Analysis of Soil Degradation Causes in Phyllostachys edulis Forests with Different Mulching Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Soil Sampling
2.4. Soil Chemical Properties
2.5. Soil Enzyme Activities
2.6. Soil Microbial Biomass
2.7. Statistical Analysis
3. Results
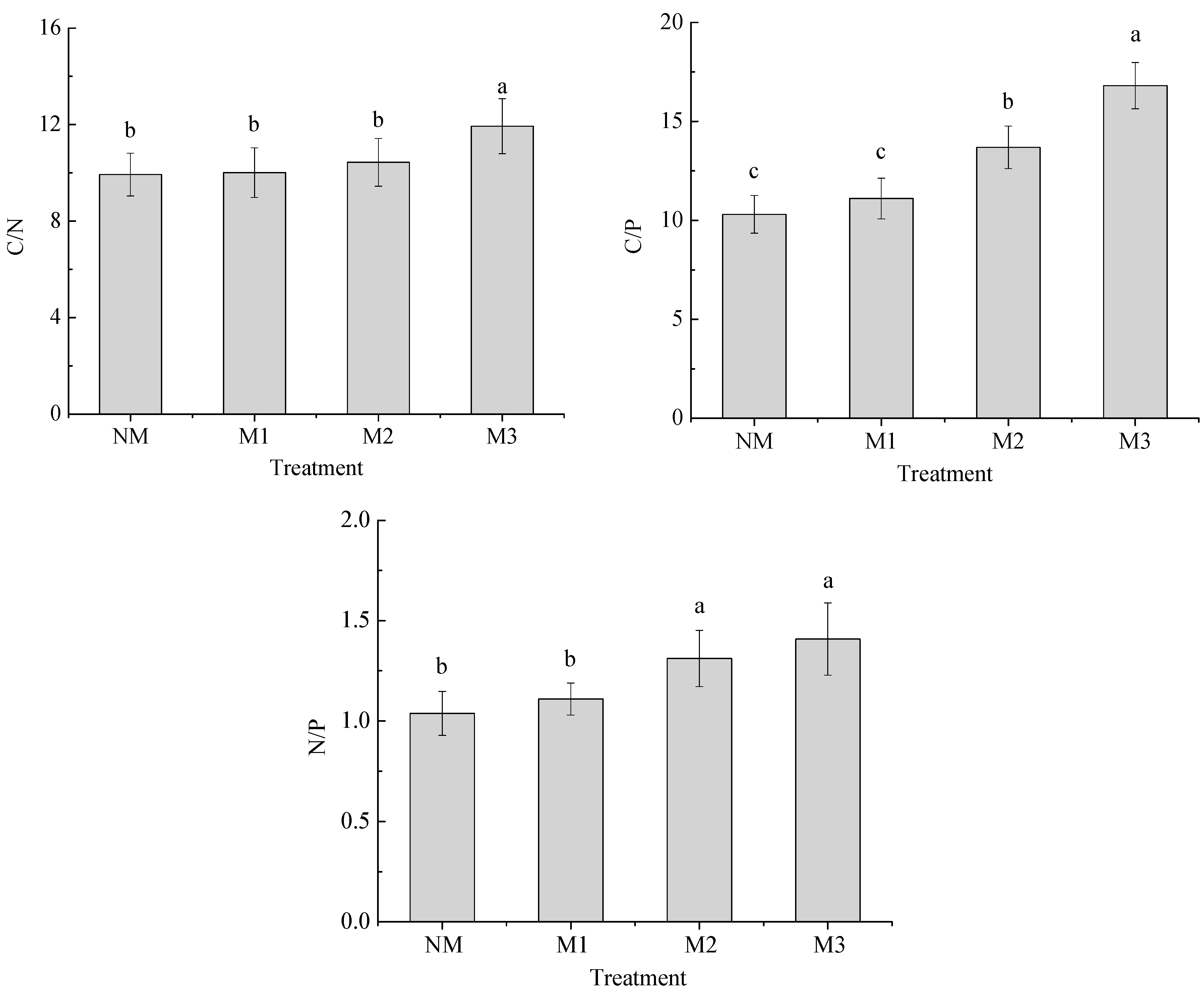
3.1. Soil Chemical Properties
3.2. Soil Enzyme Activities
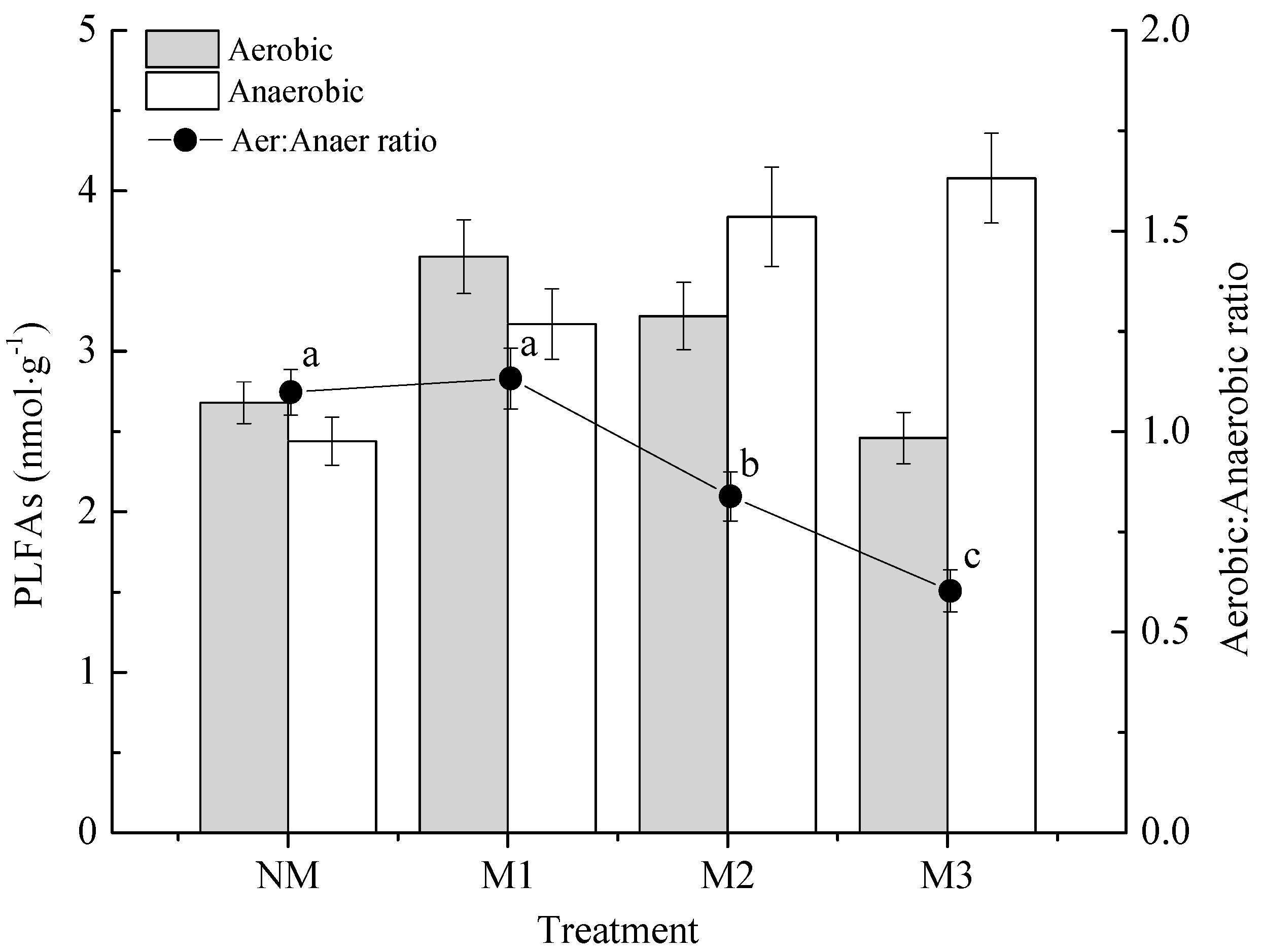
3.3. Soil Microbial Biomass
4. Discussion
4.1. Mulching Management and Soil pH
4.2. Mulching Management and Soil Nutrients
4.3. Mulching Management and Soil Enzyme Activities
4.4. Mulching Management and Soil Microbial Biomass
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, X.; Fan, S.; Qi, L.; Guan, F.; Du, M.; Zhang, H. Soil respiration and net ecosystem production in relation to intensive management in Moso bamboo forests. Catena 2016, 137, 219–228. [Google Scholar] [CrossRef]
- Zhao, J.C.; Su, W.H.; Fan, S.H.; Cai, C.J.; Zhu, X.W.; Peng, C.; Tang, X.L. Effects of various fertilization depths on ammonia volatilization in Moso bamboo (Phyllostachys edulis) forests. Plant Soil Environ. 2016, 62, 128–134. [Google Scholar]
- Chen, S.L. Thoughts on related problems of mulched technique with organic materials in moso bamboo forest for early shooting. J. Zhejiang A F Univ. 2011, 28, 799–804. [Google Scholar]
- Mertens, B.; Hua, L.; Belcher, B.; Ruiz-Pérez, M.; Fu, M.; Yang, X. Spatial patterns and processes of bamboo expansion in Southern China. Appl. Geogr. 2008, 28, 16–31. [Google Scholar] [CrossRef]
- Song, X.Z.; Zhou, G.M.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon sequestration by Chinese bamboo forests and their ecological benefits: Assessment of potential, problems, and future challenges. Environ. Rev. 2011, 19, 418–428. [Google Scholar] [CrossRef]
- Jiang, Z.H. Bamboo and Rattan in the World; China Forestry Publishing House: Beijing, China, 2007. [Google Scholar]
- Chen, J.M.; Zhu, W.; Fu, L.F.; Chen, J.M.; Ji, W.W.; Zhu, Z.J. Effect of mulching on rhizome growth and bamboo shoot yield in moso shoot productive forest. Word Bamboo Rat. 2015, 13, 20–28. [Google Scholar]
- Rathinasabapathi, B.; Ferguson, J.; Gal, M. Evaluation of allelopathic potential of wood chips for weed suppression in horticultural production systems. Hortic. Sci. 2015, 40, 711–713. [Google Scholar]
- Ni, X.; Song, W.; Zhang, H.; Yang, X.; Wang, L. Effects of mulching on soil properties and growth of tea olive (Osmanthus fragrans). PLoS ONE 2016, 11, e0158228. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.N.; Rao, V.K.; Dimri, D.C.; Sharma, S.K. Effect of mulching on soil properties, growth and yield of strawberry cv. Chandler under mid hill conditions of Uttarakhand. Prog. Hortic. 2016, 48, 42–47. [Google Scholar] [CrossRef]
- Mulumba, L.N.; Lal, R. Mulching effects on selected soil physical properties. Soil Tillage Res. 2008, 98, 106–111. [Google Scholar] [CrossRef]
- Jordán, A.; Zavala, L.M.; Gil, J. Effects of mulching on soil physical properties and runoff under semi-arid conditions in southern Spain. Catena 2010, 81, 77–85. [Google Scholar] [CrossRef]
- Nzeyimana, I.; Hartemink, A.E.; Ritsema, C.; Stroosnijder, L.; Lwanga, E.H.; Geissen, V. Mulching as a strategy to improve soil properties and reduce soil erodibility in coffee farming systems of Rwanda. Catena 2017, 149, 43–51. [Google Scholar] [CrossRef]
- Li, T. Effects of Mulching on Soil Physicochemistry Properties and Quality of Bamboo Shoots in Phyllostachys heterocycla Stand. Master’s Thesis, Sichuan Agricultural University, Chengdu, China, 2016. [Google Scholar]
- Zhong, Z.L.; Zhu, W.Q.; Wu, L.D. Effects of removing the mulching in mid and late December on Phyllostachys heterocycla cv. pubescens yield and growth. J. Northeast For. Univ. 2016, 44, 7–10. [Google Scholar]
- Wang, B.; Wang, K.H.; Li, Q.; Zhu, Z.J.; Ding, X.Z.; Yang, J. A preliminary study of the effect of mulching on growth of Phyllostachys edulis. Word Bamboo Rat. 2012, 10, 20–22. [Google Scholar]
- Tang, X.; Fan, S.; Qi, L.; Guan, F.; Liu, G.; Du, M. Effects of understory removal on root production, turnover and total belowground carbon allocation in Moso bamboo forests. For.-Biogeosci. For. 2015, 9, 187–194. [Google Scholar] [CrossRef]
- Tang, X.; Fan, S.; Qi, L.; Guan, F.; Su, W.; Du, M. A comparison of soil respiration, carbon balance and root carbon use efficiency in two managed Moso bamboo forests in subtropical China. Ann. For. Res. 2016, 59, 3–20. [Google Scholar] [CrossRef]
- Fu, J. Moso bamboo in China. ABS Mag. 2000, 21, 12–17. [Google Scholar]
- Zhou, B.; Li, Z.; Wang, X.; Gao, Y.; An, Y.; Deng, Z.; Letu, G.; Gu, L. Impact of the 2008 ice storm on Moso bamboo plantations in southeast China. J. Geophys. Res. Biogeosci. 2011, 116, G00H06. [Google Scholar] [CrossRef]
- Fan, S.; Guan, F.; Xu, X.; Forrester, D.I.; Ma, W.; Tang, X. Ecosystem carbon stock loss after land use change in subtropical forests in China. Forests 2016, 7, 142. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Guan, S.Y. Soil Enzyme and Its Research Methods; China Agriculture Press: Beijing, China, 1986. (In Chinese) [Google Scholar]
- Cycoń, M.; Wójcik, M.; Borymski, S.; Zofia, P.S. Short-term effects of the herbicide napropamide on the activity and structure of the soil microbial community assessed by the multi-approach analysis. Appl. Soil Ecol. 2013, 66, 8–18. [Google Scholar] [CrossRef]
- Wu, C.; Qin, Z.; Huang, J.; Zhou, R. Characterization of microbial community in Daqu by PLFA method. Food Sci. Technol. Res. 2014, 20, 147–154. [Google Scholar] [CrossRef]
- Francisco, R.; Stone, D.; Creamer, R.E.; Sousa, J.P.; Morais, P.V. European scale analysis of phospholipid fatty acid composition of soils to establish operating ranges. Appl. Soil Ecol. 2016, 97, 49–60. [Google Scholar] [CrossRef]
- Carrasco, L.; Gattinger, A.; Fließbach, A.; Roldán, A.; Schloter, M.; Caravaca, F. Estimation by PLFA of microbial community structure associated with the rhizosphere of Lygeum spartum and Piptatherum miliaceum growing in semiarid mine tailings. Microb. Ecol. 2010, 60, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Molineux, C.J.; Gange, A.C.; Connop, S.P.; Newport, D.J. Are microbial communities in green roof substrates comparable to those in post-industrial sites?—A preliminary study. Urban Ecosyst. 2015, 18, 1245–1260. [Google Scholar] [CrossRef]
- Moche, M.; Gutknecht, J.; Schulz, E.; Langer, U.; Rinklebe, J. Monthly dynamics of microbial community structure and their controlling factors in three floodplain soils. Soil Biol. Biochem. 2015, 90, 169–178. [Google Scholar] [CrossRef]
- Si, G.; Peng, C.; Yuan, J.; Xu, X.; Zhao, S.; Xu, D.; Wu, J. Changes in soil microbial ommunity composition and organic carbon fractions in an integrated rice-crayfish farming system in subtropical China. Sci. Rep. 2017, 7, 2856. [Google Scholar] [CrossRef] [PubMed]
- Liu, L. Study on Degradation Characteristics and the Improvement of Soil in Covered Phyllostachys praecox F. Prevernalis Forest. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2009. [Google Scholar]
- Álvarez, E.; Fernández-Marcos, M.L.; Monterroso, C.; Fernández-Sanjurjo, M.J. Application of aluminium toxicity indices to soils under various forest species. For. Ecol. Manag. 2005, 211, 227–239. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Impact of mulches on landscape plants and environment—A review. J. Environ. Hortic. 2007, 25, 239–249. [Google Scholar]
- Guo, Z.W.; Wang, W.Y.; Yang, Q.P.; Li, Y.C.; Chen, S.L. Effects of mulching management on stoichiometry of soil C, N and P in Phyllostachys praecox plantations. Guihaia 2013, 33, 627–632. [Google Scholar]
- Guan, F.; Xia, M.; Tang, X.; Fan, S. Spatial variability of soil nitrogen, phosphorus and potassium contents in Moso bamboo forests in Yong’an City, China. Catena 2017, 150, 161–172. [Google Scholar] [CrossRef]
- Jiang, P.K.; Xu, Q.F.; Qian, X.B.; Zhang, R.H.; Yang, F.F. Dynamic changes of chemical properties of warmer soil covered with different organic materials in Phyllostachys praecox forests. J. Zhejiang For. Coll. 1999, 16, 123–130. [Google Scholar]
- Guo, Z.W.; Chen, S.L.; Yang, Q.P.; Li, Y.C. Responses of N and P stoichiometry on mulching management in the stand of Phyllostachys praecox. Acta Ecol. Sin. 2012, 32, 6361–6368. [Google Scholar]
- Zhao, J.; Shen, X.; Li, X.; Hu, J.J.; Wang, H.Q.; Chen, L.B. Correlation between soil pH and the contents of available nutrients in selected soils from three pear orchards in Wendeng. North. Hourtic. 2009, 33, 5–8. [Google Scholar]
- Qian, X.; Gu, J.; Pan, H.; Zhang, K.; Sun, W.; Wang, X. Effects of living mulches on the soil nutrient contents, enzyme activities, and bacterial community diversities of apple orchard soils. Eur. J. Soil Biol. 2015, 70, 23–30. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Guo, Z.W.; Yu, W.X.; Chen, S.L.; Li, Y.C.; Yang, Q.P. Influence of mulching management on soil microbe and its relationship with soil nutrient in Phyllostachys praecox stand. Acta Ecol. Sin. 2013, 33, 5623–5630. [Google Scholar]
- Wardle, D.A.; Yeates, G.W.; Bonner, K.I.; Nicholson, K.S.; Watson, R.N. Impacts of ground vegetation management strategies in a kiwifruit orchard on the composition and functioning of the soil biota. Soil Biol. Biochem. 2001, 33, 893–905. [Google Scholar] [CrossRef]
- Unger, I.M.; Kennedy, A.C.; Muzika, R.M. Flooding effects on soil microbial communities. Appl. Soil Ecol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- Smith, R.S.; Shiel, R.S.; Bardgett, R.D.; Millward, D.; Corkhill, P.; Evans, P.; Quirk, H.; Hobbs, P.J.; Kometa, S.T. Long-term change in vegetation and soil microbial communities during the phased restoration of traditional meadow grassland. J. Appl. Ecol. 2008, 45, 670–679. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Shen, J.; Luo, Y.; Scheu, S.; Ke, X. Tillage residue burning and crop rotation alter soil fungal community and water-stable aggregation in arable fields. Soil Tillage Res. 2010, 107, 71–79. [Google Scholar] [CrossRef]


| Treatment | Density (individual·ha−2) | Mean DBH (cm) | Mean Height (m) | Age Structure (I:II:III) |
|---|---|---|---|---|
| NM | 2542 ± 81 b | 10.14 ± 0.52 b | 14.45 ± 1.78 c | 0.42:0.31:0.27 |
| M1 | 2509 ± 84 b | 10.31 ± 0.68 a | 15.22 ± 1.89 a | 0.39:0.32:0.29 |
| M2 | 2580 ± 79 a | 9.93 ± 0.76 b | 14.89 ± 1.52 b | 0.38:0.31:0.31 |
| M3 | 2587 ± 93 a | 10.42 ± 0.65 a | 15.08 ± 1.94 b | 0.41:0.29:0.30 |
| Treatment | pH | SOC (g·kg−1) | TN (g·kg−1) | TP (g·kg−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) |
|---|---|---|---|---|---|---|---|
| NM | 5.83 ± 0.21 a | 13.50 ± 0.92 c | 1.36 ± 0.13 d | 1.31 ± 0.14 c | 76.56 ± 3.24 b | 29.45 ± 1.33 b | 59.27 ± 3.51 b |
| M1 | 5.72 ± 0.22 a | 15.21 ± 1.21 c | 1.52 ± 0.28 c | 1.37 ± 0.25 c | 91.07 ± 4.81 a | 40.18 ± 1.53 a | 75.32 ± 4.24 a |
| M2 | 5.27 ± 0.15 b | 21.08 ± 1.39 b | 2.02 ± 0.37 b | 1.54 ± 0.31 b | 79.06 ± 3.83 b | 28.79 ± 1.47 b | 57.39 ± 3.84 b |
| M3 | 5.03 ± 0.11 c | 27.56 ± 1.57 a | 2.31 ± 0.33 a | 1.64 ± 0.34 a | 65.98 ± 3.43 c | 21.32 ± 1.29 c | 38.45 ± 2.25 c |
| Treatment | Invertase Activity (mg·g−1) | Urease Activity (mg·g−1) | Acid Phosphatase Activity (mg·g−1) |
|---|---|---|---|
| NM | 13.1 ± 0.95 b | 0.81 ± 0.05 b | 1.77 ± 0.11 b |
| M1 | 14.8 ± 1.03 a | 0.86 ± 0.04 a | 1.95 ± 0.15 a |
| M2 | 13.6 ± 0.84 b | 0.79 ± 0.03 b | 1.81 ± 0.12 b |
| M3 | 10.5 ± 0.82 c | 0.65 ± 0.03 c | 1.40 ± 0.10 c |
| Treatment | Total PLFAs (nmol·g−1) | Total Bacteria (nmol·g−1) | Aerobic Bacteria (nmol·g−1) | Anaerobic Bacteria (nmol·g−1) | Total Fungi (nmol·g−1) | Total Actinomycetes (nmol·g−1) |
|---|---|---|---|---|---|---|
| NM | 39.64 ± 2.13 b | 21.34 ± 1.05 b | 2.68 ± 0.13 c | 2.44 ± 0.15 d | 10.64 ± 0.79 c | 7.66 ± 0.55 b |
| M1 | 54.62 ± 2.47 a | 28.28 ± 1.24 a | 3.59 ± 0.23 a | 3.17 ± 0.22 c | 13.23 ± 0.93 b | 13.11 ± 0.93 a |
| M2 | 50.05 ± 2.51 a | 26.51 ± 1.52 a | 3.22 ± 0.21 b | 3.84 ± 0.31 b | 15.52 ± 1.21 a | 8.02 ± 0.62 b |
| M3 | 41.62 ± 1.97 b | 19.37 ± 1.13 b | 2.46 ± 0.16 c | 4.08 ± 0.28 a | 16.74 ± 0.98 a | 5.51 ± 0.47 c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, B.; Li, Q.; Yang, H.; Xu, K. Analysis of Soil Degradation Causes in Phyllostachys edulis Forests with Different Mulching Years. Forests 2018, 9, 149. https://doi.org/10.3390/f9030149
Zhao J, Wang B, Li Q, Yang H, Xu K. Analysis of Soil Degradation Causes in Phyllostachys edulis Forests with Different Mulching Years. Forests. 2018; 9(3):149. https://doi.org/10.3390/f9030149
Chicago/Turabian StyleZhao, Jiancheng, Bo Wang, Qin Li, Hejun Yang, and Kang Xu. 2018. "Analysis of Soil Degradation Causes in Phyllostachys edulis Forests with Different Mulching Years" Forests 9, no. 3: 149. https://doi.org/10.3390/f9030149





