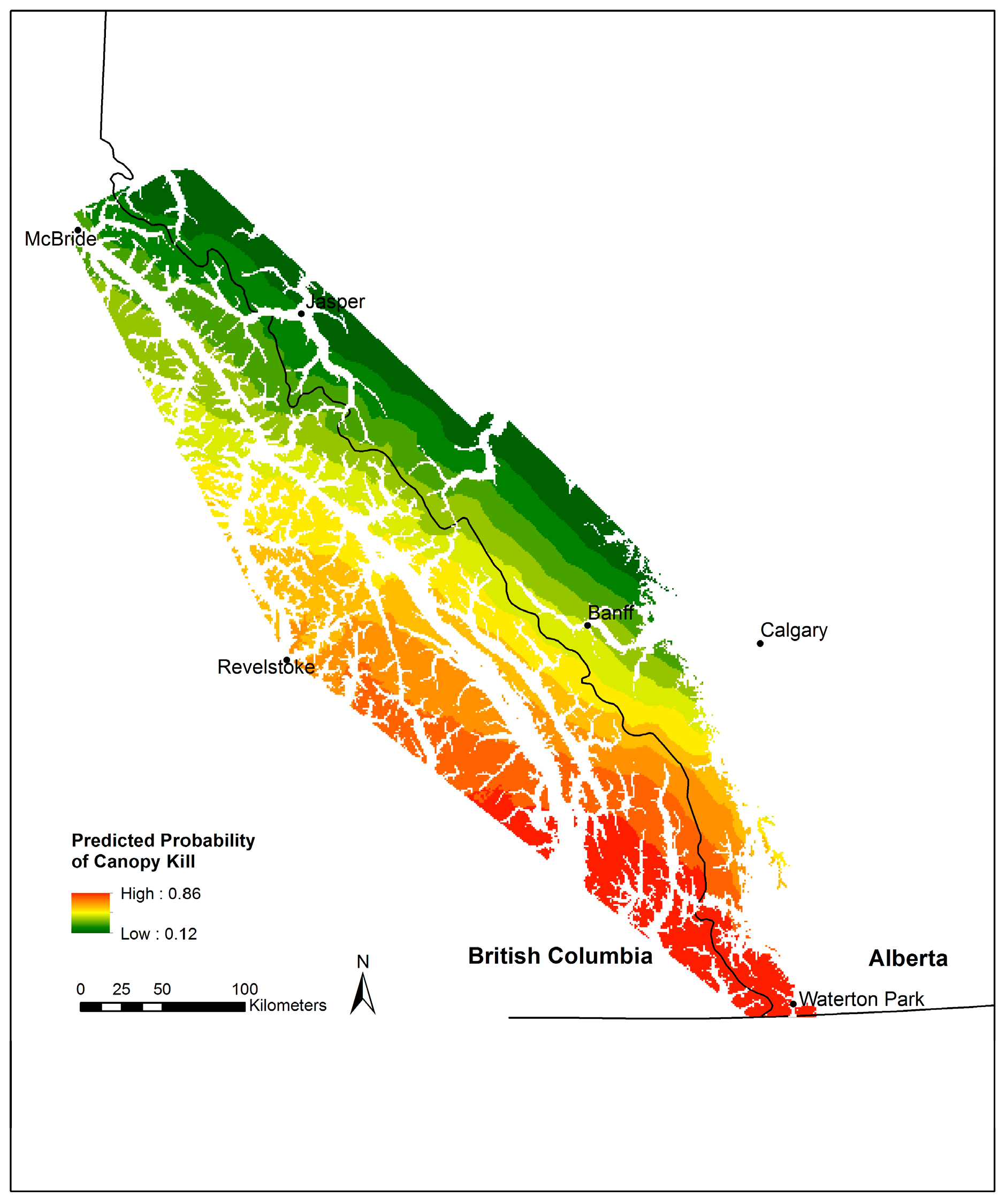
3.4. Whitebark Pine Canopy Kill
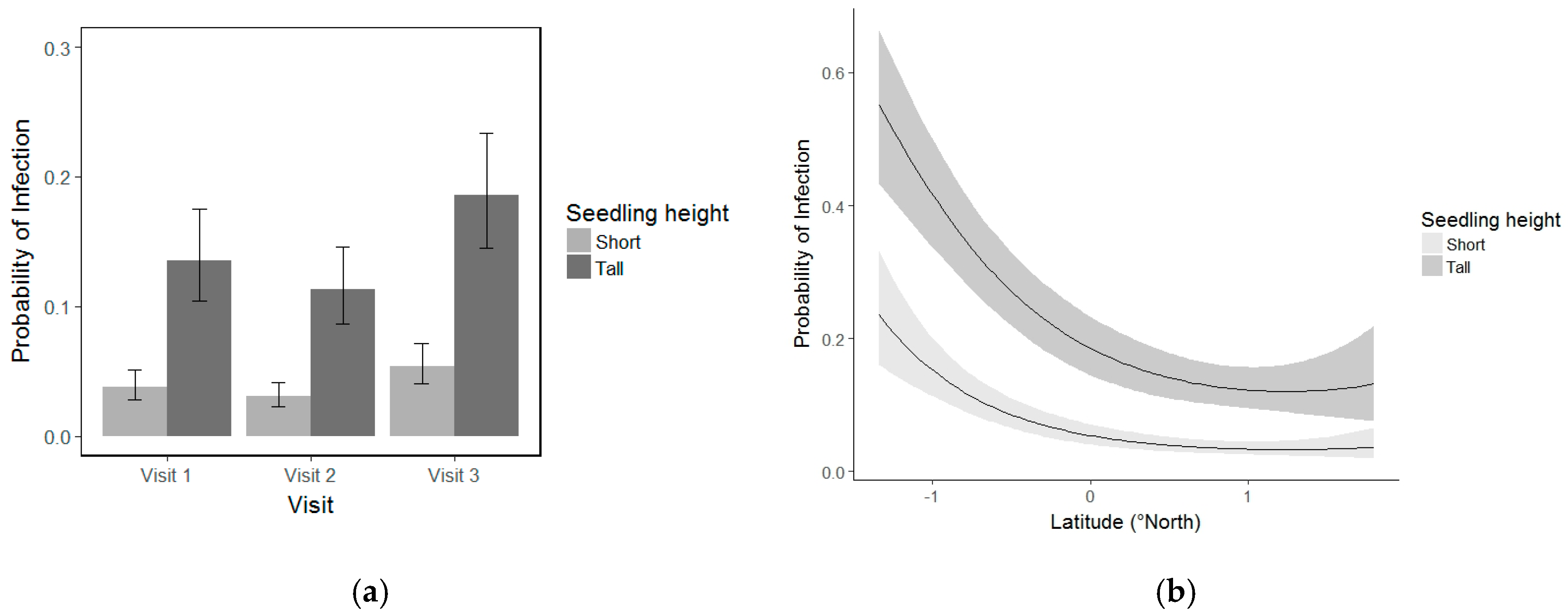
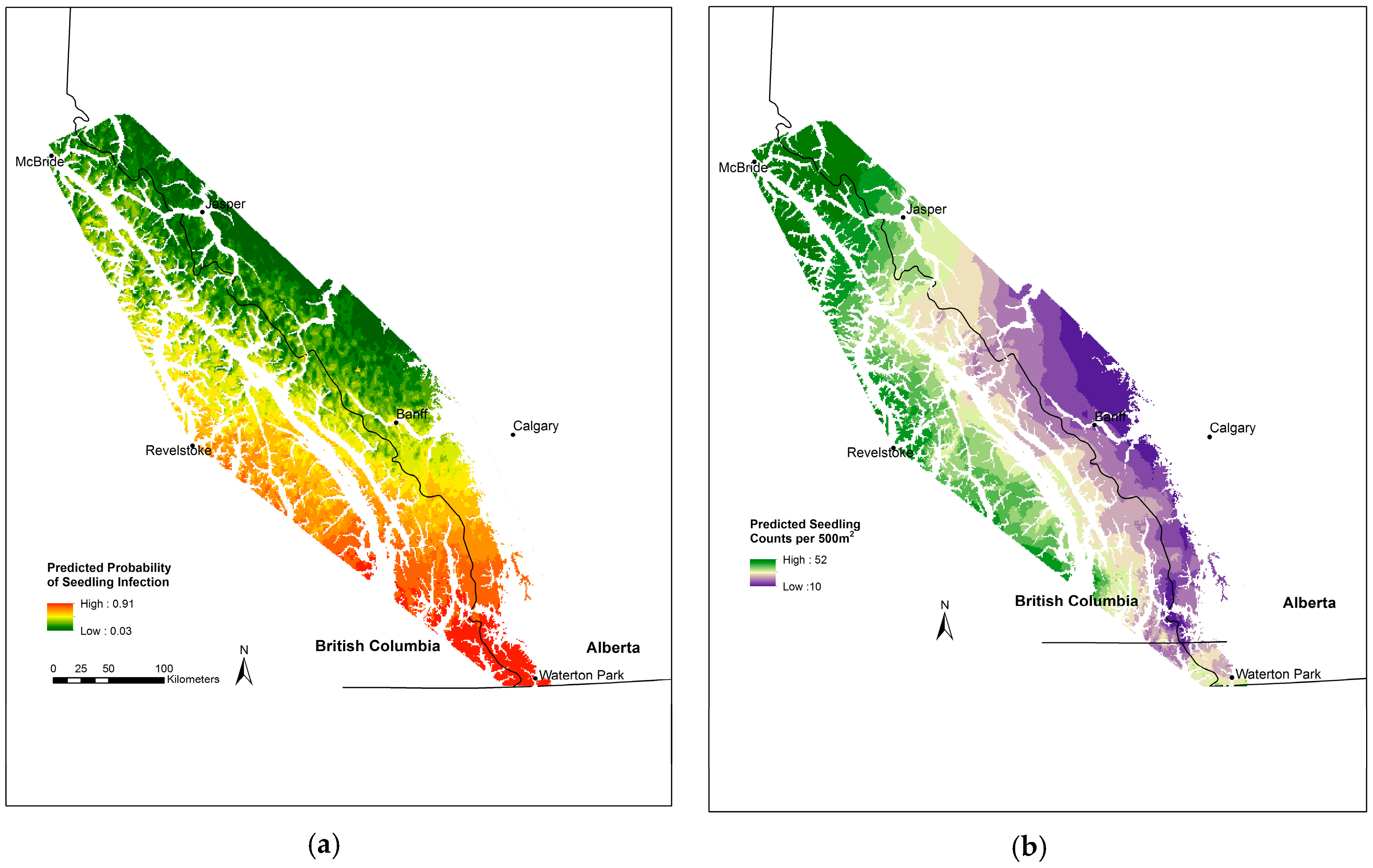
3.5. Whitebark Pine Seedling Infection
3.6. Whitebark Pine Natural Regeneration
4. Discussion
4.1. Blister Rust and Mountain Pine Beetle Relationships
4.2. Regeneration Relationships
4.3. Restoration Implications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Committee on the Status of Endangered Wildlife in Canada. COSEWIC Assessment and Status Report on the Whitebark Pine (Pinus albicaulis) in Canada-2010; Committee on the Status of Endangered Wildlife in Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- Keane, R.E.; Holsinger, L.M.; Mahalovich, M.F.; Tomback, D.F. Restoring Whitebark Pine Ecosystems in the Face of Climate Change; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2017.
- Tomback, D.F.; Kendall, K.C. Biodiversity losses: The downward spiral. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 243–262. [Google Scholar]
- Tomback, D.F.; Arno, S.F.; Keane, R.E. Whitebark Pine Communities: Ecology and Restoration; Island Press: Washington, DC, USA, 2001. [Google Scholar]
- Tomback, D.F.; Chipman, K.G.; Resler, L.M.; Smith-McKenna, E.K.; Smith, C.M. Relative abundance and functional role of whitebark pine at treeline in the Northern Rocky Mountains. Arct. Antarct. Alpine Res. 2014, 46, 407–418. [Google Scholar] [CrossRef]
- Tomback, D.F.; Achuff, P. Blister rust and western forest biodiversity: Ecology, values and outlook for white pines. For. Pathol. 2010, 40, 186–225. [Google Scholar] [CrossRef]
- Smith-McKenna, E.K.; Malanson, G.P.; Resler, L.M.; Carstensen, L.W.; Prisley, S.P.; Tomback, D.F. Cascading effects of feedbacks, disease, and climate change on alpine treeline dynamics. Environ. Model. Softw. 2014, 62, 85–96. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. Recovery Strategy for the Whitebark Pine (Pinus albicus) in Canada [Proposed]; Environment and Climate Change Canada: Ottawa, ON, Canada, 2017. [Google Scholar]
- Keane, R.E.; Tomback, D.F.; Aubry, C.A.; Bower, A.D.; Campbell, E.M.; Cripps, C.L.; Jenkins, M.B.; Mahalovich, M.F.; Manning, M.; McKinney, S.T.; et al. A Range-Wide Restoration Strategy for Whitebark Pine (Pinus albicaulis); US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2012. [Google Scholar]
- Alberta Whitebark Pine Recovery Team. Alberta Whitebark Pine Recovery Team. Alberta Whitebark Pine Recovery Plan 2012/2013–2017/2018. In Alberta Recovery Plan No. 34; Alberta Whitebark Pine Recovery Team: Edmonton, AB, USA, 2012; p. 44. [Google Scholar]
- Smith, C.M.; Shepherd, B.; Gillies, C.; Stuart-Smith, J. Changes in blister rust infection and mortality in whitebark pine over time. Can. J. For. Res. 2012, 43, 90–96. [Google Scholar] [CrossRef]
- Smith, C.M.; Wilson, B.; Rasheed, S.; Walker, R.C.; Carolin, T.; Shepherd, B. Whitebark pine and white pine blister rust in the Rocky Mountains of Canada and northern Montana. Can. J. For. Res. 2008, 38, 982–995. [Google Scholar] [CrossRef]
- Zeglen, S. Whitebark pine and white pine blister rust in British Columbia, Canada. Can. J. For. Res. 2002, 32, 1265–1274. [Google Scholar] [CrossRef]
- Campbell, E.M.; Antos, J.A. Distribution and severity of white pine blister rust and mountain pine beetle on whitebark pine in British Columbia. Can. J. For. Res. 2000, 30, 1051–1059. [Google Scholar] [CrossRef]
- Cooke, B.J.; Carroll, A.L. Predicting the risk of mountain pine beetle spread to eastern pine forests: Considering uncertainty in uncertain times. For. Ecol. Manag. 2017, 396, 11–25. [Google Scholar] [CrossRef]
- Kendall, K.C.; Keane, R.E. Whitebark pine decline: Infection, mortality, and population trends. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 221–242. [Google Scholar]
- Six, D.L.; Adams, J. White pine blister rust severity and selection of individual whitebark pine by the mountain pine beetle (Coleoptera: Curculionidae, Scolytinae). J. Entomol. Sci. 2007, 42, 345–353. [Google Scholar] [CrossRef]
- Barringer, L.E.; Tomback, D.F.; Wunder, M.B.; McKinney, S.T. Whitebark Pine Stand Condition, Tree Abundance, and Cone Production as Predictors of Visitation by Clark’s Nutcracker. PLoS ONE 2012, 7, e37663. [Google Scholar] [CrossRef] [PubMed]
- Tomback, D.F.; Keane, R.E.; McCaughey, W.W.; Smith, C.M. Methods for Surveying and Monitoring Whitebark Pine for Blister Rust Infection and Damage. Whitebark Pine Ecosystem Foundation: Missoula, Mont. Available online: whitebarkfound.org/monitoring.html (accessed on 8 December 2006).
- Hoff, R.J. How to Recognize Blister Rust Infection on Whitebark Pine; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 1992; pp. 1–8.
- Larson, E.R. Influences of the biophysical environment on blister rust and mountain pine beetle, and their interactions, in whitebark pine forests. J. Biogeogr. 2011, 38, 453–470. [Google Scholar] [CrossRef]
- Smith-Mckenna, E.K.; Resler, L.M.; Tomback, D.F.; Zhang, H.; Malanson, G.P. Topographic influences on the distribution of white pine blister rust in Pinus albicaulis treeline communities. Ecoscience 2013, 20, 215–229. [Google Scholar] [CrossRef]
- Smith, C.M.; Langor, D.W.; Myrholm, C.; Weber, J.; Gillies, C.; Stuart-Smith, J. Changes in white pine blister rust infection and mortality in limber pine over time. Can. J. For. Res. 2013, 43, 919–928. [Google Scholar] [CrossRef]
- Gelderman, M.S.; Macdonald, S.E.; Gould, A.J. Regeneration niche of whitebark pine in the Canadian Rocky Mountains: The basis to restoring an endangered species. Arct. Antarct. Alpine Res. 2016, 48, 279–292. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.; Carroll, C. Locally downscaled and spatially customizable climate data for historical and future periods for North America. PLoS ONE 2016, 11, e0156720. [Google Scholar] [CrossRef] [PubMed]
- National Geographic, Esri, DeLorme, NAVTEQ, UNEP-WCMC, USGS, NASA, ESA, METI, NRCAN, GEBCO, NOAA, iPC. 2011. National Geographic World Map, Digital Topographic Basemap of the World. Available online: http://www.arcgis.com/home/item.html?id=b9b1b422198944fbbd5250b3241691b6 (accessed on 13 March 2018).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Burnham, K.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 1998. [Google Scholar]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Pearce, J.; Ferrier, S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000, 133, 225–245. [Google Scholar] [CrossRef]
- McKinney, S.T.; Tomback, D.F. The influence of white pine blister rust on seed dispersal in whitebark pine. Can. J. For. Res. 2007, 37, 1044–1057. [Google Scholar] [CrossRef]
- Marshall, S.J.; White, E.C.; Demuth, M.N.; Bolch, T.; Wheate, R.; Menounos, B.; Beedle, M.J.; Shea, J.M. Glacier water resources on the eastern slopes of the Canadian Rocky Mountains. Can. Water Resour. J. 2011, 36, 109–134. [Google Scholar] [CrossRef]
- McDonald, G.I.; Hoff, R.J. Blister rust: An introduced plague. In Whitebark Pine Communities: Ecology and Restoration; Tomback, D.F., Arno, S.F., Keane, R.E., Eds.; Island Press: Washington, DC, USA, 2001; pp. 193–220. [Google Scholar]
- Van Arsdel, E.; Riker, A.; Patton, R. The effects of temperature and moisture on the spread of white pine blister rust. Phytopathology 1956, 46, 307–318. [Google Scholar]
- McDonald, G.I.; Hoff, R.J.; Wykoff, W.R. Computer Simulation of White Pine Blister Rust Epidemics; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 1981.
- Spector, D.A. The Influence of Forest Structure on Cone Production in Whitebark Pine throughout the Greater Yellowstone Ecosystem; Montana State University-Bozeman, College of Letters & Science: Bozeman, MT, USA, 1999. [Google Scholar]
- Larson, E.R.; Kipfmueller, K.F. Patterns in whitebark pine regeneration and their relationships to biophysical site characteristics in southwest Montana, central Idaho, and Oregon, USA. Can. J. For. Res. 2010, 40, 476–487. [Google Scholar] [CrossRef]
- Tomback, D.F.; Clary, J.K.; Koehler, J.; Hoff, R.J.; Arno, S.F. The effects of blister rust on post-fire regeneration of whitebark pine: The Sundance Burn of northern Idaho (USA). Conserv. Biol. 1995, 9, 654–664. [Google Scholar] [CrossRef]
- Cleaver, C.M.; Jacobi, W.R.; Burns, K.S.; Means, R.E. Limber Pine Regeneration and White Pine Blister Rust in the Central and Southern Rocky Mountains. For. Sci. 2017, 63, 151–164. [Google Scholar] [CrossRef]
- Mulvey, R.; Hansen, E. Castilleja and Pedicularis confirmed as telial hosts for Cronartium ribicola in whitebark pine ecosystems of Oregon and Washington. For. Pathol. 2011, 41, 453–463. [Google Scholar] [CrossRef]
- Wong, C.M.; Daniels, L.D. Novel forest decline triggered by multiple interactions among climate, an introduced pathogen and bark beetles. Glob. Chang. Biol. 2017, 23, 1926–1941. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, P.; Braumandl, T.F. Historic Influence of the Mountain Pine Beetle on Stand Dynamics in Canada's Rocky Mountain Parks; Natural Resources Canada: Victoria, BC, Canada, 2006. [Google Scholar]
- Perkins, D.L.; Jorgensen, C.L.; Rinella, M.J. Verbenone decreases whitebark pine mortality throughout a mountain pine beetle outbreak. For. Sci. 2015, 61, 747–752. [Google Scholar] [CrossRef]
- Moody, R.J. Post-Fire Regeneration and Survival of Whitebark Pine (Pinus albicaulis Engelm.); University of British Columbia: Vancouver, BC, Canada, 2006. [Google Scholar]
- McCaughey, W.W.; Tomback, D.F. The natural regeneration process. In Whitebark Pine Communities: Ecology and Restoration; Island Press: Washington, DC, USA, 2001. [Google Scholar]
- Tomback, D.F.; Anderies, A.J.; Carsey, K.S.; Powell, M.L.; Mellmann-Brown, S. Delayed seed germination in whitebark pine and regeneration patterns following the Yellowstone fires. Ecology 2001, 82, 2587–2600. [Google Scholar] [CrossRef]
- Keane, R.E.; Ryan, K.C.; Veblen, T.T.; Allen, C.D.; Logan, J.A.; Hawkes, B.; Barron, J. Cascading Effects of Fire Exclusion in the Rocky Mountain Ecosystems: A Literature Review; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2002; p. 24.
- Keane, R.E. Successional dynamics: Modeling an anthropogenic threat. In Whitebark Pine Communities: Ecology and Restoration; Island Press: Washington, DC, USA, 2001; pp. 159–192. [Google Scholar]
- Pigott, D.; Moody, R.; Clason, A. Promoting Whitebark Pine Recovery in British Columbia; Society for Ecosystem Restoration: Washington, DC, USA, 2015; p. 108. [Google Scholar]
- Cripps, C.L.; Antibus, R.K. Native ectomycorrhizal fungi of limber and whitebark pine: Necessary for forest sustainability. In Proceedings of the The Future of High-Elevation, Five-Needle White Pines in Western North America: High Five Symposium, Fort Collins, CO, USA, 28–30 June 2010; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011. [Google Scholar]
- McDermid, G.; Smith, I. Mapping the distribution of whitebark pine (Pinus albicaulis) in Waterton Lakes National Park using logistic regression and classification tree analysis. Can. J. Remote Sens. 2008, 34, 356–366. [Google Scholar] [CrossRef]
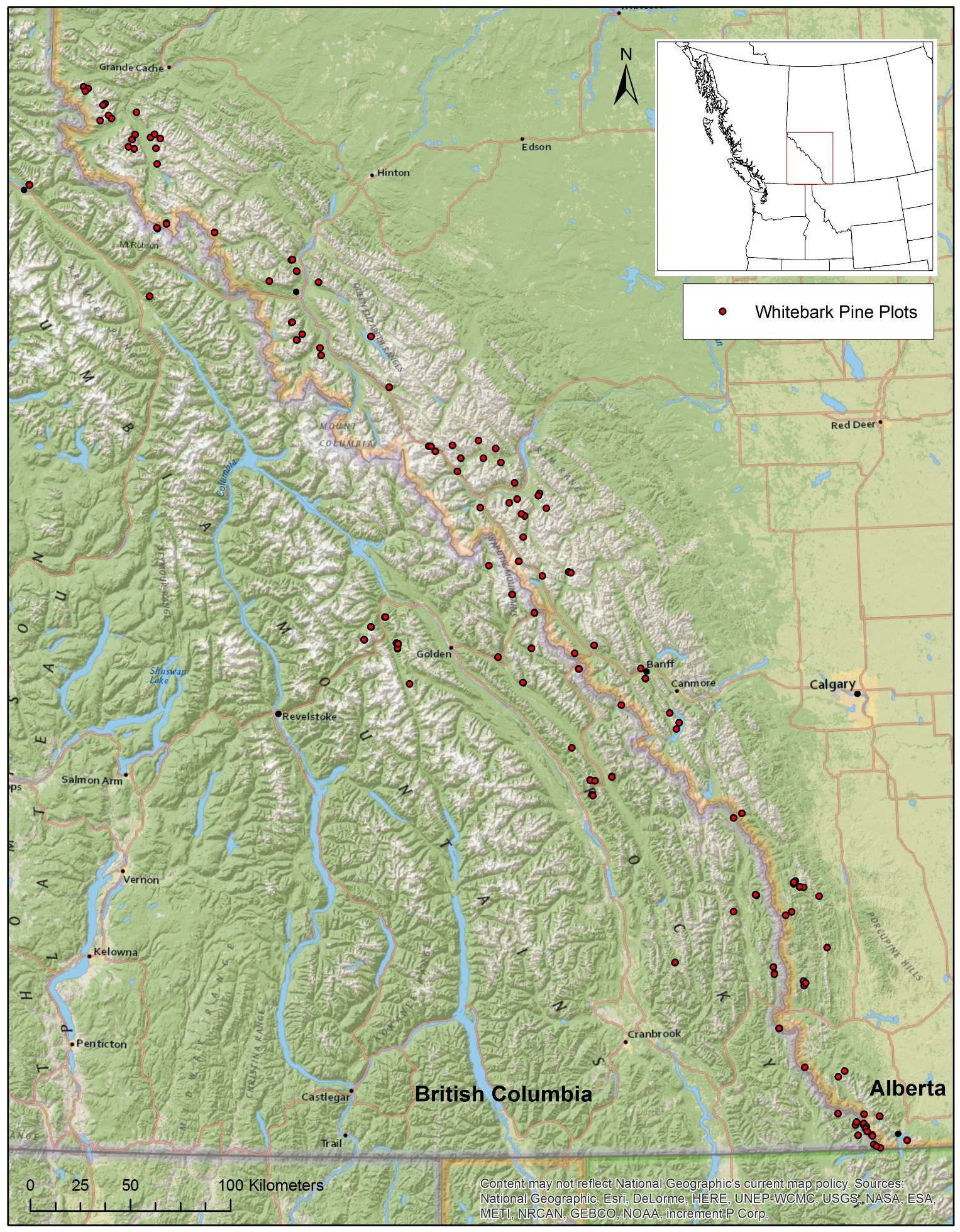

| Date Source 1 | Variable | Description | Variable Type |
|---|---|---|---|
| Field | Visit | Visit 1, 2, 3 (2003–2007, 2008–2012, 2014) | Predictor |
| GIS | Divide | Distance to divide (km) | Predictor |
| Field | Latitude | Latitude (°) | Predictor |
| Field and GIS | Elevation | Elevation (m) | Predictor |
| Field and GIS | Slope | Slope (0–90°) | Predictor |
| Field | Aspect | Azimuth converted (0–1) | Predictor |
| Field | DBH | Tree diameter at breast height (cm) 2 | Predictor |
| ClimateWNA | SHM | Summer heat moisture index | Predictor |
| ClimateWNA | bFFP | Day frost-free period begins (day of year) | Predictor |
| ClimateWNA | DD5 | Degree days above 5 °C 3 | Predictor |
| ClimateWNA | MWMT | Mean warmest month temperature (°C) | Predictor |
| ClimateWNA | Rad_sp | Spring solar radiation (MJ m−2 day−1) | Predictor |
| ClimateWNA | PAS_sp | Spring snow (mm) | Predictor |
| ClimateWNA | PPT_sp | Spring precipitation (mm) | Predictor |
| Field | Basal area | Basal area of whitebark pine (m2/ha) | Predictor |
| Field | Height class | Seedling height class (short ≤ 50 cm, tall > 50 cm) | Predictor |
| Field | Plot | Plot ID | Random effect |
| GIS | Location | Location ID for plots | Random effect |
| Field | Disease | Live tree status (0 = WPBR absent, 1 = WPBR present) | Response |
| Field | Mortality | Tree status (0 = dead, 1 = live) | Response |
| Field | Canopy Kill | Mean proportion of canopy kill by plot | Response |
| Field | Regeneration | Seedling count by plot | Response |
| Model and Fixed Effects | Coefficients | Standard Error | p Value |
|---|---|---|---|
| Live Tree Infection | |||
| Latitude | −0.3633 | 0.1372 | 0.0081 ** |
| Divide | −1.0462 | 0.1916 | <0.0001 *** |
| Divide2 | −0.3138 | 0.1091 | 0.0040 ** |
| Visit 2 | 0.5419 | 0.0495 | <0.0001 *** |
| Visit 3 | 0.9990 | 0.0496 | <0.0001 *** |
| DD5 | 0.9010 | 0.1298 | <0.0001 *** |
| DBH | 0.8532 | 0.0266 | <0.0001 *** |
| Tree Mortality | |||
| Latitude | −0.6510 | 0.1398 | <0.0001 *** |
| Latitude2 | 0.4494 | 0.1474 | 0.0023 ** |
| Divide | −0.9149 | 0.1667 | <0.0001 *** |
| Divide2 | −0.2863 | 0.0953 | 0.0026 ** |
| Visit 2 | 0.5860 | 0.0525 | <0.0001 *** |
| Visit 3 | 0.8589 | 0.0526 | <0.0001 *** |
| DD5 | 0.3260 | 0.1486 | 0.0282 * |
| Seedling Infection | |||
| Latitude | −0.8150 | 0.1160 | <0.0001 *** |
| Latitude2 | 0.3269 | 0.1153 | 0.0046 ** |
| Divide | −0.3342 | 0.1153 | 0.0037 ** |
| Visit 2 | −0.2103 | 0.0754 | 0.0053 ** |
| Visit 3 | 0.3715 | 0.0740 | <0.0001 *** |
| Rad_sp | 0.2447 | 0.1235 | 0.0476 * |
| Height class | 1.3810 | 0.0647 | <0.0001 *** |
| Canopy Kill | |||
| Latitude | 0.0186 | 0.0656 | 0.7760 |
| Visit 2 | 0.0405 | 0.0488 | 0.4070 |
| Visit 3 | 0.7766 | 0.0471 | <0.0001 *** |
| Divide | −0.4169 | 0.0823 | <0.0001 *** |
| Divide2 | −0.1319 | 0.0564 | 0.0190 * |
| DD5 | 0.3059 | 0.0599 | <0.0001 *** |
| DD52 | 0.1082 | 0.0447 | 0.0150 * |
| Latitude at Visit 2 | −0.2040 | 0.0472 | <0.0001 *** |
| Latitude at Visit 3 | −0.4154 | 0.0477 | <0.0001 *** |
| Regeneration | |||
| Latitude | −0.1422 | 0.1094 | 0.1937 |
| Latitude2 | 0.3398 | 0.1051 | 0.0012 ** |
| Divide | −0.2570 | 0.1120 | 0.0217 * |
| Visit 2 | 0.0188 | 0.0503 | 0.7082 |
| Visit 3 | 0.2359 | 0.0624 | 0.0002 *** |
| Aspect | 0.2271 | 0.0914 | 0.0130 * |
| DD5 | −0.3318 | 0.1270 | 0.0090 ** |
| DD52 | −0.1818 | 0.0718 | 0.0113 * |
| Canopy kill | −0.1342 | 0.0458 | 0.0034 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepherd, B.; Jones, B.; Sissons, R.; Cochrane, J.; Park, J.; Smith, C.M.; Stafl, N. Ten Years of Monitoring Illustrates a Cascade of Effects of White Pine Blister Rust and Focuses Whitebark Pine Restoration in the Canadian Rocky and Columbia Mountains. Forests 2018, 9, 138. https://doi.org/10.3390/f9030138
Shepherd B, Jones B, Sissons R, Cochrane J, Park J, Smith CM, Stafl N. Ten Years of Monitoring Illustrates a Cascade of Effects of White Pine Blister Rust and Focuses Whitebark Pine Restoration in the Canadian Rocky and Columbia Mountains. Forests. 2018; 9(3):138. https://doi.org/10.3390/f9030138
Chicago/Turabian StyleShepherd, Brenda, Brad Jones, Robert Sissons, Jed Cochrane, Jane Park, Cyndi M. Smith, and Natalie Stafl. 2018. "Ten Years of Monitoring Illustrates a Cascade of Effects of White Pine Blister Rust and Focuses Whitebark Pine Restoration in the Canadian Rocky and Columbia Mountains" Forests 9, no. 3: 138. https://doi.org/10.3390/f9030138





