Human Cytomegalovirus Nuclear Capsids Associate with the Core Nuclear Egress Complex and the Viral Protein Kinase pUL97
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and HCMV Infection
2.2. Antibodies
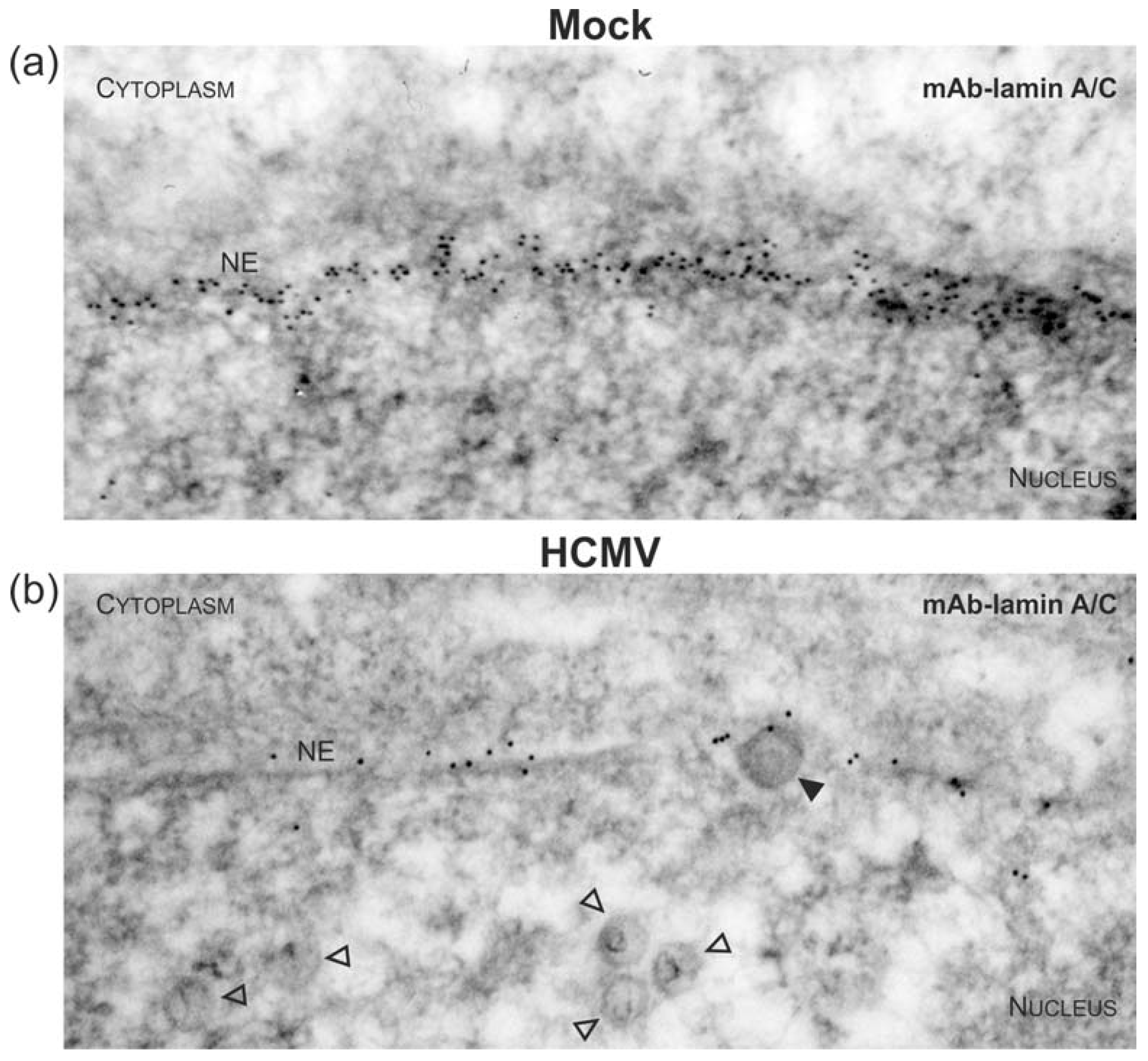
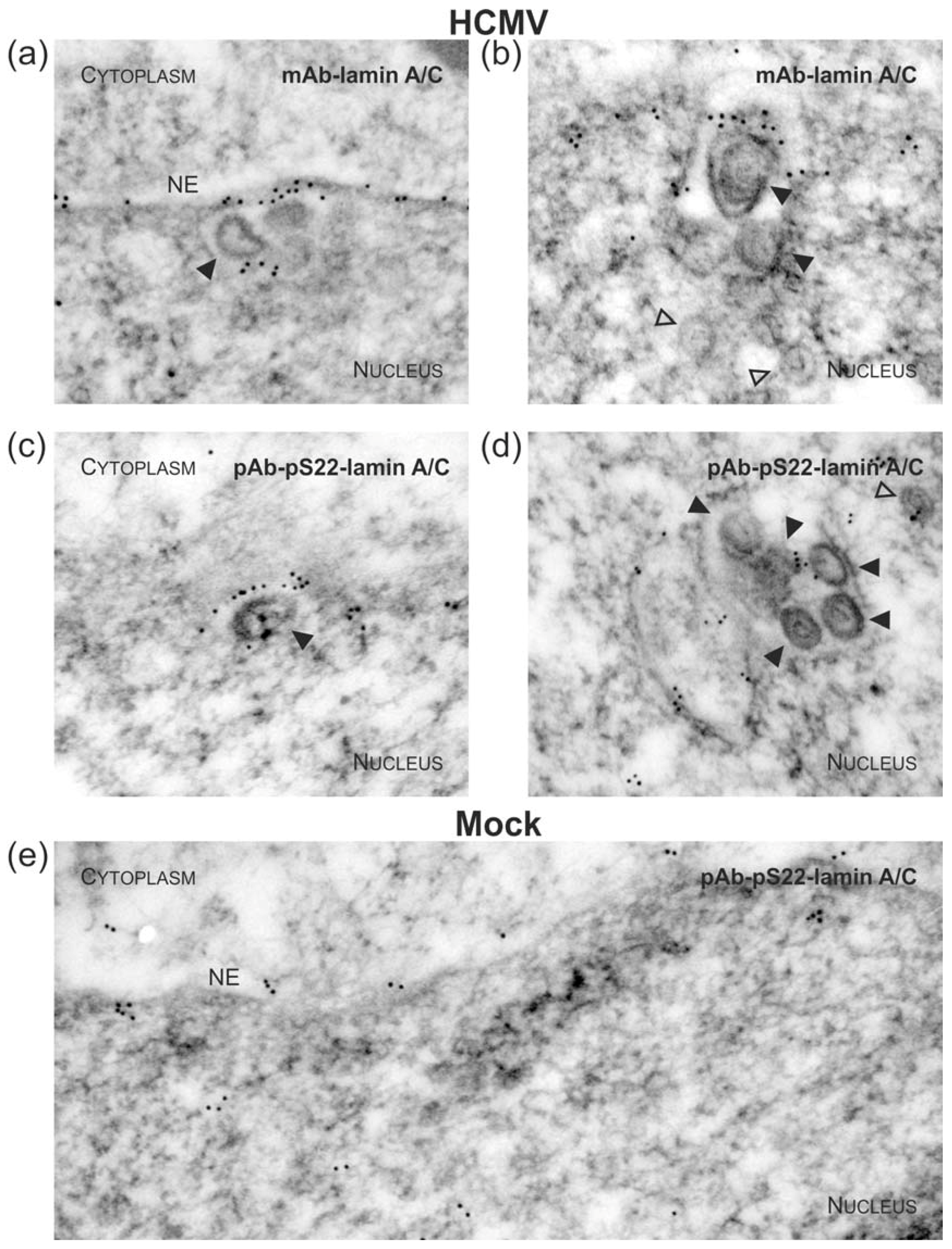
2.3. Immunogold Labelling and Transmission Electron Microscopy (TEM)
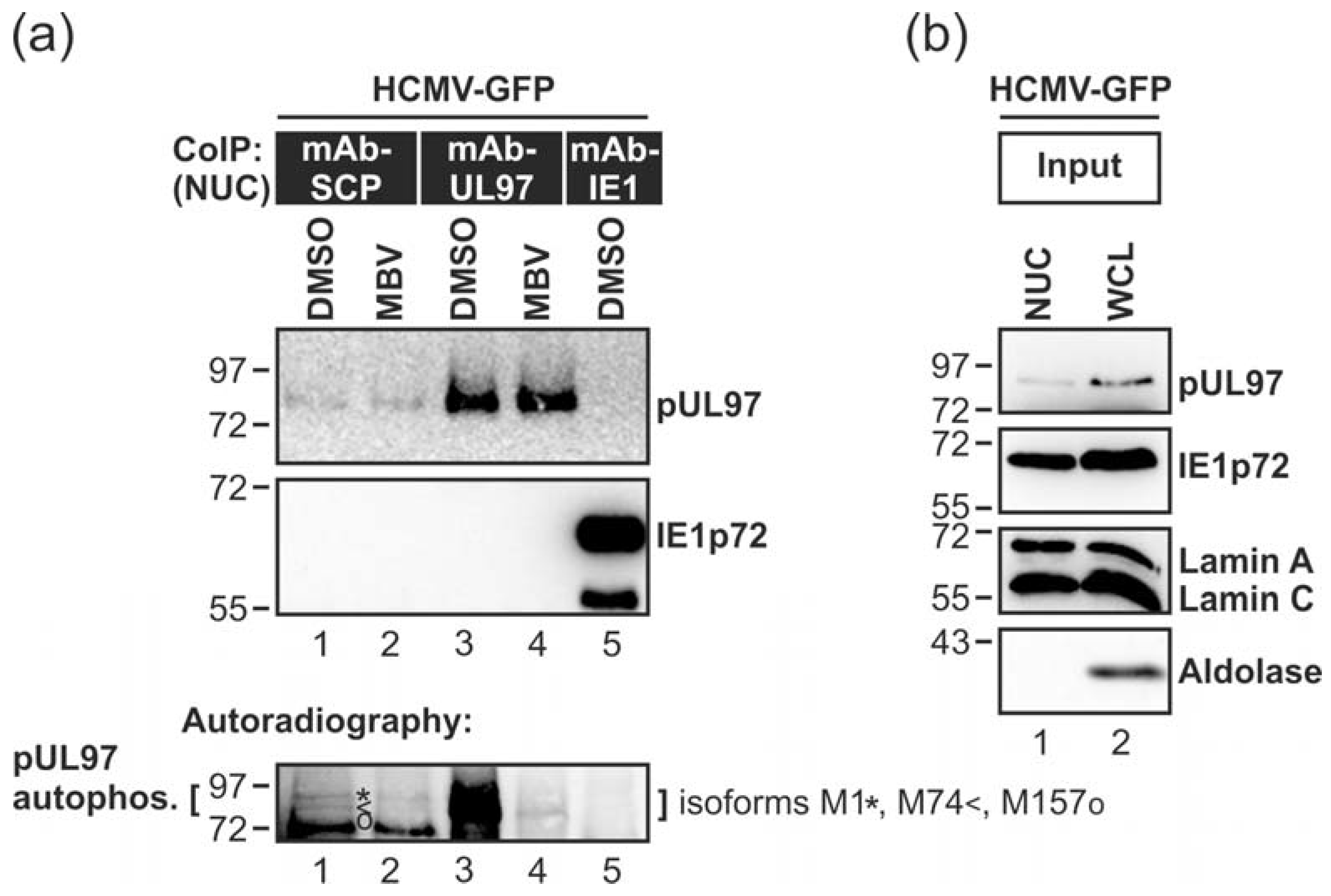
2.4. Co-immunoprecipitation (CoIP) Assay
2.5. In Vitro Kinase Assay (IVKA)
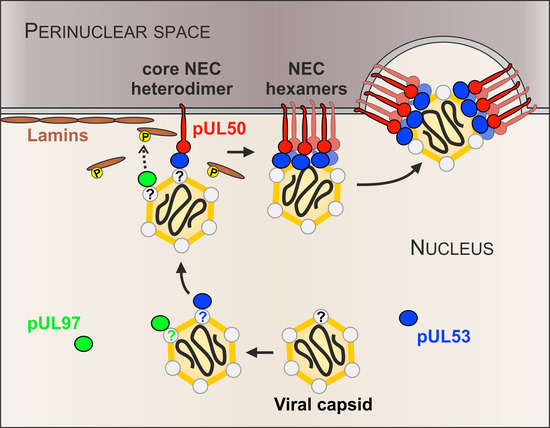
3. Results
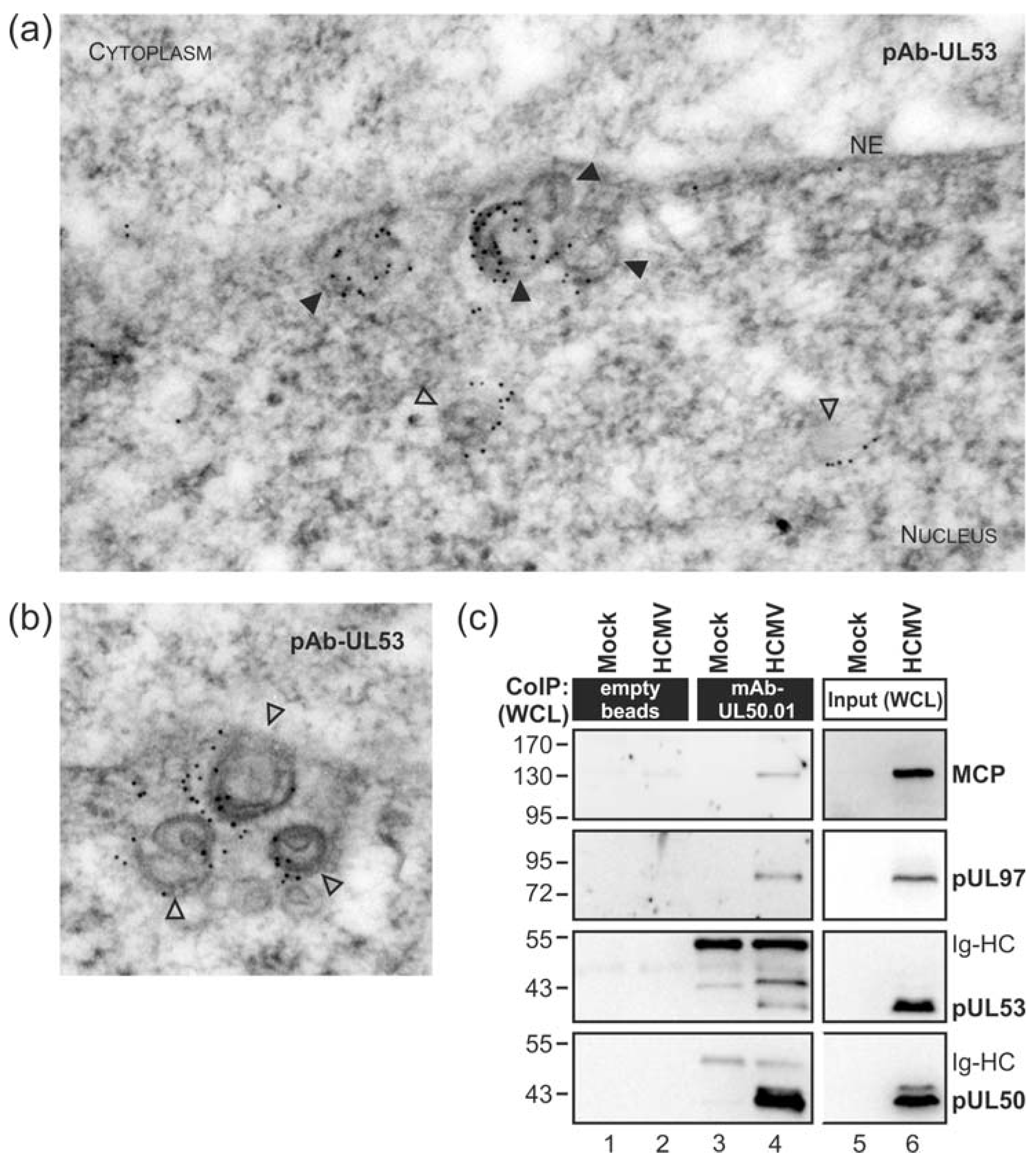
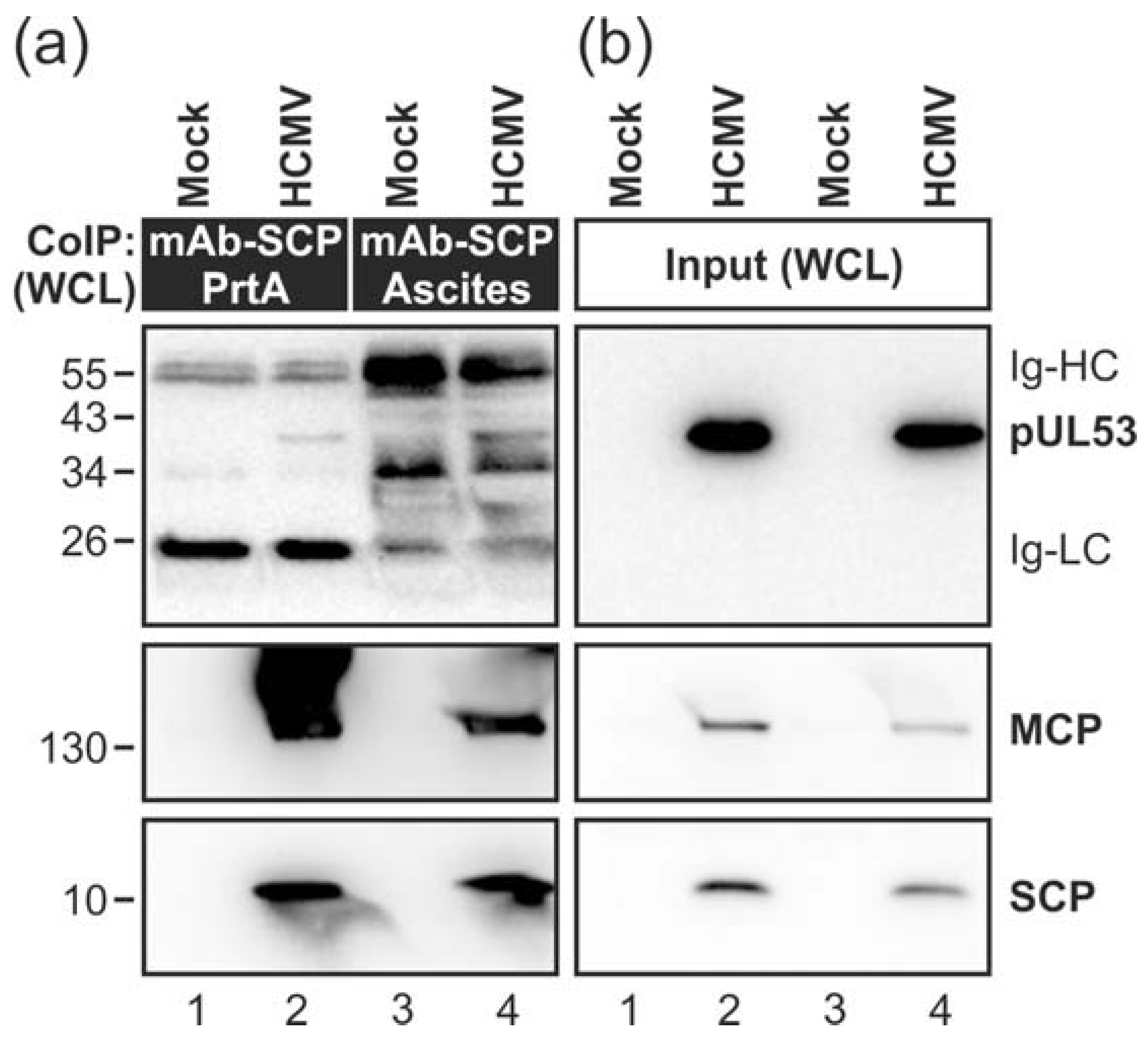
3.1. Association of HCMV Capsids with the Core NEC Constituent pUL53 Visualized by Immuno-Gold Electron Microscopy
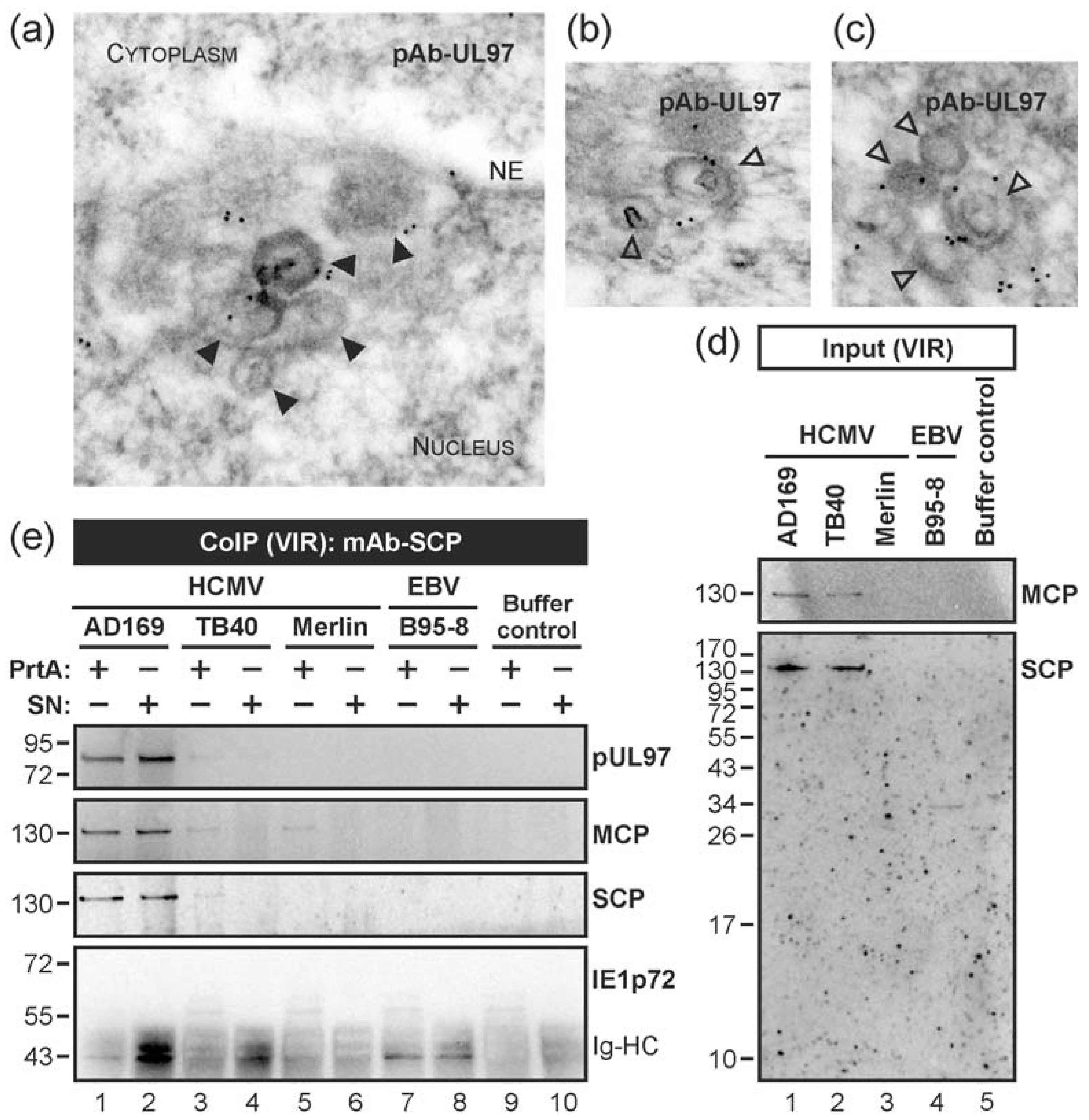
3.2. HCMV Kinase pUL97 Associates with Viral Capsids Already in the nucleus of Infected Cells
3.3. Targeted Mode of Lamina Disassembly by Capsid-Associated pUL97
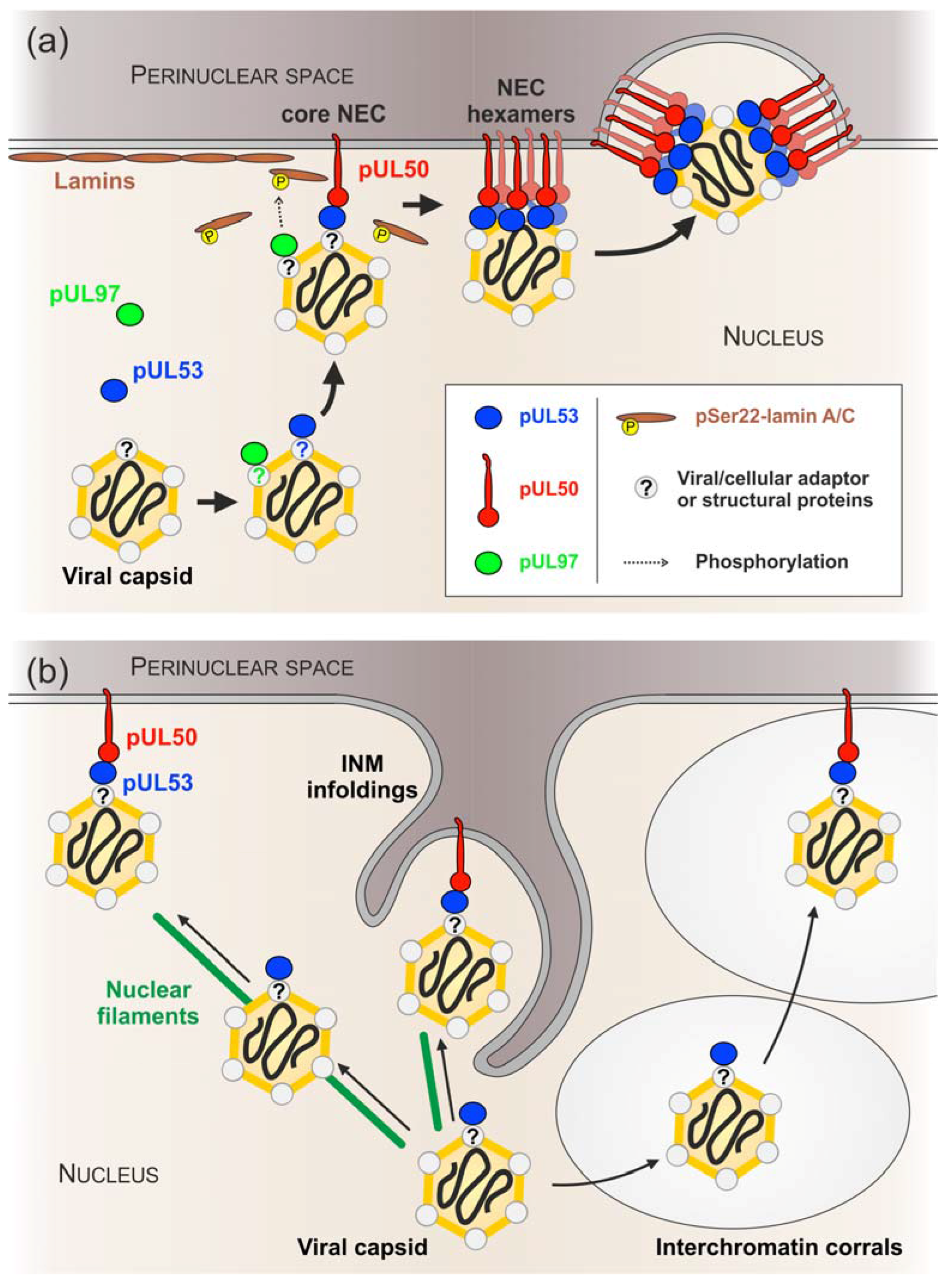
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mocarski, E.S.; Shenk, T.; Griffiths, P.D.; Pass, R.F. Cytomegaloviruses. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Wolters Kluwer Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1960–2014. [Google Scholar]
- Griffiths, P.; Baraniak, I.; Reeves, M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Milbradt, J.; Kraut, A.; Hutterer, C.; Sonntag, E.; Schmeiser, C.; Ferro, M.; Wagner, S.; Lenac, T.; Claus, C.; Pinkert, S.; et al. Proteomic analysis of the multimeric nuclear egress complex of human cytomegalovirus. Mol. Cell. Proteom. 2014, 13, 2132–2146. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Coen, D.M. Comparison of effects of inhibitors of viral and cellular protein kinases on human cytomegalovirus disruption of nuclear lamina and nuclear egress. J. Virol. 2014, 88, 10982–10985. [Google Scholar] [CrossRef] [PubMed]
- Lemnitzer, F.; Raschbichler, V.; Kolodziejczak, D.; Israel, L.; Imhof, A.; Bailer, S.M.; Koszinowski, U.; Ruzsics, Z. Mouse cytomegalovirus egress protein pM50 interacts with cellular endophilin-a2. Cell. Microbiol. 2013, 15, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Steingruber, M.; Kraut, A.; Socher, E.; Sticht, H.; Reichel, A.; Stamminger, T.; Amin, B.; Coute, Y.; Hutterer, C.; Marschall, M. Proteomic interaction patterns between human cyclins, the cyclin-dependent kinase ortholog pUL97 and additional cytomegalovirus proteins. Viruses 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.; Dent, K.C.; Zeev-Ben-Mordehai, T.; Grange, M.; Bosse, J.B.; Whittle, C.; Klupp, B.G.; Siebert, C.A.; Vasishtan, D.; Bauerlein, F.J.; et al. Structural basis of vesicle formation at the inner nuclear membrane. Cell 2015, 163, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Hennig, T.; O’Hare, P. Viruses and the nuclear envelope. Curr. Opin. Cell Biol. 2015, 34, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Baines, J.D. Herpesviruses remodel host membranes for virus egress. Nat. Rev. Microbiol. 2011, 9, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Chen, M.R. Escape of herpesviruses from the nucleus. Rev. Med. Virol. 2010, 20, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C. Vesicular nucleo-cytoplasmic transport-herpesviruses as pioneers in cell biology. Viruses 2016, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Lye, M.F.; Wilkie, A.R.; Filman, D.J.; Hogle, J.M.; Coen, D.M. Getting to and through the inner nuclear membrane during herpesvirus nuclear egress. Curr. Opin. Cell Biol. 2017, 46, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, T.; Passvogel, L.; Schulz, K.S.; Klupp, B.G.; Mettenleiter, T.C. Nuclear egress of herpesviruses: The prototypic vesicular nucleocytoplasmic transport. Adv. Virus Res. 2016, 94, 81–140. [Google Scholar] [PubMed]
- Bigalke, J.M.; Heldwein, E.E. Nuclear exodus: Herpesviruses lead the way. Annu. Rev. Virol. 2016, 3, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Marschall, M.; Muller, Y.A.; Diewald, B.; Sticht, H.; Milbradt, J. The human cytomegalovirus nuclear egress complex unites multiple functions: Recruitment of effectors, nuclear envelope rearrangement, and docking to nuclear capsids. Rev. Med. Virol. 2017, 27. [Google Scholar] [CrossRef] [PubMed]
- Bigalke, J.M.; Heuser, T.; Nicastro, D.; Heldwein, E.E. Membrane deformation and scission by the hsv-1 nuclear egress complex. Nat. Commun. 2014, 5, 4131. [Google Scholar] [CrossRef] [PubMed]
- Bigalke, J.M.; Heldwein, E.E. Have nec coat, will travel: Structural basis of membrane budding during nuclear egress in herpesviruses. Adv. Virus Res. 2017, 97, 107–141. [Google Scholar] [PubMed]
- Marschall, M.; Marzi, A.; aus dem Siepen, P.; Jochmann, R.; Kalmer, M.; Auerochs, S.; Lischka, P.; Leis, M.; Stamminger, T. Cellular p32 recruits cytomegalovirus kinase pul97 to redistribute the nuclear lamina. J. Biol. Chem. 2005, 280, 33357–33367. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, E.; Hamilton, S.T.; Bahsi, H.; Wagner, S.; Jonjic, S.; Rawlinson, W.D.; Marschall, M.; Milbradt, J. Cytomegalovirus pUL50 is the multi-interacting determinant of the core nuclear egress complex (NEC) that recruits cellular accessory nec components. J. Gen. Virol. 2016, 97, 1676–1685. [Google Scholar] [PubMed]
- Sonntag, E.; Milbradt, J.; Svrlanska, A.; Strojan, H.; Hage, S.; Kraut, A.; Hesse, A.M.; Amin, B.; Sonnewald, U.; Coute, Y.; et al. Protein kinases responsible for the phosphorylation of the nuclear egress core complex of human cytomegalovirus. J. Gen. Virol. 2017, 98, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Bowman, B.R.; Baker, M.L.; Rixon, F.J.; Chiu, W.; Quiocho, F.A. Structure of the herpesvirus major capsid protein. EMBO J. 2003, 22, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yu, X.; Gong, H.; Jiang, X.; Abenes, G.; Liu, H.; Shivakoti, S.; Britt, W.J.; Zhu, H.; Liu, F.; et al. The smallest capsid protein mediates binding of the essential tegument protein pp150 to stabilize DNA-containing capsids in human cytomegalovirus. PLoS Pathog. 2013, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jih, J.; Jiang, J.; Zhou, Z.H. Atomic structure of the human cytomegalovirus capsid with its securing tegument layer of pp150. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Britt, W.J. The interaction between the major capsid protein and the smallest capsid protein of human cytomegalovirus is dependent on two linear sequences in the smallest capsid protein. J. Virol. 2003, 77, 2730–2735. [Google Scholar] [CrossRef] [PubMed]
- Heming, J.D.; Conway, J.F.; Homa, F.L. Herpesvirus capsid assembly and DNA packaging. Adv. Anat. Embryol. Cell Biol. 2017, 223, 119–142. [Google Scholar] [PubMed]
- Cardone, G.; Winkler, D.C.; Trus, B.L.; Cheng, N.; Heuser, J.E.; Newcomb, W.W.; Brown, J.C.; Steven, A.C. Visualization of the herpes simplex virus portal in situ by cryo-electron tomography. Virology 2007, 361, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Schmid, M.F.; Rixon, F.J.; Chiu, W. Electron cryotomography reveals the portal in the herpesvirus capsid. J. Virol. 2007, 81, 2065–2068. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, W.W.; Juhas, R.M.; Thomsen, D.R.; Homa, F.L.; Burch, A.D.; Weller, S.K.; Brown, J.C. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 2001, 75, 10923–10932. [Google Scholar] [CrossRef] [PubMed]
- Trus, B.L.; Cheng, N.; Newcomb, W.W.; Homa, F.L.; Brown, J.C.; Steven, A.C. Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1. J. Virol. 2004, 78, 12668–12671. [Google Scholar] [CrossRef] [PubMed]
- Abbotts, A.P.; Preston, V.G.; Hughes, M.; Patel, A.H.; Stow, N.D. Interaction of the herpes simplex virus type 1 packaging protein UL15 with full-length and deleted forms of the UL28 protein. J. Gen. Virol. 2000, 81, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Beard, P.M.; Taus, N.S.; Baines, J.D. DNA cleavage and packaging proteins encoded by genes U(L)28, U(L)15, and U(L)33 of herpes simplex virus type 1 form a complex in infected cells. J. Virol. 2002, 76, 4785–4791. [Google Scholar] [CrossRef] [PubMed]
- Higgs, M.R.; Preston, V.G.; Stow, N.D. The UL15 protein of herpes simplex virus type 1 is necessary for the localization of the UL28 and UL33 proteins to viral DNA replication centres. J. Gen. Virol. 2008, 89, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Koslowski, K.M.; Shaver, P.R.; Casey, J.T., 2nd; Wilson, T.; Yamanaka, G.; Sheaffer, A.K.; Tenney, D.J.; Pederson, N.E. Physical and functional interactions between the herpes simplex virus UL15 and UL28 DNA cleavage and packaging proteins. J. Virol. 1999, 73, 1704–1707. [Google Scholar] [PubMed]
- Reynolds, A.E.; Fan, Y.; Baines, J.D. Characterization of the U(L)33 gene product of herpes simplex virus 1. Virology 2000, 266, 310–318. [Google Scholar] [CrossRef] [PubMed]
- White, C.A.; Stow, N.D.; Patel, A.H.; Hughes, M.; Preston, V.G. Herpes simplex virus type 1 portal protein UL6 interacts with the putative terminase subunits UL15 and UL28. J. Virol. 2003, 77, 6351–6358. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.H.; Roberts, A.P.; McElwee, M.; Bhella, D.; Rixon, F.J.; Lauder, R. The large tegument protein pUL36 is essential for formation of the capsid vertex-specific component at the capsid-tegument interface of herpes simplex virus 1. J. Virol. 2015, 89, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Huet, A.; Makhov, A.M.; Huffman, J.B.; Vos, M.; Homa, F.L.; Conway, J.F. Extensive subunit contacts underpin herpesvirus capsid stability and interior-to-exterior allostery. Nat. Struct. Mol. Biol. 2016, 23, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Mocarski, E.S. Viral and host control of cytomegalovirus maturation. Trends Microbiol. 2012, 20, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Loveland, A.N.; Nguyen, N.L.; Brignole, E.J.; Gibson, W. The amino-conserved domain of human cytomegalovirus UL80a proteins is required for key interactions during early stages of capsid formation and virus production. J. Virol. 2007, 81, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Oien, N.L.; Thomsen, D.R.; Wathen, M.W.; Newcomb, W.W.; Brown, J.C.; Homa, F.L. Assembly of herpes simplex virus capsids using the human cytomegalovirus scaffold protein: Critical role of the c terminus. J. Virol. 1997, 71, 1281–1291. [Google Scholar] [PubMed]
- Nguyen, N.L.; Loveland, A.N.; Gibson, W. Nuclear localization sequences in cytomegalovirus capsid assembly proteins (UL80 proteins) are required for virus production: Inactivating nls1, nls2, or both affects replication to strikingly different extents. J. Virol. 2008, 82, 5381–5389. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.J.; Baxter, M.K.; Plafker, S.M.; Gibson, W. Human cytomegalovirus capsid assembly protein precursor (pUL80.5) interacts with itself and with the major capsid protein (pUL86) through two different domains. J. Virol. 1997, 71, 179–190. [Google Scholar] [PubMed]
- Neuber, S.; Wagner, K.; Goldner, T.; Lischka, P.; Steinbrueck, L.; Messerle, M.; Borst, E.M. Mutual interplay between the human cytomegalovirus terminase subunits pUL51, pUL56, and pUL89 promotes terminase complex formation. J. Virol. 2017, 91, e02384-16. [Google Scholar] [CrossRef] [PubMed]
- Borst, E.M.; Wagner, K.; Binz, A.; Sodeik, B.; Messerle, M. The essential human cytomegalovirus gene UL52 is required for cleavage-packaging of the viral genome. J. Virol. 2008, 82, 2065–2078. [Google Scholar] [CrossRef] [PubMed]
- Koppen-Rung, P.; Dittmer, A.; Bogner, E. Intracellular distribution of capsid-associated pUL77 of human cytomegalovirus and interactions with packaging proteins and pUL93. J. Virol. 2016, 90, 5876–5885. [Google Scholar] [CrossRef] [PubMed]
- Meissner, C.S.; Koppen-Rung, P.; Dittmer, A.; Lapp, S.; Bogner, E. A “coiled-coil” motif is important for oligomerization and DNA binding properties of human cytomegalovirus protein UL77. PLoS ONE 2011, 6, e25115. [Google Scholar] [CrossRef] [PubMed]
- Holzenburg, A.; Dittmer, A.; Bogner, E. Assembly of monomeric human cytomegalovirus pul104 into portal structures. J. Gen. Virol. 2009, 90, 2381–2385. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, E.; Luftig, M.; Chase, M.R.; Weicksel, S.; Cahir-McFarland, E.; Illanes, D.; Sarracino, D.; Kieff, E. Proteins of purified epstein-barr virus. Proc. Natl. Acad. Sci. USA 2004, 101, 16286–16291. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.H.; Wu, M.C.; Wu, C.C.; Chen, Y.C.; Lin, S.F.; Hsu, J.T.; Yang, C.S.; Tsai, C.H.; Takada, K.; Chen, M.R.; et al. Epstein-barr virus BALF3 has nuclease activity and mediates mature virion production during the lytic cycle. J. Virol. 2014, 88, 4962–4975. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, S.; Feederle, R.; Gartner, K.; Fuchs, W.; Granzow, H.; Delecluse, H.J. An epstein-barr virus mutant produces immunogenic defective particles devoid of viral DNA. J. Virol. 2013, 87, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Liu, P.T.; Kung, H.N.; Su, M.T.; Chua, H.H.; Chang, Y.H.; Chang, C.W.; Tsai, C.H.; Liu, F.T.; Chen, M.R. The escrt machinery is recruited by the viral bfrf1 protein to the nucleus-associated membrane for the maturation of epstein-barr virus. PLoS Pathog. 2012, 8, e1002904. [Google Scholar] [CrossRef] [PubMed]
- Milbradt, J.; Webel, R.; Auerochs, S.; Sticht, H.; Marschall, M. Novel mode of phosphorylation-triggered reorganization of the nuclear lamina during nuclear egress of human cytomegalovirus. J. Biol. Chem. 2010, 285, 13979–13989. [Google Scholar] [CrossRef] [PubMed]
- Buser, C.; Walther, P.; Mertens, T.; Michel, D. Cytomegalovirus primary envelopment occurs at large infoldings of the inner nuclear membrane. J. Virol. 2007, 81, 3042–3048. [Google Scholar] [CrossRef] [PubMed]
- Villinger, C.; Neusser, G.; Kranz, C.; Walther, P.; Mertens, T. 3D analysis of hcmv induced-nuclear membrane structures by fib/sem tomography: Insight into an unprecedented membrane morphology. Viruses 2015, 7, 5686–5704. [Google Scholar] [CrossRef] [PubMed]
- Marschall, M.; Feichtinger, S.; Milbradt, J. Regulatory roles of protein kinases in cytomegalovirus replication. Adv. Virus Res. 2011, 80, 69–101. [Google Scholar] [PubMed]
- Milbradt, J.; Hutterer, C.; Bahsi, H.; Wagner, S.; Sonntag, E.; Horn, A.H.; Kaufer, B.B.; Mori, Y.; Sticht, H.; Fossen, T.; et al. The prolyl isomerase Pin1 promotes the herpesvirus-induced phosphorylation-dependent disassembly of the nuclear lamina required for nucleocytoplasmic egress. PLoS Pathog. 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Marschall, M.; Freitag, M.; Weiler, S.; Sorg, G.; Stamminger, T. Recombinant green fluorescent protein-expressing human cytomegalovirus as a tool for screening antiviral agents. Antimicrob. Agents Chemother. 2000, 44, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Ranade, D.; Koul, S.; Thompson, J.; Prasad, K.B.; Sengupta, K. Chromosomal aneuploidies induced upon lamin B2 depletion are mislocalized in the interphase nucleus. Chromosoma 2017, 126, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Mullers, E.; Silva Cascales, H.; Burdova, K.; Macurek, L.; Lindqvist, A. Residual CDK1/2 activity after DNA damage promotes senescence. Aging Cell 2017, 16, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bristol, J.A.; Iwahori, S.; Hagemeier, S.R.; Meng, Q.; Barlow, E.A.; Fingeroth, J.D.; Tarakanova, V.L.; Kalejta, R.F.; Kenney, S.C. Hsp90 inhibitor 17-dmag decreases expression of conserved herpesvirus protein kinases and reduces virus production in epstein-barr virus-infected cells. J. Virol. 2013, 87, 10126–10138. [Google Scholar] [CrossRef] [PubMed]
- Andreoni, M.; Faircloth, M.; Vugler, L.; Britt, W.J. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 1989, 23, 157–167. [Google Scholar] [CrossRef]
- Plachter, B.; Britt, W.; Vornhagen, R.; Stamminger, T.; Jahn, G. Analysis of proteins encoded by ie regions 1 and 2 of human cytomegalovirus using monoclonal antibodies generated against recombinant antigens. Virology 1993, 193, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Hutterer, C.; Wandinger, S.K.; Wagner, S.; Muller, R.; Stamminger, T.; Zeittrager, I.; Godl, K.; Baumgartner, R.; Strobl, S.; Marschall, M. Profiling of the kinome of cytomegalovirus-infected cells reveals the functional importance of host kinases Aurora A, Abl and Ampk. Antivir. Res. 2013, 99, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Hutterer, C.; Hamilton, S.; Steingruber, M.; Zeittrager, I.; Bahsi, H.; Thuma, N.; Naing, Z.; Orfi, Z.; Orfi, L.; Socher, E.; et al. The chemical class of quinazoline compounds provides a core structure for the design of anticytomegaloviral kinase inhibitors. Antivir. Res. 2016, 134, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Kamil, J.P.; Coen, D.M. Human cytomegalovirus protein kinase UL97 forms a complex with the tegument phosphoprotein pp65. J. Virol. 2007, 81, 10659–10668. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.C.; Krosky, P.M.; Coen, D.M. Relationship between autophosphorylation and phosphorylation of exogenous substrates by the human cytomegalovirus UL97 protein kinase. J. Virol. 2002, 76, 11943–11952. [Google Scholar] [CrossRef] [PubMed]
- Webel, R.; Hakki, M.; Prichard, M.N.; Rawlinson, W.D.; Marschall, M.; Chou, S. Differential properties of cytomegalovirus pUL97 kinase isoforms affect viral replication and maribavir susceptibility. J. Virol. 2014, 88, 4776–4785. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; He, Y.S.; Kim, Y.; Chu, L.; Ohmstede, C.; Biron, K.K.; Coen, D.M. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J. Virol. 1997, 71, 405–411. [Google Scholar] [PubMed]
- Steingruber, M.; Socher, E.; Hutterer, C.; Webel, R.; Bergbrede, T.; Lenac, T.; Sticht, H.; Marschall, M. The interaction between cyclin b1 and cytomegalovirus protein kinase pUL97 is determined by an active kinase domain. Viruses 2015, 7, 4582–4601. [Google Scholar] [CrossRef] [PubMed]
- Dal Monte, P.; Pignatelli, S.; Zini, N.; Maraldi, N.M.; Perret, E.; Prevost, M.C.; Landini, M.P. Analysis of intracellular and intraviral localization of the human cytomegalovirus UL53 protein. J. Gen. Virol. 2002, 83, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Rechter, S.; Konig, T.; Auerochs, S.; Thulke, S.; Walter, H.; Dornenburg, H.; Walter, C.; Marschall, M. Antiviral activity of arthrospira-derived spirulan-like substances. Antivir. Res. 2006, 72, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Konig, P.; Buscher, N.; Steingruber, M.; Socher, E.; Sticht, H.; Tenzer, S.; Plachter, B.; Marschall, M. Dynamic regulatory interaction between cytomegalovirus major tegument protein pp65 and protein kinase pUL97 in intracellular compartments, dense bodies and virions. J. Gen. Virol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gibson, W.; Clopper, K.S.; Britt, W.J.; Baxter, M.K. Human cytomegalovirus (hcmv) smallest capsid protein identified as product of short open reading frame located between hcmv UL48 and UL49. J. Virol. 1996, 70, 5680–5683. [Google Scholar] [PubMed]
- Sanchez, V.; Greis, K.D.; Sztul, E.; Britt, W.J. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: Characterization of a potential site of virus assembly. J. Virol. 2000, 74, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Greis, K.D.; Gibson, W.; Hart, G.W. Site-specific glycosylation of the human cytomegalovirus tegument basic phosphoprotein (UL32) at serine 921 and serine 952. J. Virol. 1994, 68, 8339–8349. [Google Scholar] [PubMed]
- Utz, U.; Britt, W.; Vugler, L.; Mach, M. Identification of a neutralizing epitope on glycoprotein gp58 of human cytomegalovirus. J. Virol. 1989, 63, 1995–2001. [Google Scholar] [PubMed]
- Milbradt, J.; Auerochs, S.; Sticht, H.; Marschall, M. Cytomegaloviral proteins that associate with the nuclear lamina: Components of a postulated nuclear egress complex. J. Gen. Virol. 2009, 90, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Milbradt, J.; Auerochs, S.; Marschall, M. Cytomegaloviral proteins pUL50 and pUL53 are associated with the nuclear lamina and interact with cellular protein kinase c. J. Gen. Virol. 2007, 88, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.; Ott, M.; Raschbichler, V.; Nagel, C.H.; Binz, A.; Sodeik, B.; Bauerfeind, R.; Bailer, S.M. The herpes simplex virus protein pUL31 escorts nucleocapsids to sites of nuclear egress, a process coordinated by its n-terminal domain. PLoS Pathog. 2015, 11, e1004957. [Google Scholar] [CrossRef] [PubMed]
- Hamirally, S.; Kamil, J.P.; Ndassa-Colday, Y.M.; Lin, A.J.; Jahng, W.J.; Baek, M.C.; Noton, S.; Silva, L.A.; Simpson-Holley, M.; Knipe, D.M.; et al. Viral mimicry of cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 2009, 5, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuny, C.V.; Chinchilla, K.; Culbertson, M.R.; Kalejta, R.F. Cyclin-dependent kinase-like function is shared by the beta- and gamma-subset of the conserved herpesvirus protein kinases. PLoS Pathog. 2010, 6, 1001092. [Google Scholar] [CrossRef] [PubMed]
- Leelawong, M.; Guo, D.; Smith, G.A. A physical link between the pseudorabies virus capsid and the nuclear egress complex. J. Virol. 2011, 85, 11675–11684. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Baines, J.D. Selection of hsv capsids for envelopment involves interaction between capsid surface components pUL31, pUL17, and pUL25. Proc. Natl. Acad. Sci. USA 2011, 108, 14276–14281. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wills, E.; Lim, H.Y.; Zhou, Z.H.; Baines, J.D. Association of herpes simplex virus pUL31 with capsid vertices and components of the capsid vertex-specific complex. J. Virol. 2014, 88, 3815–3825. [Google Scholar] [CrossRef] [PubMed]
- Sam, M.D.; Evans, B.T.; Coen, D.M.; Hogle, J.M. Biochemical, biophysical, and mutational analyses of subunit interactions of the human cytomegalovirus nuclear egress complex. J. Virol. 2009, 83, 2996–3006. [Google Scholar] [CrossRef] [PubMed]
- Schmeiser, C.; Borst, E.; Sticht, H.; Marschall, M.; Milbradt, J. The cytomegalovirus egress proteins pUL50 and pUL53 are translocated to the nuclear envelope through two distinct modes of nuclear import. J. Gen. Virol. 2013, 94, 2056–2069. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kamil, J.P.; Coughlin, M.; Reim, N.I.; Coen, D.M. Human cytomegalovirus UL50 and UL53 recruit viral protein kinase UL97, not protein kinase c, for disruption of nuclear lamina and nuclear egress in infected cells. J. Virol. 2014, 88, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Walzer, S.A.; Egerer-Sieber, C.; Sticht, H.; Sevvana, M.; Hohl, K.; Milbradt, J.; Muller, Y.A.; Marschall, M. Crystal structure of the human cytomegalovirus pUL50-pUL53 core nuclear egress complex provides insight into a unique assembly scaffold for virus-host protein interactions. J. Biol. Chem. 2015, 290, 27452–27458. [Google Scholar] [CrossRef] [PubMed]
- Lye, M.F.; Sharma, M.; El Omari, K.; Filman, D.J.; Schuermann, J.P.; Hogle, J.M.; Coen, D.M. Unexpected features and mechanism of heterodimer formation of a herpesvirus nuclear egress complex. EMBO J. 2015, 34, 2937–2952. [Google Scholar] [CrossRef] [PubMed]
- Bigalke, J.M.; Heldwein, E.E. Structural basis of membrane budding by the nuclear egress complex of herpesviruses. EMBO J. 2015, 34, 2921–2936. [Google Scholar] [CrossRef] [PubMed]
- Zeev-Ben-Mordehai, T.; Weberruss, M.; Lorenz, M.; Cheleski, J.; Hellberg, T.; Whittle, C.; El Omari, K.; Vasishtan, D.; Dent, K.C.; Harlos, K.; et al. Crystal structure of the herpesvirus nuclear egress complex provides insights into inner nuclear membrane remodeling. Cell. Rep. 2015, 13, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Klupp, B.G.; Granzow, H.; Keil, G.M.; Mettenleiter, T.C. The capsid-associated UL25 protein of the alphaherpesvirus pseudorabies virus is nonessential for cleavage and encapsidation of genomic DNA but is required for nuclear egress of capsids. J. Virol. 2016, 80, 6235–6246. [Google Scholar] [CrossRef] [PubMed]
- Borst, E.M.; Bauerfeind, R.; Binz, A.; Stephan, T.M.; Neuber, S.; Wagner, K.; Steinbruck, L.; Sodeik, B.; Lenac Rovis, T.; Jonjic, S.; et al. The essential human cytomegalovirus proteins pUL77 and pUL93 are structural components necessary for viral genome encapsidation. J. Virol. 2016, 90, 5860–5875. [Google Scholar] [CrossRef] [PubMed]
- DeRussy, B.M.; Boland, M.T.; Tandon, R. Human cytomegalovirus pUL93 links nucleocapsid maturation and nuclear egress. J. Virol. 2016, 90, 7109–7117. [Google Scholar] [CrossRef] [PubMed]
- Bosse, J.B.; Enquist, L.W. The diffusive way out: Herpesviruses remodel the host nucleus, enabling capsids to access the inner nuclear membrane. Nucleus 2016, 7, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Roller, R.J.; Baines, J.D. Herpesvirus nuclear egress. Adv. Anat. Embryol. Cell Biol. 2017, 223, 143–169. [Google Scholar] [PubMed]
- Sere, K.M.; Janssen, M.P.; Willems, G.M.; Tans, G.; Rosing, J.; Hackeng, T.M. Purified protein s contains multimeric forms with increased apc-independent anticoagulant activity. Biochemistry 2001, 40, 8852–8860. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.F.; Adie, K.; Chaubert, P.; Bird, A.P. Engineering a high-affinity methyl-cpg-binding protein. Nucleic Acids Res. 2006, 34, e96. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Goldstein, B.; Gnanakaran, S. Quantifying intramolecular binding in multivalent interactions: A structure-based synergistic study on grb2-sos1 complex. PLoS Comput. Biol. 2011, 7, e1002192. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.Y.; Shaw, A.S.; Chan, A.C. Analysis of the interaction of zap-70 and syk protein-tyrosine kinases with the t-cell antigen receptor by plasmon resonance. Proc. Natl. Acad. Sci. USA 1995, 92, 5106–5110. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.; Poirson, J.; Foltz, C.; Chebaro, Y.; Schrapp, M.; Meyer, A.; Bonetta, A.; Forster, A.; Jacob, Y.; Masson, M.; et al. Targeting the two oncogenic functional sites of the hpv e6 oncoprotein with a high-affinity bivalent ligand. Angew. Chem. Int. Ed. Engl. 2015, 54, 7958–7962. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K. Assessment of weak sugar-binding ability using lectin tetramer and membrane-based glycans. Methods Mol. Biol. 2014, 1200, 413–418. [Google Scholar] [PubMed]
- Lindborg, M.; Dubnovitsky, A.; Olesen, K.; Bjorkman, T.; Abrahmsen, L.; Feldwisch, J.; Hard, T. High-affinity binding to staphylococcal protein a by an engineered dimeric affibody molecule. Protein Eng. Des. Select. 2013, 26, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lu, Z.; Raso, S.W.; Entrican, C.; Tangarone, B. Dimers and multimers of monoclonal igg1 exhibit higher in vitro binding affinities to fcgamma receptors. mAbs 2009, 1, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; von Wronski, M.A.; Sato, A.K.; Dransfield, D.T.; Sexton, D.; Bogdan, N.; Pillai, R.; Nanjappan, P.; Song, B.; Marinelli, E.; et al. A distinct strategy to generate high-affinity peptide binders to receptor tyrosine kinases. Protein Eng. Des. Select. 2005, 18, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Merritt, E.A.; Zhang, Z.; Pickens, J.C.; Ahn, M.; Hol, W.G.; Fan, E. Characterization and crystal structure of a high-affinity pentavalent receptor-binding inhibitor for cholera toxin and e. Coli heat-labile enterotoxin. J. Am. Chem. Soc. 2002, 124, 8818–8824. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.K.; Zhang, Z.S.; Minke, W.E.; Hou, Z.; Verlinde, C.; Hol, W.G.J. High-affinity pentavalent ligands of escherichia coli heat-labile enterotoxin by modular structure-based design. J. Am. Chem. Soc. 2000, 122, 2663–2664. [Google Scholar] [CrossRef]
- Kochin, V.; Shimi, T.; Torvaldson, E.; Adam, S.A.; Goldman, A.; Pack, C.G.; Melo-Cardenas, J.; Imanishi, S.Y.; Goldman, R.D.; Eriksson, J.E. Interphase phosphorylation of lamin A. J. Cell Sci. 2014, 127 Pt 12, 2683–2696. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev. Med. Virol. 2009, 19, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Kalejta, R.F. Tegument proteins of human cytomegalovirus. Microbiol. Mol. Biol. Rev. MMBR 2008, 72, 249–265. [Google Scholar] [CrossRef] [PubMed]
| Type of Protein | Alpha-Herpesvirus a HSV-1 | Beta-Herpesvirus b HCMV | Gamma-Herpesvirus c EBV |
|---|---|---|---|
| Capsid portal | pUL6 | pUL104 | BBRF1 |
| Capsid scaffolding | pUL26.5 | pUL80.5/pUL80a | BdRF1 |
| CVSC | pUL17 | pUL93 | BGLF1 |
| pUL25 | pUL77 | BVRF1 | |
| pUL36 | nd | nd | |
| Terminase complex | pUL15 | pUL89 | BGRF1/BDRF1 |
| pUL28 | pUL56 | BALF3 | |
| pUL33 | pUL51 | BFRF1A | |
| Cleavage-packaging | pUL32 d | pUL52 d | BFLF1 d |
| NEC core protein | pUL31 | pUL53 e | nd |
| Protein kinase | nd | pUL97 e | nd |
| Designation | Type of Antibody * | Type of Antigen | Demonstrated Reactivity ** | References |
|---|---|---|---|---|
| mAb-lamin A/C, (EPR4100) | mAb, rabbit | synthetic peptide, aa 500–600 | WB, IF, IEM | [3,56,58] |
| pAb-pS22-lamin A/C (#2026) | pAb, rabbit | synthetic phosphopeptide, pS22 | WB, IF, IEM | [3,59,60] |
| mAb-IE1p72 (P63-27) | mAb, mouse IgG | purified infected cell nuclei | WB, IF, IP | [61,62,63,64] |
| pAb-UL97 (Boston) | pAb, rabbit | baculovirus-expressed pUL97, full-length 1-707 | WB, IF, IEM, IP, MS, IV | [6,65,66,67,68] |
| mAb-UL97 (97.01) | mAb, mouse kappa IgG1 | baculovirus-expressed MBP-UL97, full-length 1-707 | WB, IF, IP, MS, IV, EL | [6,63,69] |
| pAb-UL53 (Bologna) | pAb, mouse | bacterially expressed β-gal-UL53, full-length 1-292 | WB, IF, IEM, IP, EL | [19,20,70] |
| mAb-UL53 (53.01) | mAb, mouse kappa IgG1 | bacterially expressed pUL53, fragment 50-292 | WB, IF, IP, EL | [3,19,20] |
| mAb-UL50 (50.01) | mAb, mouse kappa IgG1 | bacterially expressed pUL50, fragment 1-181 | WB, IF, IP, EL | [3,19,20] |
| mAb-MCP (28-4) | mAb, mouse | gradient-purified virions | WB, IF, IP, CA, EL | [22,24,71,72] |
| mAb-SCP (11-2-23) | mAb, mouse | gradient-purified virions | WB, IF, IP, IV, CA, EL | [22,73] |
| mAb-pp150 (36-14/XPA) | mAb, mouse | bacterially expressed pp150/pUL32 | WB, IF, IP, CA, EL | [74,75] |
| mAb-gB (27-287) | mAb, mouse | bacterially expressed gp58/gB | WB, IF, IP, EL | [63,76] |
| First Binding Partner | Second Binding Partner | Type of Complex Formed | Gain in Affinity | References |
|---|---|---|---|---|
| S protein | Phospholipid bilayer | Multimeric protein S | >250 fold | [97] |
| MBD protein | DNA CpG sites | Tetrameric MBD | >50 fold | [98] |
| Grb SH3 domain | Proline-rich motif of SOS | Two SH3 domains and five proline-rich motifs | >100 fold | [99] |
| ZAP70 SH2 domain | ITAM motifs of Syk kinase | Two SH2 domains and two ITAM motifs | >100 fold | [100] |
| HPV E6 protein | Peptidic ligand | Bivalent peptidic ligand | >300 fold | [101] |
| Lectins | Sugar ligands | Multimeric lectins and ligands | 1–3 orders of magnitude | [102] |
| Staphylococcal protein A | Affibody | Dimeric affibody | 3 orders of magnitude | [103] |
| Monoclonal IgG I | Fcγ receptors RIIA and RIIIB | Dimeric IgG I | 200–800 fold | [104] |
| Receptor tyrosine kinase VEGFR-2 | Peptidic ligand | Dimeric peptidic ligand | 6–500 fold | [105] |
| E. coli heat-labile enterotoxin (pentamer) | Galactose (monomer) | Five galactose moieties linked by a pentavalent scaffold | >104 fold | [106,107] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milbradt, J.; Sonntag, E.; Wagner, S.; Strojan, H.; Wangen, C.; Lenac Rovis, T.; Lisnic, B.; Jonjic, S.; Sticht, H.; Britt, W.J.; et al. Human Cytomegalovirus Nuclear Capsids Associate with the Core Nuclear Egress Complex and the Viral Protein Kinase pUL97. Viruses 2018, 10, 35. https://doi.org/10.3390/v10010035
Milbradt J, Sonntag E, Wagner S, Strojan H, Wangen C, Lenac Rovis T, Lisnic B, Jonjic S, Sticht H, Britt WJ, et al. Human Cytomegalovirus Nuclear Capsids Associate with the Core Nuclear Egress Complex and the Viral Protein Kinase pUL97. Viruses. 2018; 10(1):35. https://doi.org/10.3390/v10010035
Chicago/Turabian StyleMilbradt, Jens, Eric Sonntag, Sabrina Wagner, Hanife Strojan, Christina Wangen, Tihana Lenac Rovis, Berislav Lisnic, Stipan Jonjic, Heinrich Sticht, William J. Britt, and et al. 2018. "Human Cytomegalovirus Nuclear Capsids Associate with the Core Nuclear Egress Complex and the Viral Protein Kinase pUL97" Viruses 10, no. 1: 35. https://doi.org/10.3390/v10010035