1. Introduction
Natural regeneration is a key component for securing the sustainability of forests and forest dynamics [
1,
2,
3] because it has the potential for the development of future generations, the storage of carbon, and it leads to the recovery of the ecosystem functions [
4,
5,
6]. The research conducted in sub-tropical forests of India showed that an absence or a small number of seedlings and saplings of tree species in a forest stand indicates poor regeneration; on the other hand, a greater number of seedlings and saplings shows successful regeneration [
7]. The maintenance of a forest stand with sufficient regeneration is the main target in conservation ecology [
2].
Natural regeneration has also been used as a tool in the implementation of large-scale restoration programs in degraded forest areas [
8,
9], especially in the tropical regions [
10,
11]. When we consider species distribution and the diversity of forests in tropical ecology, natural regeneration should also be included in the assessment because it can help us to envisage the upcoming forest stand. Therefore, understanding the mechanism and nature of natural regeneration facilitates the enhancement of natural regeneration, as well as the changes in future forest structure [
12].
Natural regeneration could be affected by both abiotic (environment) and biotic (overstory structure) factors [
1,
2], and securing a sufficient regeneration of a forest is a great challenge in forest management. A reduction in precipitation, especially drought, can limit the regeneration potential and composition of plants because almost all species are very susceptible to environmental factors at the seedling stages [
8,
13,
14]. Osem et al. [
12] reported that species composition at regeneration stages varied in regions with an annual rainfall of 300 to 900 mm. Mok et al. [
15] also estimated, by simulation analysis, that species diversity and composition at the regeneration stage could change in upcoming decades due to an increase in temperature and decrease in precipitation. On the other hand, changes in overstory structure, such as species distribution and canopy cover, could directly affect the diversity [
2], survivorship [
16,
17,
18,
19], and composition [
20,
21] of natural regeneration of some species. Therefore, monitoring the changes in natural regeneration in response to different overstory structures and climatic regions has been essential in ecological studies.
Only a few studies have focused on natural regeneration ecology. Among them, some studies have addressed the effect of overstory structure on natural regeneration at relatively small scales in Acadian forests [
22], disturbed forests [
23], and Amazonian forests [
24]. Although the factors driving the structure and composition of natural regeneration have been studied by modeling approaches [
9], there is also no convincing evidence to date relating to which overstory factors have a stronger relationship with natural regeneration than others, especially in Asian regions. Beside this, the regeneration-rainfall gradient hypothesis has—to our knowledge—only been tested in a few studies. The way in which abiotic and biotic factors affect the structure and composition of natural regeneration by actual field surveys is still not well understood [
25,
26].
This study focused on cause-and-effect relationships between environmental factors and natural regeneration, and those between overstory structure and natural regeneration of tropical forests in Myanmar. So, the research questions were set up as: (1) Do species diversity, richness, density, and status of natural regeneration vary across a precipitation gradient in Myanmar? (2) Do environmental and overstory structural factors affect the status of natural regeneration? and (3) If so, how do these factors vary?
The objectives of the study were:
- (1)
To observe the natural regeneration status of tropical forests in Myanmar across a precipitation gradient;
- (2)
To extrapolate the effects of the environmental factors and overstory structure on natural regeneration of tropical forests; and
- (3)
To identify the most influential environmental and overstory structure factors on natural regeneration of tropical forests.
4. Discussion
The assessment of natural regeneration plays a vital role in sustainable forest management, because the regeneration stage is very important for the successful establishment of the mature community [
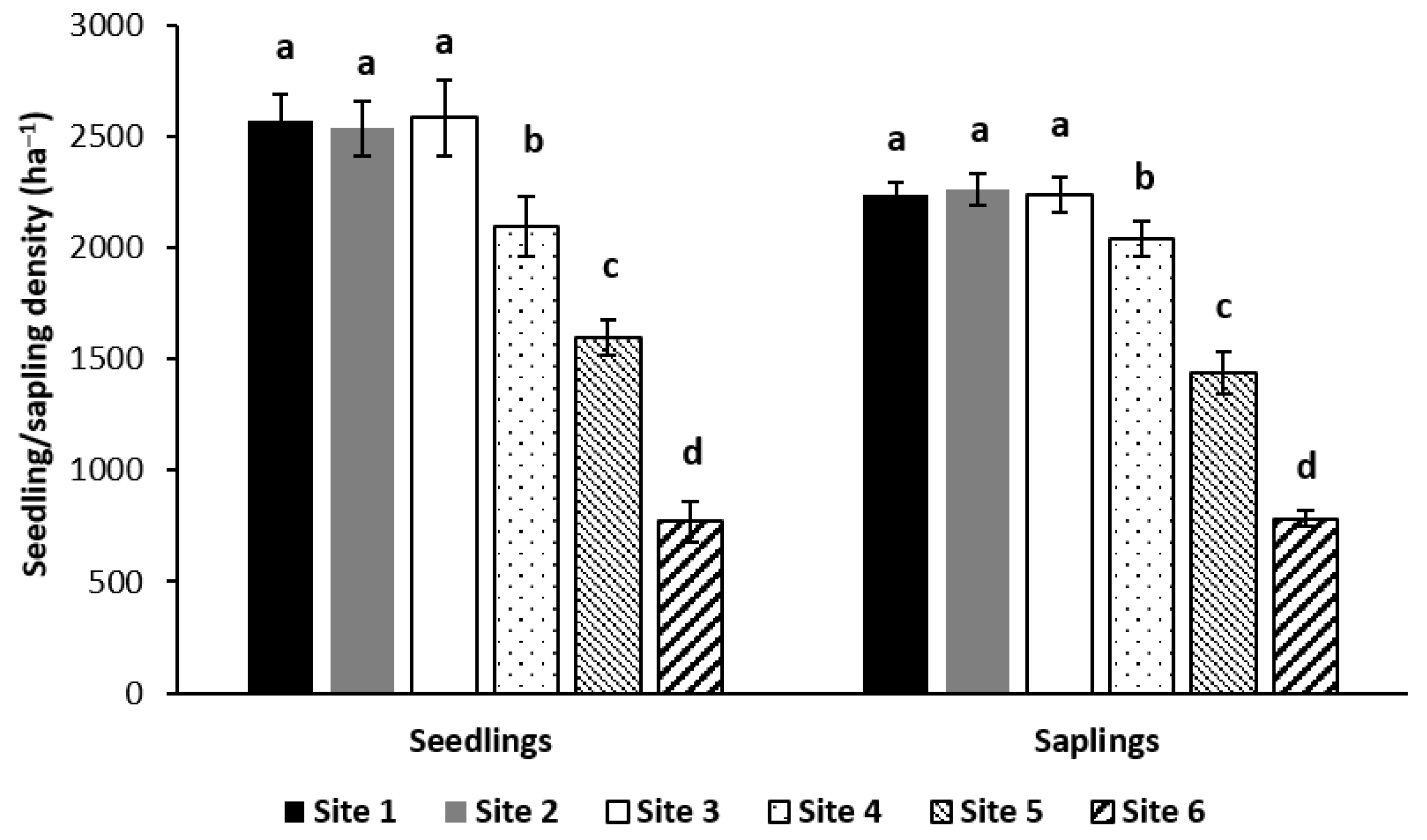
2]. In this study, the densities of both seedlings and saplings at all sites were good enough for future regeneration from a management perspective, even though the density at lower rainfall regions (sites 4–6) was significantly lower than for the other sites.
Within the lower rainfall sites, the seedling and sapling densities of the forests with 1029 mm of annual rainfall and 843 mm of annual rainfall were 2.5 times and two times higher than those of the forests with 625 mm of annual rainfall, respectively. This finding supported the research by Galiano et al. [
45] and Parada and Lusk [
46], which explored how drought or reduced rainfall limited the survival of plants at the seedling stage. In comparison with other studies, the density of natural regeneration at our study sites was lower than that of semi-evergreen forests in Myanmar studied by Oo [
47], and higher than that of the secondary forests in Vietnam studied by Woo et al. [
48].
The richness of understory species in this study ranged from 17 species in the low rainfall region to 74 species in the high rainfall region, showing an increasing trend from low to high rainfall. Nonetheless, the species richness of forests at all sites in this study was lower than the richness found in semi-evergreen forests [
47], although it was higher than that of dry forests in a semiarid region, where twelve regenerating species occurred [
12].
Similar observations were made for species diversity. The results throughout the study sites showed an increasing trend with rainfall. Moreover, a noticeable difference (a significant increase) was found when annual rainfall changed from 625–1029 mm to 1411 mm. There was no evidence of a significance difference between forests with 625–1029 mm of annual rainfall.
Our study supported the statement developed by Mok et al. [
15] that climatic variation was one of the factors that resulted in a shift in species niches, showing that species composition varied among forests in different rainfall regions. Moreover, this study showed that some species were distributed throughout a wide range of rainfall regions, whereas some species were adapted to a particular area. The natural regeneration of
T. grandis and
X. dolabriformis occurred throughout the regions where the annual rainfall was 843–2035 mm, showing that
X. dolabriformis exhibited higher regeneration than
T. grandis (
Table S1). In addition,
T. grandis occurred abundantly at sites 1–3 only, while
X. dolabriformis occurred abundantly at sites 1–5. Therefore, the distribution range of
X. dolabriformis was wider than that of
T. grandis. However, those two species did not occur in a region with 625 mm of annual rainfall.
Furthermore, in a region with 625 mm of annual rainfall, a higher percentage of regeneration occurred for M. tinctoria and A. catechu species. However, those species were not observed in the high rainfall regions, particularly M. tinctoria, which was not observed in regions with 843–2035 mm of annual rainfall, while A. catechu did not occur in regions with 1029–2035 mm of annual rainfall. Therefore, low rainfall regions could be the preferred habitat of these species.
Most of the species at all sites had a good regeneration status, although a few species had a poor regeneration status or no natural regeneration. Numerically, 1.33% of total species at site 1, 1.37% at site 2, 2.04% at site 4, 4.55% at site 5, and 10.53% at site 6 revealed no natural regeneration. Moreover, 1.37% of total species at site 2, 4.35% at site 3, 4.08% at site 4, and 4.55% at site 5 demonstrated poor regeneration. The regeneration status found in this study was better than that of subtropical forests found by Khumbongmayum et al. [
7], who reported that 2–16% of total species had no regeneration, and 13–29% had poor regeneration.
The spatial distribution of forests varied among sites, although the values did not differ significantly from each other. Within a range of 625–2035 mm of annual rainfall, the spatial distribution at all study sites was found to be regular, without showing any decreasing or increasing trends with annual rainfall.
Generally, natural regeneration largely depends on not only abiotic (temperature, rainfall, fertility, etc.), but also biotic (diversity, richness, composition, etc.) factors. In terms of abiotic factors, it is commonly accepted that the amount of rainfall is one of the essential factors for plant survival [
49,
50,
51]. Our study supports that statement, in that the diversity and density of natural regeneration increased with rainfall. This study was also consistent with Osem et al. [
12] findings that the density and richness increased linearly with rainfall in the regions where annual rainfall ranged from 300 to 900 mm.
This study highlighted that natural regeneration was more susceptible to the impact of rainfall in low rainfall regions than high rainfall regions, because the density of natural regeneration was significantly lower when annual rainfall decreased within the lower rainfall regions; however, a significant effect of rainfall on natural regeneration did not occur within high rainfall regions (
Figure 2). Among climatic factors, average rainfall tended to be the most influential factor on the natural regeneration of forests, followed by maximum rainfall and average temperature.
In terms of biotic effects, understory species richness depends on overstory diversity [
2,
52]. Within a range of 625 to 2035 mm annual rainfall, the richer the overstory diversity of the forest, the richer the understory species recorded. The correlation of the diversity of mature trees to the diversities of seedlings and saplings was strong (
r = 0.961,
p ≤ 0.01 for seedlings, and
r = 0.99,
p ≤ 0.01 for saplings). Natural generation status was better in forests with a range of 843–2035 mm of annual rainfall than that in forests with 625 mm of annual rainfall.
Luo et al. [
3] expressed that stand density was one of the factors that affected regeneration status. In this study, the overstory structure parameters that affected the natural regeneration of a forest were the overstory species richness, density, species diversity, and ecosystem complexity. Additionally, this study explored the combination of the influential overstory parameters that significantly affected species diversity and richness of natural regeneration, with the combination of two overstory factors, overstory density × richness and overstory richness × ecosystem complexity, showing significant effects.
The results from this study exhibited the variation of natural regeneration in response to different overstory structures across a rainfall gradient, which may indicate that there is potential for greater species diversity, richness, and density of upcoming forest stands with 1411–2035 mm of annual rainfall than those of forests with 625–1029 mm of annual rainfall. Although the density of upcoming forests with 1029 mm of annual rainfall is expected to be higher than those of forests with 625–843 mm of annual rainfall, the species diversity and richness of forests may remain the same between those regions, if there are no other disturbances. Overall, the possibility of a more diverse forest stand may occur in regions with 1411–2035 mm of annual rainfall than the other regions (625–1029 mm) in the future generation, and the overstory structure may control the natural regeneration of forests. The results from this study may form a basis for creating the next generation. A forest management approach, including silvicultural operations which can enhance natural regeneration such as canopy opening, climber cutting, weeding, enrichment planting, etc., may be given for maintaining forest biodiversity or creating the upcoming forest stands as a diverse forest with intended species.
5. Conclusions
The status of natural regeneration in tropical forests of Myanmar differed along rainfall gradients (a range of 625 to 2035 mm annual rainfall), although it demonstrated a good regeneration status, and regular species distributions, within that range of annual rainfall. The impact of climate was significant on species diversity, richness, and density of natural regeneration; an increasing trend occurred in regions with 625–2035 mm of annual rainfall. Forests with 1411 to 2035 mm of annual rainfall have a significantly higher density and species diversity at natural regeneration stages than those forests with an annual rainfall of 625 to 1029 mm. This study also revealed that species distributions, as well as the regeneration status of species at natural regeneration stages, varied from site to site, with a change of dominant species in the overstory structure. Both abiotic and biotic factors influenced the regeneration status. Among the environmental factors, average annual rainfall, followed by maximum rainfall and the temperature difference, strongly influenced the density of natural regeneration, although the average annual rainfall, followed by maximum rainfall and average annual temperature, influenced the diversity of natural regeneration. Nevertheless, the spatial distribution of natural regeneration could not be determined by the environmental factors. Biotic factors, such as: (1) the overstory density × richness interaction, and (2) the overstory richness × ecosystem complexity interaction, significantly influenced the density of natural regeneration. All combination effects of overstory density, richness, diversity, and ecosystem complexity significantly influenced species richness and the diversity of natural regeneration. It can be concluded that the natural regeneration of forests varied with both: (1) different rainfall gradients, and (2) overstory structure parameters, in terms of density, species richness, diversity, and ecosystem complexity. To control or conserve the natural regeneration of forests, amount of rainfall and overstory parameters should be taken into consideration in the forest management system.