Progress and Challenges of Protecting North American Ash Trees from the Emerald Ash Borer Using Biological Control
Abstract
:1. Introduction
2. Rationale for Selection of Emerald Ash Borer as Target for Biological Control
3. The Role of Natural Enemies in Suppressing EAB in Its Native Range
4. Development of an EAB Biological Control Program in North America
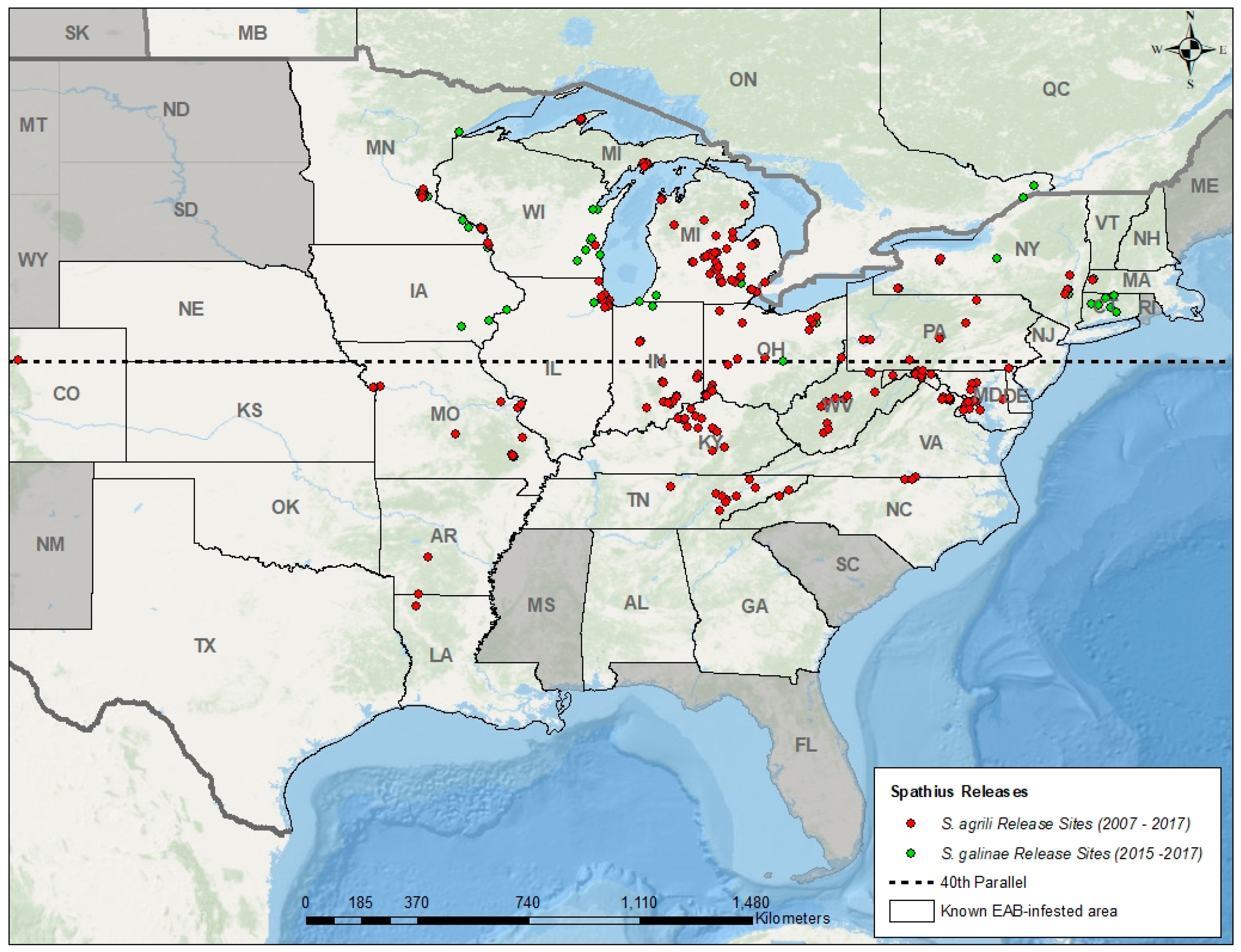
5. Introduction and Establishment of EAB Biocontrol Agents
6. Impact of EAB Biocontrol Agents on Target Pest Populations
7. Ash Recovery and Regeneration after EAB invasion with Biological Control
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Pimentel, D. Biological invasions of plants and animals in agriculture and forestry. In Ecology of Biological Invasions of North America and Hawaii; Mooney, H.A., Drake, J.A., Eds.; Ecological Studies 58; Springer: New York, NY, USA, 1986; pp. 149–162. [Google Scholar]
- Aukema, J.E.; McCullough, D.G.; Von Holle, B.; Liebhold, A.M.; Britton, K.; Frankel, S.J. Historical accumulation of non-indigenous forest pests in the continental United States. BioScience 2010, 60, 886–897. [Google Scholar] [CrossRef]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The consequence of tree pests and diseases for ecosystem services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef] [PubMed]
- Liebhold, A.M.; MacDonald, W.L.; Bergdahl, D.; Mastro, V.C. Invasion by exotic forest pests: A threat to forest ecosystems. For. Sci. Monogr. 1995, 41, 1–49. [Google Scholar]
- Haack, R.A. Exotic bark and wood-boring Coleoptera in the United States: Recent establishments and interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Haack, R.A.; Britton, K.O.; Brockerhoff, E.G.; Cavey, J.F.; Garrett, L.J.; Kimberley, M.; Lowenstein, F.; Nuding, A.; Olson, L.J.; Turner, J.; et al. Effectiveness of the international phytosanitary standard ISPM15 on reducing wood borer infestation rates in wood packaging material entering the US. PLoS ONE 2014, 9, e96611. [Google Scholar] [CrossRef] [PubMed]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Soc. Am. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.; Fitter, A. The varying success of invaders. Ecology 1996, 77, 1661–1666. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Liebhold, A.M. Ecology of forest insect invasions. Biol. Invasions 2017, 19, 3141–3159. [Google Scholar] [CrossRef]
- Haack, R.A.; Jendek, E.; Liu, H.; Marchant, K.R.; Petrice, T.R.; Poland, T.M.; Ye, H. The emerald ash borer: A new exotic pest in North America. Newsl. Mich. Entomol. Soc. 2002, 47, 1–5. [Google Scholar]
- Federal Register. Emerald Ash Borer; Quarantine and Regulations. 2003; 7 CFR Part 301 [Docket Number 02-125-1]. Available online: https://www.federalregister.gov/documents/2003/10/14/03-25881/emerald-ash-borer-quarantine-and-regulations (accessed on 18 December 2017).
- Cappaert, D.L.; McCullough, D.G.; Poland, T.M.; Siegert, N.W. Emerald ash borer in North America: A research and regulatory challenge. Am. Entomol. 2005, 51, 152–165. [Google Scholar] [CrossRef]
- GAO. Invasive Forest Pests: Lessons Learned from Three Recent Infestations May Aid in Managing Future Efforts. Report of the United States Government Accounting Office. 2006; GAO-06-353. Available online: https://www.gao.gov/assets/250/249776.pdf (accessed on 5 January 2018).
- Siegert, N.W.; McCullough, D.G.; Liebhold, A.M.; Telewski, F.W. Dendrochronological reconstruction of the epicentre and early spread of emerald ash borer in North America. Divers. Distrib. 2014, 20, 847–858. [Google Scholar] [CrossRef]
- Poland, T.M.; McCullough, D.G. Emerald ash borer: Invasion of the urban forest and the threat to North America’s ash resource. J. For. 2006, 104, 118–124. [Google Scholar]
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impact and management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Morin, R.S.; Liebhold, A.M.; Pugh, S.A.; Crocker, S.J. Regional assessment of emerald ash borer, Agrilus planipennis, impacts in forests of the Eastern United States. Biol. Invasions 2017, 19, 703–711. [Google Scholar] [CrossRef]
- Bauer, L.S.; Liu, H.P.; Miller, D.L.; Gould, J. Developing a classical biological control program for Agrilus planipennis (Coleoptera: Buprestidae), an invasive ash pest in North America. Newsl. Mich. Entomol. Soc. 2008, 53, 38–39. [Google Scholar]
- Bauer, L.S.; Duan, J.J.; Gould, J.R. Emerald ash borer Agrilus planipennis Fairmaire Coleoptera: Buprestidae. In The Use of Classical Biological Control to Preserve Forests in North America; FHTET-2013-2; Van Driesche, R., Reardon, R., Eds.; United States Department of Agriculture, Forest Service, Forest Health and Technology Enterprise Team: Morgantown, WV, USA, 2014; pp. 189–209. Available online: https://www.nrs.fs.fed.us/pubs/48051 (accessed on 30 January 2018).
- Bauer, L.S.; Duan, J.J.; Gould, J.R.; Van Driesche, R.G. Progress in the classical biological control of Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) in North America. Can. Entomol. 2015, 147, 300–317. [Google Scholar] [CrossRef]
- Sadof, C.S.; Hughes, G.P.; Witte, A.R.; Peterson, D.J.; Ginzel, M.D. Tools for staging and managing emerald ash borer in the urban forest. Arboric. Urban For. 2017, 43, 15–26. [Google Scholar]
- Mercader, R.J.; McCullough, D.G.; Storer, A.J.; Bedford, J.; Poland, T.M.; Katovich, S. Evaluation of the potential use of a systemic insecticide and girdled trees in area wide management of the emerald ash borer. For. Ecol. Manag. 2015, 350, 70–80. [Google Scholar] [CrossRef]
- Rigsby, C.M.; Showalter, D.N.; Herms, D.A.; Koch, J.L.; Bonello, P.; Cipollini, D. Physiological responses of emerald ash borer larvae to feeding on different ash species reveal putative resistance mechanisms and insect counter-adaptations. J. Insect Physiol. 2015, 78, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Villari, C.; Herms, D.A.; Whitehill, J.G.A.; Cipollini, D.; Bonello, P. Progress and gaps in understanding mechanisms of ash tree resistance to emerald ash borer; a model for wood-boring insects that kill angiosperms. New Phytol. 2016, 209, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.; Bauer, L.S.; Poland, T.M.; Haack, R.A.; Cognato, A.I.; Smith, J.J. Genetic analysis of emerald ash borer (Agrilus planipennis Fairmaire) populations in Asia and North America. Biol. Invasions 2011, 13, 2869–2887. [Google Scholar] [CrossRef]
- Liu, H.; Bauer, L.S.; Gao, R.; Zhao, T.; Petrice, T.R.; Haack, R.A. Exploratory survey for the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), and its natural enemies in China. Great Lakes Entomol. 2003, 36, 191–204. [Google Scholar]
- Eyles, A.; Jones, W.; Riedl, K.; Cipollini, D.; Schwartz, S.; Chan, K.; Herms, D.A.; Bonello, P. Comparative phloem chemistry of Manchurian Fraxinus mandshurica and two North American ash species Fraxinus americana and Fraxinus pennsylvanica. J. Chem. Ecol. 2007, 33, 1430–1448. [Google Scholar] [CrossRef] [PubMed]
- Rebek, E.J.; Herms, D.A.; Smitley, D.R. Interspecific variation in resistance to emerald ash borer (Coleoptera: Buprestidae) among North American and Asian ash (Fraxinus spp.). Environ. Entomol. 2008, 37, 242–246. [Google Scholar] [CrossRef]
- Klooster, W.S.; Herms, D.A.; Knight, K.S.; Herms, C.P.; McCullough, D.G.; Smith, A.S.; Gandhi, K.J.K.; Cardina, J. Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol. Invasions 2014, 16, 859–873. [Google Scholar] [CrossRef]
- Tanis, S.R.; McCullough, D.G. Differential persistence of blue ash (Fraxinus quadrangulata) and white ash (Fraxinus americana) following emerald ash borer (Agrilus planipennis) invasion. Can. J. For. Res. 2012, 42, 1542–1550. [Google Scholar] [CrossRef]
- Tanis, S.R.; McCullough, D.G. Host resistance of five Fraxinus species to Agrilus planipennis (Coleoptera: Buprestidae) and effects of paclobutrazol and fertilization. Environ. Entomol. 2015, 41, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Knight, K.S.; Brown, J.P.; Long, R.P. Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (Agrilus planipennis). Biol. Invasions 2013, 15, 371–383. [Google Scholar] [CrossRef]
- Cipollini, D. White fringetree as a novel larval host for emerald ash borer. J. Econ. Entomol. 2015, 108, 370–375. [Google Scholar] [CrossRef] [PubMed]
- USDA–FS (United States Department of Agriculture, Forest Service). Silvics of Forest Trees of the United States; Compiler; Agriculture Handbook 271; Folwells, H.A., Ed.; United States Department of Agriculture, Forest Service: Washington, DC, USA, 1965; pp. 181–196. Available online: https://catalog.hathitrust.org/Record/001507718 (accessed on 20 December 2017).
- Nowak, D.; Crane, D.; Stevens, J.; Walton, J. Potential Damage from Emerald Ash Borer; United States Department of Agriculture, Forest Service, Northern Research Station: Syracuse, NY, USA, 2003; pp. 1–5. Available online: https://www.nrs.fs.fed.us/disturbance/invasive_species/eab/local-resources/downloads/EAB_potential.pdf (accessed on 18 December 2017).
- Eyre, F.H. (Ed.) Forest Cover Types of the United States and Canada; Society of American Foresters: Washington, DC, USA, 1980; 148p, ISBN 13: 978-0686306979. [Google Scholar]
- Harlow, W.M.; Harrar, E.S.; Hardin, J.W.; White, F.M. Textbook of Dendrology, 8th ed.; McGraw Hill Book Company: New York, NY, USA, 1996; ISBN 13: 978-0070265721. [Google Scholar]
- USDA–NRCS. Plants Profile for Fraxinus in North America. 2017. Available online: https://plants.usda.gov/core/profile?symbol=fraxi (accessed on 18 December 2017).
- Koenig, W.D.; Liebhold, A.M.; Bonter, D.N.; Hachachka, W.M.; Dicknson, J.L. Effects of the emerald ash borer on four species of birds. Biol. Invasions 2013, 15, 2095–2103. [Google Scholar] [CrossRef]
- Wagner, D.L.; Todd, K.J. New ecological assessment for the emerald ash borer: A cautionary tale about unvetted host-plant literature. Am. Entomol. 2016, 62, 26–35. [Google Scholar] [CrossRef]
- USDA–APHIS. Initial County EAB Detection Map. 2018. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b/downloads/MultiState.pdf (accessed on 7 February 2018).
- Emerald Ash Borer Information. Emerald Ash Borer Information Network. 2016. Available online: http://www.emeraldashborer.info/ (accessed on 18 December 2017).
- IUCN (International Union for Conservation of Nature). IUCN Red List of Threatened Species. Available online: http://www.iucnredlist.org/ (accessed on 18 December 2017).
- Flower, C.E.; Knight, K.S.; Gonzalez-Meler, M.A. Impacts of the emerald ash borer (Agrilus planipennis) induced ash (Fraxinus spp.) mortality on forest carbon cycling and successional dynamics in the eastern United States. Biol. Invasions 2013, 15, 931–944. [Google Scholar] [CrossRef]
- Jennings, D.E.; Duan, J.J.; Bean, D.; Gould, J.R.; Kimberly, A.R.; Shrewsbury, P.M. Monitoring the establishment and abundance of introduced parasitoids of emerald ash borer larvae in Maryland, U.S.A. Biol. Control 2016, 101, 138–144. [Google Scholar] [CrossRef]
- Stephens, J.P.; Berven, K.A.; Tiegs, S.D. Anthropogenic changes to leaf litter input affect the fitness of a larval amphibian. Freshw. Biol. 2013, 58, 1631–1646. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Klooster, W.S.; Barrington, W.T.; Herms, D.A. Impacts of emerald ash borer-induced tree mortality on leaf litter arthropods and exotic earthworms. Pedobiologia 2011, 54, 261–265. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Barrington, W.T.; Hoebeke, E.R.; Herms, D.A. Vertically stratified ash-limb beetle fauna in northern Ohio. Psyche 2012, 2012, 215891. [Google Scholar] [CrossRef]
- Kovacs, F.K.; Haight, R.G.; McCullough, D.G.; Mercader, R.J.; Siegert, N.W.; Leibhold, A.M. Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol. Econ. 2010, 69, 569–578. [Google Scholar] [CrossRef]
- Aukema, J.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic impacts of non-native forest insects in the United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.J.; Bauer, L.S.; Poland, T.M.; Windell, K. Flight performance of Agrilus planipennis (Coleoptera: Buprestidae) on a flight mill and in free flight. J. Insect Behav. 2010, 23, 128–148. [Google Scholar] [CrossRef]
- USDA–APHIS. Emerald Ash Borer Federal Regulations and Quarantine Notices. 2017. Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/plant-pest-and-disease-programs/pests-and-diseases/emerald-ash-borer/ct_quarantine (accessed on 30 January 2018).
- CFIA. Canadian Food Inspection Agency. Emerald Ash Borer. 2018. Available online: http://www.inspection.gc.ca/plants/plant-pests-invasive-species/insects/emerald-ash-borer/eng/1337273882117/1337273975030 (accessed on 29 January 2018).
- USDA–APHIS. Emerald Ash Borer Program Manual, Agrilus planipennis (Fairmaire), ver. 1.6. 2015. Available online: https://www.aphis.usda.gov/import_export/plants/manuals/domestic/downloads/emerald_ash_borer_manual.pdf (accessed on 7 February 2018).
- Flower, C.E.; Dalton, J.E.; Knight, K.S.; Brikha, M.; Gonzalez-Meler, M.A. To treat or not to treat: Diminishing effectiveness of emamectin benzoate tree injections in ash trees heavily infested by emerald ash borer. Urban For. Urban Green. 2015, 14, 790–795. [Google Scholar] [CrossRef]
- O’Brien, E.M. Conserving Ash (Fraxinus) Populations and Genetic Variation in Forests Invaded by Emerald Ash Borer Using Large-Scale Insecticide Applications. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2017. [Google Scholar]
- Davidson, W.; Rieske, L.K. Establishment of classical biological control targeting emerald ash borer is facilitated by use of insecticides, with little effect on native arthropod communities. Biol. Control 2016, 101, 78–86. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.Y.; Li, M.L.; Yang, Z.Q.; Zeng, F.Z.; Wang, H.Y.; Bai, L.; Liu, S.J.; Sun, J. Biology and mass rearing of Sclerodermus pupariae Yang et Yao (Hymenoptera: Bethylidae), an important ectoparasitoid of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae) in China. Acta Entomol. Sin. 2008, 51, 46–54. [Google Scholar]
- Yang, Z.Q.; Wang, X.Y.; Yao, Y.X.; Gould, J.R.; Cao, L.M. A new species of Sclerodermus (Hymenoptera: Bethylidae) parasitizing Agrilus planipennis (Coleoptera: Buprestidae) from China, with a key to Chinese species in the genus. Ann. Entomol. Soc. Am. 2012, 105, 619–627. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Achterberg, C.V.; Choi, W.Y.; Marsh, P.M. First recorded parasitoid from China of Agrilus planipennis: A new species of Spathius (Hymenoptera: Braconidae: Doryctinae). Ann. Entomol. Soc. Am. 2005, 98, 636–642. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Yao, Y.X.; Wang, X.Y. A new species of emerald ash borer parasitoid from China belonging to the genus Tetrastichus (Hymneoptera: Eulophidae). Proc. Entomol. Soc. Wash. 2006, 108, 550–558. [Google Scholar]
- Zhang, Y.Z.; Huang, D.W.; Zhao, T.H.; Liu, H.P.; Bauer, L.S. Two new species of egg parasitoids (Hymenoptera: Encyrtidae) of wood-boring beetle pests from China. Phytoparasitica 2005, 53, 253–260. [Google Scholar] [CrossRef]
- Belokobylskij, S.A.; Yurchenko, G.I.; Strazanac, J.S.; Zaldi’var-Riveron, A.L.; Mastro, V. A new emerald ash borer (Coleoptera: Buprestidae) parasitoid species of Spathius Nees (Hymenoptera: Braconidae: Doryctinae) from the Russian Far East and South Korea. Ann. Entomol. Soc. Am. 2012, 105, 165–178. [Google Scholar] [CrossRef]
- Duan, J.J.; Yurchenko, G.; Fuester, R.W. Occurrence of emerald ash borer (Coleoptera: Buprestidae) and biotic factors affecting its immature stages in the Russian Far East. Environ. Entomol. 2012, 41, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.X.; Duan, J.J.; Hopper, K.R.; Mottern, J.L.; Gates, M.W. A new species of Oobius Trjapitzin (Hymenoptera: Encyrtidae) from the Russian Far East that parasitizes eggs of emerald ash borer (Coleoptera: Buprestidae). Ann. Entomol. Soc. Am. 2016, 106, 629–638. [Google Scholar] [CrossRef]
- Wang, X.Y.; Cao, L.M.; Yang, Z.Q.; Duan, J.J.; Gould, J.R.; Bauer, L.S. Natural enemies of emerald ash borer (Coleoptera: Buprestidae) in northeast China, with notes on two species of parasitic Coleoptera. Can. Entomol. 2016, 148, 329–342. [Google Scholar] [CrossRef]
- Liu, H.; Bauer, L.S.; Miller, D.L.; Zhao, T.; Gao, R.; Song, L.; Luan, Q.; Jin, R.; Gao, C. Seasonal abundance of Agrilus planipennis (Coleoptera: Buprestidae) and its natural enemies Oobius agrili (Hymenoptera: Encyrtidae) and Tetrastichus planipennisi (Hymenoptera: Eulophidae) in China. Biol. Control 2007, 42, 61–71. [Google Scholar] [CrossRef]
- NAPPO. (North American Plant Protection Organization) NAPPO Regional Standards for Phytosanitary Measures (RSPM). RSPM 12: Guidelines for Petition for First Release of Non-Indigenous Entomophagous Biological Control Agents. 2015. Available online: https://www.nappo.org/files/1814/4065/2949/RSPM12_30-07-2015-e.pdf (accessed on 19 January 2018).
- Mason, P.G.; Kabaluk, J.T.; Spence, B.; Gillespie, D.R. Regulation of Biological Control in Canada. In Biological Control Programmes in Canada 2001–2012; Mason, P.G., Gillespie, D.R., Eds.; CABI: Wallingford, UK, 2013; pp. 1–5. [Google Scholar]
- Montgomery, M. Understanding federal regulations as guidelines for classical biological control programs. In Implementation and Status of Biological Control of the Hemlock Woolly Adelgid; Onken, B., Reardon, R., Eds.; FHTET-2011-04; United States Department of Agriculture, Forest Service: Morgantown, WV, USA, 2011; pp. 25–40. Available online: http://www.fs.fed.us/nrs/pubs/jrnl/2011/nrs_2011_montgomery_001.pdf (accessed on 19 January 2018).
- Federal Register. Availability of an environmental assessment for the proposed release of three parasitoids for the biological control of the emerald ash borer Agrilus planipennis in the Continental United States. Fed. Regist. 2007, 72, 28947–28948. Available online: http://www.regulations.gov/#!documentDetail;D=APHIS-2007-0060-0043 (accessed on 18 December 2017).
- Federal Register. Availability of an environmental assessment for field release of the parasitoid Spathius galinae for the biological control of the emerald ash borer (Agrilus planipennis) in the contiguous United States. Fed. Regist. 2015, 80, 7827–7828. Available online: https://www.regulations.gov/docket?D=APHIS-2014-0094 (accessed on 18 December 2017).
- Duan, J.J.; Gould, J.R.; Fuester, R.W. Evaluation of the host specificity of Spathius galinae (Hymenoptera: Braconidae), a larval parasitoid of the emerald ash borer (Coleoptera: Buprestidae) in Northeast Asia. Biol. Control 2015, 89, 91–97. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Wang, X.Y.; Gould, J.R.; Wu, H. Host specificity of Spathius agrili Yang (Hymenoptera: Braconidae), an important parasitoid of the emerald ash borer. Biol. Control 2008, 47, 216–221. [Google Scholar] [CrossRef]
- Bellamy, C.L. World catalogue and bibliography of the jewel beetles (Coleoptera: Buprestidae). In Agrilinae: Agrilina through Trachyini; Pensoft Series Faunistica #79; Pensoft Publishers: Sofia, Bulgaria; Moscow, Russia, 2008; Volume 4, pp. 1932–2684. ISBN 9789546423214. [Google Scholar]
- Nelson, G.H.; Walters, G.C., Jr.; Haines, R.D.; Bellamy, C.L. A Catalog and Bibliography of the Buprestoidea of America North of Mexico; The Coleopterists Society, Special Publication: North Potomac, MD, USA, 2008; pp. 1–274. [Google Scholar]
- Johnson, T.D.; Lelito, J.P.; Raffa, K.F. Responses of two parasitoids, the exotic Spathius agrili Yang and the native Spathius floridanus Ashmead, to volatile cues associated with the emerald ash borer, Agrilus planipennis Fairmaire. Biol. Control 2014, 79, 110–117. [Google Scholar] [CrossRef]
- Jennings, D.E.; Duan, J.J.; Bean, D.; Kimberly, A.R.; Williams, G.L.; Bells, S.K.; Shurtleff, A.S.; Shrewsbury, P.M. Effects of the emerald ash borer invasion on the community composition of arthropods associated with ash tree boles in Maryland, U.S.A. Agric. For. Entomol. 2017, 19, 122–129. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Van Driesche, R.G. Emerald ash borer biocontrol in ash saplings: The potential for early stage recovery of North American ash trees. For. Ecol. Manag. 2017, 394, 64–72. [Google Scholar] [CrossRef]
- Margulies, E.; Bauer, L.; Ibanez, I. Buying time: Preliminary assessment of biocontrol in the recovery of native forest vegetation in the aftermath of the invasive emerald ash borer. Forests 2017, 8, 369. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Lelito, J.P.; Van Driesche, R.G. Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: Potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2013, 106, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Abell, K.J.; Bauer, L.S.; Duan, J.J.; Van Driesche, R.G. Long-term monitoring of the introduced emerald ash borer (Coleoptera: Buprestidae) egg parasitoid, Oobius agrili (Hymenoptera: Encyrtidae), in Michigan, USA and evaluation of a newly developed monitoring technique. Biol. Control 2014, 79, 36–42. [Google Scholar] [CrossRef]
- USDA–APHIS/ARS/FS. USDA Animal Plant Health Inspection Service/Agricultural Research Service/Forest Service. Emerald Ash Borer Biological Control Release and Recovery Guidelines. 2016. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b/downloads/EAB-FieldRelease-Guidelines.pdf (accessed on 18 January 2018).
- MapBioControl.org. Agent Release Tracking and Data Management for Federal, State, and Researchers Releasing Biocontrol Agents for Management of the Emerald Ash Borer. 2018. Available online: http://www.mapbiocontrol.org/ (accessed on 16 January 2018).
- Duan, J.J.; Van Driesche, R.G.; Bauer, L.S.; Reardon, R.; Gould, J.; Elkinton, J.S. The Role of Biocontrol of Emerald Ash Borer in Protecting Ash Regeneration after Invasion; FHAAST-2017-02; United States Department of Agriculture, Forest Service, Forest Health Assessment and Applied Sciences Team: Morgantown, WV, USA, 2017; pp. 1–10. [Google Scholar]
- Duan, J.J.; Van Driesche, R.G.; Bauer, L.S.; Kashian, D.M.; Herms, D.A. Risk to ash from emerald ash borer: Can biological control prevent the loss of ash stands? In Biology and Control of Emerald Ash Borer; van Driesche, R.G., Reardon, R.C., Eds.; FHTET-2014-9; United States Department of Agriculture Forest Service, Forest Health Technology Enterprise Team: Morganton, WV, USA, 2015; pp. 153–163. Available online: https://www.nrs.fs.fed.us/pubs/49310 (accessed on 30 January 2018).
- Lindell, C.A.; McCullough, D.G.; Cappaert, D.; Apostolou, N.M.; Roth, M.B. Factors influencing woodpecker predation on emerald ash borer. Am. Midl. Nat. 2008, 159, 434–444. [Google Scholar] [CrossRef]
- Flower, C.E.; Long, L.C.; Knight, K.S.; Rebbeck, J.; Brown, J.S.; Gonzalez-Meler, M.A.; Whelan, C.J. Native bark-foraging birds preferentially forage in infected ash (Fraxinus spp.) and prove effective predators of the invasive emerald ash borer (Agrilus planipennis Fairmaire). For. Ecol. Manag. 2014, 313, 300–306. [Google Scholar] [CrossRef]
- Jennings, D.E.; Gould, J.R.; Vandenberg, J.D.; Duan, J.J.; Shrewsbury, P.M. Quantifying the impact of woodpecker predation on population dynamics of the emerald ash borer (Agrilus planipennis). PLoS ONE 2013, 8, e83491. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D.E.; Duan, J.J.; Abell, K.J.; Bauer, L.S. Life table evaluation of change in emerald ash borer populations due to biological control. In Biology and Control of Emerald Ash Borer; Van Driesche, R.G., Reardon, R.C., Eds.; FHTET-2014-9; United States Department of Agriculture Forest Service, Forest Health Technology Enterprise Team: Morganton, WV, USA, 2015; pp. 139–151. Available online: https://www.nrs.fs.fed.us/pubs/49312 (accessed on 6 March 2018).
- Liu, H.P.; Bauer, L.S. Susceptibility of Agrilus planipennis (Coleoptera: Buprestidae) to Beauveria bassiana and Metarhizium anisopliae. J. Econ. Entomol. 2006, 99, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, L.A.; Bauer, L.S.; Houping, L.P.; Griggs, M.H.; Vandenberg, J.D. Characterization of Beauveria bassiana (Ascomycota: Hypocreales) isolates associated with Agrilus planipennis (Coleoptera: Buprestidae) populations in Michigan. Biol. Control 2010, 54, 135–140. [Google Scholar] [CrossRef]
- Wu, H.; Li, M.L.; Yang, Z.Q.; Wang, X.Y. Research on cold hardiness of emerald ash borer and its two parasitoids, Spathius agrili Yang (Hym., Braconidae) and Tetrastichus planipennisi Yang (Hym., Eulophidae). Chin. J. Biol. Control 2007, 23, 119–122. (In Chinese) [Google Scholar]
- Abell, K.J.; Bauer, L.S.; Miller, D.L.; Duan, J.J.; Van Driesche, R.G. Monitoring the establishment and flight phenology of parasitoids of emerald ash borer (Coleoptera: Buprestidae) in Michigan by using sentinel eggs and larvae. Fla. Entomol. 2016, 99, 667–672. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Van Driesche, R.G. Population responses of hymenopteran parasitoids to the emerald ash borer (Coleoptera: Buprestidae) in recently invaded areas in north central United States. BioControl 2012, 57, 199–209. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Ulyshen, M.D.; Van Driesche, R.G. Population dynamics of an invasive forest insect and associated natural enemies in the aftermath of invasion: Implications for biological control. J. Appl. Ecol. 2015, 52, 1246–1254. [Google Scholar] [CrossRef]
- Bauer, L.S.; Duan, J.J.; Lelito, J.P.; Liu, H.P.; Gould, J.R. Biology of emerald ash borer parasitoids. In Biology and Control of Emerald Ash Borer; van Driesche, R.G., Reardon, R.C., Eds.; FHTET-2014-9; United States Department of Agriculture Forest Service, Forest Health Technology Enterprise Team: Morganton, WV, USA, 2015; Chapter 6; pp. 97–112. Available online: https://www.nrs.fs.fed.us/pubs/49294 (accessed on 30 January 2018).
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Van Driesche, R.G. Natural enemies implicated in the regulations of an invasive pest: A life table analysis of the population dynamics of the invasive emerald ash borer. Agric. For. Entomol. 2014, 16, 406–416. [Google Scholar] [CrossRef]
- Mercader, R.; Siegert, N.W.; Liebhold, A.M.; McCullough, D.G. Dispersal of the emerald ash borer, Agrilus planipennis, in newly colonized sites. Agric. For. Entomol. 2009, 11, 421–424. [Google Scholar] [CrossRef]
- Siegert, N.W.; McCullough, D.G.; Williams, D.W.; Fraser, I.; Poland, T.M. Dispersal of Agrilus planipennis (Coleoptera: Buprestidae) from discrete epicenters in two outlier sites. Environ. Entomol. 2010, 39, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Abell, K.J.; Duan, J.J.; Bauer, L.S.; Lelito, J.P.; Van Driesche, R.G. The effect of bark thickness on the effectiveness of Tetrastichus planipennisi (Hymen: Eulophidae) and Atanycolus spp. (Hymen: Braconidae) two parasitoids of emerald ash borer (Coleop: Buprestidae). Biol. Control 2012, 63, 320–325. [Google Scholar] [CrossRef]
- Murphy, T.C.; Van Dreische, R.G.; Gould, J.R.; Elkinton, J. Can Spathius galinae attack emerald ash borer larvae feeding in large ash trees? Biol. Control 2017, 114, 8–14. [Google Scholar] [CrossRef]
- Larson, K.M.; Duan, J.J. Differences in the reproductive biology and diapause of two congeneric species of egg parasitoids (Hymenoptera: Encyrtidae) from northeast Asia: Implications for biological control of the invasive emerald ash borer (Coleoptera: Buprestidae). Biol. Control 2016, 101, 39–45. [Google Scholar] [CrossRef]
- Wei, X.; Reardon, D.; Wu, Y.; Sun, J.H. Emerald ash borer, Agrilus planipennis, in China: A review and distribution survey. Acta Entomol. Sin. 2004, 47, 679–685. [Google Scholar]
- Kashian, D.M.; Witter, J.A. Assessing the potential for ash canopy tree replacement via current regeneration following emerald ash borer-caused mortality on southeastern Michigan landscapes. For. Ecol. Manag. 2011, 261, 480–488. [Google Scholar] [CrossRef]
- Kashian, D.M. Sprouting and seed production may promote persistence of green ash in the presence of the emerald ash borer. Ecosphere 2016, 7, e01332. [Google Scholar] [CrossRef]

| Geographic Region | Natural Enemies | EAB Stage(s) Attacked | Rate of Attack (Parasitism or Predation) | References |
|---|---|---|---|---|
| Northeast China: Heilongjiang, Jilin, and Liaoning provinces | Oobius agrili | eggs | 12–62% | [26,62,66] |
| Oencyrtus sp. | eggs | 1–2% | [19] | |
| Tetrastichus planipennisi | 3rd and 4th instars | 3–44% | [26,61,66,67] | |
| Spathius agrili | 3rd and 4th instars | 0–13% | [26,60,66] | |
| Atanycolus spp. Foerster (Hymenoptera: Braconidae) | 3rd and 4th instars | 0–23% | [66] | |
| Xorides sp. Latreille (Hymenoptera: Ichneumonidae) | 3rd and 4th instars | 0–11% | [66] | |
| Tenerus sp. Laporte (Coleoptera: Cleridae) | JLand pupae | 0–21% | [66] | |
| Xenoglena quadrisignata Mannerheim (Coleoptera: Trogossitidae) | JL and pupae | 0–1.2% | [66] | |
| Northcentral China: Beijing and Tianjin cities | Oobius agrili | eggs | 0–4.0% | [62,66] |
| Tetrastichus planipennisi | 3rd and 4th instars | 0–7% | [26,61,66,67] | |
| Spathius agrili | 3rd and 4th instars | 44–67% | [61,66] | |
| Atanycolus sp. | 3rd and 4th instars | 0–5% | [66] | |
| Metapelma sp. Westwood (Hymenoptera: Eupelmidae) | 3rd and 4th instars | 0–4% | [66] | |
| Sclerodermus pupariae Yang and Yao (Hymenoptera: Bethylidae) | 3rd and pupae | 1–1.3% | [66] | |
| Russia: Primorsky Kray | Oobius primorskyensis | egg | 23–44% | JJD (unpublished data) |
| Tetrastichus planipennisi | 3rd and 4th instars | 0–7% | [64] | |
| Spathius galinae | 3rd and 4th instars | 0–78% | [64] | |
| Atanycolus nigriventris Vojnovskaja-Krieger (Braconidae: Braconinae) | 3rd and 4th instars | 0–55% | [64] | |
| Atanycolus sp. | 3rd and 4th instars | 0–1% | [64] |
| EAB Parasitoids from Asia | Insect Orders Tested | Insect Families Tested | Insect Species Tested | Agrilus Species Tested | The Only Non-Targets Attacked Were Agrilus Species |
|---|---|---|---|---|---|
| Oobius agrili 1 | 2 | 6 | 18 | 6 | 3 |
| Tetrastichus planipennisi 1 | 3 | 6 | 14 | 5 | 0 |
| Spathius agrili 1 | 2 | 6 | 18 | 9 | 5 |
| Spathius galinae 2 | 3 | 6 | 15 | 6 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, J.J.; Bauer, L.S.; Van Driesche, R.G.; Gould, J.R. Progress and Challenges of Protecting North American Ash Trees from the Emerald Ash Borer Using Biological Control. Forests 2018, 9, 142. https://doi.org/10.3390/f9030142
Duan JJ, Bauer LS, Van Driesche RG, Gould JR. Progress and Challenges of Protecting North American Ash Trees from the Emerald Ash Borer Using Biological Control. Forests. 2018; 9(3):142. https://doi.org/10.3390/f9030142
Chicago/Turabian StyleDuan, Jian J., Leah S. Bauer, Roy G. Van Driesche, and Juli R. Gould. 2018. "Progress and Challenges of Protecting North American Ash Trees from the Emerald Ash Borer Using Biological Control" Forests 9, no. 3: 142. https://doi.org/10.3390/f9030142




