Viroid Diseases in Pome and Stone Fruit Trees and Koch’s Postulates: A Critical Assessment
Abstract
:1. Introduction
2. Viroid Pathogenicity Requires Fulfilment of Koch’s Postulates
3. Viroid Diseases Caused by Apple Scar Skin Viroid (ASSVd)
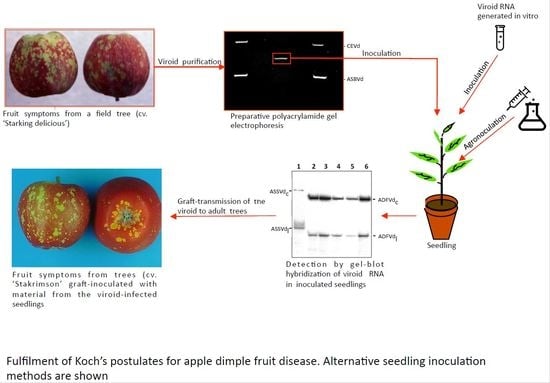
4. Viroid Disease Caused by Apple Dimple Fruit Viroid (ADFVd)
5. Viroid Diseases Caused by Apple Fruit Crinkle Viroid (AFCVd)
 family Pospiviroidae) [9]. AFCVd has not been yet classified as a novel species since no discriminating biological traits have been reported with respect to AGVd.
family Pospiviroidae) [9]. AFCVd has not been yet classified as a novel species since no discriminating biological traits have been reported with respect to AGVd.6. Viroid Disease Caused by Pear Blister Canker Viroid (PBCVd)
7. Viroid Diseases Associated with Hop Stunt Viroid (HSVd)
8. Viroid Diseases Caused by Peach Latent Mosaic Viroid (PLMVd)
9. Concluding Remarks
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Diener, T.O. Potato spindle tuber “virus”. IV. A replicating, low molecular weight RNA. Virology 1971, 45, 411–428. [Google Scholar] [CrossRef]
- Semancik, J.S.; Weathers, L.G. Exocortis virus: An infectious free-nucleic acid plant virus with unusual properties. Virology 1972, 47, 456–466. [Google Scholar] [CrossRef]
- Ivanowski, D. Concerning the mosaic disease of the tobacco plant. St. Petersburg Acad. Imp. Sci. Bul. 1942, 7, 27–30. [Google Scholar]
- Beijerinck, M.W. Concerning a contagium vivum fluidum as cause of the spot disease of tobacco leaves. Verhandelingen der Koninklijke Akademie Wetenschappen te Amsterdam 1898, 65, 3–21, English edition; Phytopathological Classics. American Phytopathological Society, St. Paul, MN, USA 1942, 7, 33–52. [Google Scholar]
- Flores, R.; Minoia, S.; Carbonell, A.; Gisel, A.; Delgado, S.; López-Carrasco, A.; Navarro, B.; Di Serio, F. Viroids, the simplest RNA replicons: How they manipulate their hosts for being propagated and how their hosts react for containing the infection. Virus Res. 2015, 209, 136–145. [Google Scholar] [CrossRef] [PubMed]
- López-Carrasco, A.; Flores, R. Dissecting the secondary structure of the circular RNA of a nuclear viroid in vivo: A “naked” rod-like conformation similar but not identical to that observed in vitro. RNA Biol. 2017, 14, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- López-Carrasco, A.; Flores, R. The predominant circular form of avocado sunblotch viroid accumulates in planta as a free RNA adopting a rod-shaped secondary structure unprotected by tightly bound host proteins. J. Gen. Virol. 2017, 98, 1913–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores, R.; Hernández, C.; De Alba, A.E.M.; Daròs, J.A.; Di Serio, F. Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 2005, 43, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, F.; Flores, R.; Verhoeven, J.T.J.; Li, S.F.; Pallás, V.; Randles, J.W.; Sano, T.; Vidalakis, G.; Owens, R.A. Current status of viroid taxonomy. Arch. Virol. 2014, 159, 3467–3478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Serio, F.; Li, S.F.; Matoušek, J.; Owens, R.A.; Pallás, V.; Randles, J.W.; Sano, T.; Verhoeven, J.T.J.; Vidalakis, G.; Flores, R.; et al. ICTV virus taxonomy profile: Avsunviroidae. J. Gen. Virol. 2018, 99, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Hammond, R.W. Economic significance of viroids in vegetable and field crops. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 5–13. ISBN 9780128014981. [Google Scholar]
- Hadidi, A.; Vidalakis, G.; Sano, T. Economic significance of fruit tree and grapevine viroids. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 15–25. ISBN 9780128014981. [Google Scholar]
- Verhoeven, J.T.J.; Hammond, R.W.; Stancanelli, G. Economic significance of viroids in ornamental Crops. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 27–38. ISBN 9780128014981. [Google Scholar]
- Rodriguez, M.J.B.; Vadamalai, G.; Randles, J.W. Economic significance of palm tree viroids. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 39–49. ISBN 9780128014981. [Google Scholar]
- Szychowski, J.A.; McKenry, M.V.; Walker, M.A.; Wolpert, J.A.; Credi, R.; Semancik, J.S. The vein-banding disease syndrome: A synergistic reaction between grapevine viroids and fanleaf virus. Vitis 1995, 34, 229–234. [Google Scholar]
- Diener, T.O.; Smith, D.R.; O’Brien, M.J. Potato spindle tuber viroid. VII. Susceptibility of several solanaceous plant species to infection with low molecular-weight RNA. Virology 1972, 48, 844–846. [Google Scholar] [CrossRef]
- Diener, T.O. Potato spindle tuber viroid. VIII. Correlation of infectivity with a UV-absorbing component and thermal denaturation properties of the RNA. Virology 1972, 50, 606–609. [Google Scholar] [CrossRef]
- Semancik, J.S.; Wheathers, L. Properties of the infectious forms of exocortis virus of Citrus. Phytopathology 1970, 60, 732–736. [Google Scholar] [CrossRef]
- Semancik, J.S.; Morris, T.J.; Weathers, L.G. Structure and conformation of low molecular weight pathogenic RNA from exocortis disease. Virology 1973, 53, 448–456. [Google Scholar] [CrossRef]
- Bos, L. Hundred years of Koch’s postulates and the history of etiology in plant virus research. Neth. J. Plant Pathol. 1981, 87, 91–110. [Google Scholar] [CrossRef]
- Hanold, D.; Vadamalai, G. Gel electrophoresis. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 357–367. ISBN 9780128014981. [Google Scholar]
- Schumacher, J.; Randles, J.W.; Riesner, D. A two-dimensional electrophoretic technique for the detection of circular viroids and virusoids. Anal. Biochem. 1983, 135, 288–295. [Google Scholar] [CrossRef]
- Flores, R.; Durán-Vila, N.; Pallás, V.; Semancik, J.S. Detection of viroid and viroid-like RNAs from grapevine. J. Gen. Virol. 1985, 66, 2095–2102. [Google Scholar] [CrossRef]
- Di Serio, F.; Malfitano, M.; Alioto, D.; Ragozzino, A.; Desvignes, J.C.; Flores, R. Apple dimple fruit viroid: Fulfillment of Koch’s postulates and symptom characteristics. Plant Dis. 2001, 85, 179–182. [Google Scholar] [CrossRef]
- Pallás, V.; Navarro, A.; Flores, R. Isolation of a viroid-like RNA from hop different from hop stunt viroid. J. Gen. Virol. 1987, 68, 3201–3205. [Google Scholar] [CrossRef]
- Navarro, B.; Flores, R. Chrysanthemum chlorotic mottle viroid: Unusual structural properties of a subgroup of self-cleaving viroids with hammerhead ribozymes. Proc. Natl. Acad. Sci. USA 1997, 94, 11262–11267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Peña, M.; Navarro, B.; Flores, R. Mapping the molecular determinant of pathogenicity in a hammerhead viroid: A tetraloop within the in vivo branched RNA conformation. Proc. Natl. Acad. Sci. USA 1999, 96, 9960–9965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellamy, A.R.; Ralph, R.K. Recovery and purification of nucleic acids by means of cetyltrimethylammonium bromide. Methods Enzymol. 1968, 12, 156–160. [Google Scholar] [CrossRef]
- Ambrós, S.; Hernández, C.; Desvignes, J.C.; Flores, R. Genomic structure of three phenotypically different isolates of peach latent mosaic viroid: Implications of the existence of constraints limiting the heterogeneity of viroid quasispecies. J. Virol. 1998, 72, 7397–7406. [Google Scholar] [PubMed]
- Codoñer, F.M.; Daròs, J.A.; Solé, R.V.; Elena, S.F. The fittest versus the flattest: Experimental confirmation of the quasispecies effect with subviral pathogens. PLoS Pathog. 2006, 2, e136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durán-Vila, N.; Elena, S.F.; Daròs, J.A.; Flores, R. Structure and Evolution of Viroids. In Origin and Evolution of Viruses, 2nd ed.; Domingo, E., Parrish, C., Holland, J.J., Eds.; Academic Press: London, UK, 2008; pp. 43–65. ISBN 9780123741530. [Google Scholar]
- Di Serio, F.; Navarro, B.; Flores, R. Origin and evolution of viroids. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 125–134. ISBN 9780128014981. [Google Scholar]
- Hashimoto, J.; Koganezawa, H. Nucleotide sequence and secondary structure of apple scar skin viroid. Nucleic Acids Res. 1987, 15, 7045–7052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.F.; Hadidi, A.; Yang, X.; Hammond, R.W.; Hansen, A.J. Nucleotide sequence and secondary structure of pome fruit viroids from dapple apple diseased apples, pear rusty skin diseased pears and apple scar skin symptomless pears. Acta Hortic. 1995, 386, 554–559. [Google Scholar] [CrossRef]
- Zhu, S.F.; Hadidi, A.; Hammond, R.W. Agroinfection of pear and apple with dapple apple viroid results in systemic infection. Acta Hortic. 1998, 472, 613–616. [Google Scholar] [CrossRef]
- Osaki, H.; Kudo, A.; Ohtsu, Y. Japanese pear fruit dimple disease caused by apple scar skin viroid (ASSVd). Ann. Phytopathol. Soc. Jpn. 1996, 62, 379–385. [Google Scholar] [CrossRef]
- Ito, T.; Yoshida, K. Reproduction of apple fruit crinkle disease symptoms by apple fruit crinkle viroid. Acta Hortic. 1998, 472, 587–594. [Google Scholar] [CrossRef]
- Hadidi, A.; Yang, X. Detection of pome fruit viroids by enzymatic cDNA amplification. J. Virol. Methods 1990, 30, 261–269. [Google Scholar] [CrossRef]
- Shamloul, A.M.; Yang, X.; Han, L.; Hadidi, A. Characterization of a new variant of Apple scar skin viroid associated with pear fruit crinkle disease. J. Plant Pathol. 2004, 86, 249–256. [Google Scholar]
- Kyriakopoulou, P.E.; Hadidi, A. Natural infection of wild and cultivated pears with apple scar skin viroid in Greece. Acta Hortic. 1998, 472, 617–625. [Google Scholar] [CrossRef]
- Kyriakopoulou, P.E.; Giunchedi, L.; Hadidi, A. Peach latent mosaic and pome fruit viroids in naturally infected cultivated pear Pyrus communis and wild pear P. amygdaliformis: Implications on possible origin of these viroids in the mediterranean region. J. Plant Pathol. 2001, 83, 51–62. [Google Scholar] [CrossRef]
- Ito, T.; Suzaki, K.; Nakahara, K.; Machita, I.; Matsunaka, K.; Yoshida, K. Apple fruit crinkle viroid (AFCVd) causes a graft-transmissible blister bark on apple cv. Nero 26. Ann. Phytopathol. Soc. Jpn. 1999, 65, 233–235. [Google Scholar]
- Ambrós, S.; Desvignes, J.C.; Llácer, G.; Flores, R. Pear blister canker viroid: Sequence variability and causal role in pear blister canker disease. J. Gen. Virol. 1995, 76, 2625–2629. [Google Scholar] [CrossRef] [PubMed]
- Terai, Y. Ocurrence of a new viroid disease, plum dapple fruit. Shokubutu Boeki 1990, 44, 127–129. [Google Scholar]
- Sano, T.; Hataya, T.; Terai, Y.; Shikata, E. Hop stunt viroid strains from dapple fruit disease of plum and peach in Japan. J. Gen. Virol. 1989, 70, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Hernández, C.; Desvignes, J.C.; Llácer, G. Some properties of the viroid inducing peach latent mosaic disease. Res. Virol. 1990, 141, 109–118. [Google Scholar] [CrossRef]
- Malfitano, M.; Di Serio, F.; Covelli, L.; Ragozzino, A.; Hernández, C.; Flores, R. Peach latent mosaic viroid variants inducing peach calico (extreme chlorosis) contain a characteristic insertion that is responsible for this symptomatology. Virology 2003, 313, 492–501. [Google Scholar] [CrossRef]
- Delgado, S.; Navarro, B.; Serra, P.; Gentit, P.; Cambra, M.A.; Chiumenti, M.; Di Serio, F.; Flores, R. A yellow mosaic incited by peach latent mosaic viroid: strict association with a single-nucleotide change involved in RNA silencing-mediated cleavage of the mRNA coding for a thylakoid protein. In Proceedings of the Book of Abstracts of the International Conference on Viroids and Viroid-Like RNAs, Valencia, Spain, 5–7 July 2018; Daròs, J.A., Ed.; Abstract Number O12. p. 27. [Google Scholar]
- Puchta, H.; Luckinger, R.; Yang, X.; Hadidi, A.; Sanger, H.L. Nucleotide sequence and secondary structure of apple scar skin viroid (ASSVd) from China. Plant Mol. Biol. 1990, 14, 1065–1067. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, A.; Barba, M.; Hong, N.; Hallan, V. Apple scar skin viroid. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 217–228. ISBN 9780128014981. [Google Scholar]
- Liu, F.C.; Chen, R.F.; Chen, Y.X. Apple Scar Skin Disease; Academica Sinica Printery: Beijing, China, 1957; p. 43. [Google Scholar]
- Koganezawa, H. Transmission to apple seedlings of a low molecular weight RNA extracted from apple scar skin diseased trees. Ann. Phytopathol. Soc. Jpn. 1985, 51, 176–182. [Google Scholar] [CrossRef]
- Koganezawa, H. Further evidence for viroid etiology of apple scar skin and dapple diseases. Acta Hortic. 1986, 193, 29–33. [Google Scholar] [CrossRef]
- Smith, W.W.; Barrat, J.G.; Rich, A.E. Dapple apple, an unusual fruit symptom of apples in New Hampshire. Plant Dis. Rep. 1956, 50, 765–766. [Google Scholar]
- Yamaguchi, A.; Yanase, H. Possible relationship between the causal agent of dapple apple and scar skin. Acta Hortic. 1976, 67, 249–254. [Google Scholar] [CrossRef]
- Desvignes, J.C.; Grasseau, N.; Boyé, R.; Cornaggia, D.; Aparicio, F.; Di Serio, F.; Flores, R. Biological properties of apple scar skin viroid: Isolates, host range, different sensitivity of apple cultivars, elimination, and natural transmission. Plant Dis. 1999, 83, 768–772. [Google Scholar] [CrossRef]
- Ohtsu, Y.; Sakuma, T.; Tanaka, Y.; Takahashi, K.; Isoda, T.; Sekimoto, Y.; Matsuura, E.; Taniguchi, N. A few symptoms of “Kubomi” on fruits of Japanese pear. Ann. Phytopathol. Soc. Jpn. 1990, 56, 101. [Google Scholar]
- Wang, Y.; Zhao, Y.; Niu, J. Molecular identification and seqeunce analysis of the apple scar skin viroid (ASSVd) isolated from four kinds of fruit trees in Xinjiang Province, China. Mol. Pathog. 2012, 3, 12–18. [Google Scholar]
- Kaponi, M.S.; Luigi, M.; Barba, M.; Sano, T.; Kyriakopoulou, P.E. First report and molecular analysis of apple scar skin viroid in sweet cherry. Julius-Kühn-Archiv. 2010, 427, 361–365. [Google Scholar]
- Kaponi, M.S.; Sano, T.; Kyriakopoulou, P.E. Natural infection of sweet cherry trees with apple scar skin viroid. J. Plant Pathol. 2013, 95, 429–433. [Google Scholar]
- Walia, Y.; Dhir, S.; Bhadoria, S.; Hallan, V.; Zaidi, A.A. Molecular characterization of Apple scar skin viroid from Himalayan wild cherry. For. Pathol. 2012, 42, 84–87. [Google Scholar] [CrossRef]
- Di Serio, F.; Aparicio, F.; Alioto, D.; Ragozzino, A.; Flores, R. Identification and molecular properties of a 306 nucleotide viroid associated with apple dimple fruit disease. J. Gen. Virol. 1996, 77, 2833–2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Serio, F.; Giunchedi, L.; Alioto, D.; Ragozzino, A.; Flores, R. Identification of apple dimple fruit viroid in different commercial varieties of apple grown in Italy. Acta Hortic. 1998, 472, 595–602. [Google Scholar] [CrossRef]
- Choueiri, E.; El Zammar, S.; Jreijiri, F.; Hobeika, C.; Myrta, A.; Di Serio, F. First report of apple dimple fruit viroid in Lebanon. J. Plant Pathol. 2007, 89, 304. [Google Scholar]
- Ye, T.; Chen, S.Y.; Wang, R.; Hao, L.; Chen, H.; Wang, N.; Guo, L.Y.; Fan, Z.F.; Li, S.F.; Zhou, T. Identification and molecular characterization of apple dimple fruit viroid in China. J. Plant Pathol. 2013, 95, 637–641. [Google Scholar]
- Roumi, V.; Gazel, M.; Caglayan, K. First report of apple dimple fruit viroid in apple trees in Iran. New Dis. Rep. 2017, 35, 3. [Google Scholar] [CrossRef]
- He, Y.H.; Isono, S.; Kawaguchi-Ito, Y.; Taneda, A.; Kondo, K.I.; Iijima, A.; Tanaka, K.; Sano, T. Characterization of a new apple dimple fruit viroid variant that causes yellow dimple fruit formation in “Fuji” apple trees. J. Gen. Plant Pathol. 2010, 76, 324–330. [Google Scholar] [CrossRef]
- Chiumenti, M.; Torchetti, E.M.; Di Serio, F.; Minafra, A. Identification and characterization of a viroid resembling apple dimple fruit viroid in fig (Ficus carica L.) by next generation sequencing of small RNAs. Virus Res. 2014, 188, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, F.; Torchetti, E.M.; Flores, R.; Sano, T. Other apscaviroids infecting pome fruit trees; In Viroids and, Satellites, Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 229–241. ISBN 9780128014981. [Google Scholar]
- Ito, T.; Sano, T.; Yoshida, K. Nucleotic sequence of apple fruit crinkle viroid (AFCVd). Ann. Phytopathol. Soc. Jpn. 1998, 64, 424–425. [Google Scholar]
- Koganezawa, H.; Ohnuma, Y.; Sakuma, T.; Yanase, H. “Apple fruit crinkle,” a new graft transmissible fruit disorder of apple. Bull. Fruit Tree Res. Stn. 1989, C16, 57–62. [Google Scholar]
- Ito, T.; Kanematsu, S.; Koganezawa, H.; Tsuchizaki, T.; Yoshida, K. Detection of a viroid associated with apple fruit crinkle disease. Ann. Phytopathol. Soc. Jpn. 1993, 59, 520–527. [Google Scholar] [CrossRef]
- Matsunaka, K.; Machita, I. A graft-transmissible blister bark occurring on apple cv. Nero 26. Ann. Rep. Plant Prot. North Jpn. 1987, 38, 186. [Google Scholar]
- Sano, T.; Yoshida, H.; Goshono, M.; Monma, T.; Kawasaki, H.; Ishizaki, K. Characterization of a new viroid strain from hops: Evidence for viroid speciation by isolation in different host species. J. Gen. Plant Pathol. 2004, 70, 181–187. [Google Scholar] [CrossRef]
- Nakaune, R.; Nakano, M. Identification of a new apscaviroid from Japanese persimmon. Arch. Virol. 2008, 153, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Sano, T. Apple fruit crinkle viroid (AFCVd). In The Compendium of Hop Diseases, Arthropod Pests and Other Disorders; APS Press: St. Paul, MN, USA, 2009; pp. 39–93. ISBN 978-0-89054-376-4. [Google Scholar]
- Hernández, C.; Elena, S.F.; Moya, A.; Flores, R. Pear blister canker viroid is a member of the apple scar skin subgroup (apscaviroids) and also has sequence homology with viroids from other subgroups. J. Gen. Virol. 1992, 73, 2503–2507. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, J. Problems regarding the detection of graft transmitted pear canker. Acta Hortic. 1986, 193, 251–260. [Google Scholar] [CrossRef]
- Ambrós, S.; Desvignes, J.C.; Llácer, G.; Flores, R. Peach latent mosaic and pear blister canker viroids: Detection by molecular hybridization and relationships with specific maladies affecting peach and pear trees. Acta Hortic. 1995, 386, 515–521. [Google Scholar] [CrossRef]
- Desvignes, J.C. Les maladies à virus du poirier et leur détection. Ctifl Doc. 1970, 26, 1–12. [Google Scholar]
- Flores, R.; Hernández, C.; Llácer, G.; Desvignes, J.C. Identification of a new viroid as the putative causal agent of pear blister canker disease. J. Gen. Virol. 1991, 72, 1199–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desvignes, J.C.; Cornaggia, D.; Grasseau, N.; Ambrós, S.; Flores, R. Pear blister canker viroid: Host range and improved bioassay with two new pear indicators, Fieud 37 and Fieud 110. Plant Dis. 1999, 83, 419–422. [Google Scholar] [CrossRef]
- Sasaki, M.; Shikata, E. On some properties of hop stunt disease agent, a viroid. Proc. Jpn. Acad. 1977, 53B, 109–112. [Google Scholar] [CrossRef]
- Hataya, T.; Tsushima, T.; Sano, T. Hop stunt viroid. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 199–210. ISBN 9780128014981. [Google Scholar]
- Ohno, T.; Takamatsu, N.; Meshi, T.; Okada, Y. Hop stunt viroid: Molecular cloning and nucleotide sequence of the complete cDNA copy. Nucleic Acids Res. 1983, 11, 6185–6197. [Google Scholar] [CrossRef] [PubMed]
- Kofalvi, S.A.; Marcos, J.F.; Cañizares, M.C.; Pallás, V.; Candresse, T. Hop stunt viroid (HSVd) sequence variants from Prunus species: Evidence for recombination between HSVd isolates. J. Gen. Virol. 1997, 78, 3177–3186. [Google Scholar] [CrossRef] [PubMed]
- Amari, K.; Gomez, G.; Myrta, A.; Di Terlizzi, B.; Pallás, V. The molecular characterization of 16 new sequence variants of hop stunt viroid reveals the existence of invariable regions and a conserved hammerhead-like structure on the viroid molecule. J. Gen. Virol. 2001, 82, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Terai, Y. Symptoms and graft transmission of plum dapple fruit disease. Ann. Phytopathol. Soc. Jpn. 1985, 51, 363. [Google Scholar]
- Sano, T.; Hataya, T.; Terai, Y.; Shikata, E. Detection of a viroid-like RNA from plum dapple fruit disease occurring in Japan. Proc. Jpn. Acad. 1986, 62B, 98–101. [Google Scholar] [CrossRef]
- Terai, Y.; Sano, T.; Hataya, T.; Shikata, E. Graft-transmissible relationship of PDFD and SYFD. Ann. Phytopathol. Soc. Jpn. 1987, 53, 423. [Google Scholar]
- Sano, T. Hop stunt viroid in plum and peach. In Viroids; Hadidi, A., Flores, R., Randles, W.J., Semancik, S.J., Eds.; CSIRO: Collingwood, Australia, 2003; pp. 165–167. ISBN 0643067892. [Google Scholar]
- Hernández, C.; Flores, R. Plus and minus RNAs of peach latent mosaic viroid self-cleave in vitro via hammerhead structures. Proc. Natl. Acad. Sci. USA 1992, 89, 3711–3715. [Google Scholar] [CrossRef] [PubMed]
- Ambrós, S.; Flores, R. In vitro and in vivo self-cleavage of a viroid RNA with a mutation in the hammerhead catalytic pocket. Nucleic Acids Res. 1998, 26, 1877–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrós, S.; Hernández, C.; Flores, R. Rapid generation of genetic heterogeneity in progenies from individual cDNA clones of peach latent mosaic viroid in its natural host. J. Gen. Virol. 1999, 80, 2239–2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fekih Hassen, I.; Massart, S.; Motard, J.; Roussel, S.; Parisi, O.; Kummert, J.; Fakhfakh, H.; Marrakchi, M.; Perreault, J.P.; Jijakli, M.H. Molecular features of new peach latent mosaic viroid variants suggest that recombination may have contributed to the evolution of this infectious RNA. Virology 2007, 360, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Dubé, A.; Bolduc, F.; Bisaillon, M.; Perreault, J.P. Mapping studies of the peach latent mosaic viroid reveal novel structural features. Mol. Plant Pathol. 2011, 12, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Bussière, F.; Ouellet, J.; Côté, F.; Lévesque, D.; Perreault, J.P. Mapping in solution shows the peach latent mosaic viroid to possess a new pseudoknot in a complex, branched secondary structure. J. Virol. 2000, 74, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Delgado, S.; Rodio, M.E.; Ambrós, S.; Hernández, C.; Di Serio, F. Peach latent mosaic viroid: Not so latent. Mol. Plant Pathol. 2006, 7, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Navarro, B.; Delgado, S.; Hernández, C.; Xu, W.X.; Barba, M.; Hadidi, A.; Di Serio, F. Peach latent mosaic viroid in infected peach. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 307–316. ISBN 9780128014981. [Google Scholar]
- Kyriakopoulou, P.E.; Giunchedi, L.; Barba, M.; Boubourakas, I.N.; Kaponi, M.S.; Hadidi, A. Peach latent mosaic viroid in temperate fruit trees other than peach. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 317–329. ISBN 9780128014981. [Google Scholar]
- Blodgett, E.C. Peach calico. Phytopathology 1944, 34, 650–657. [Google Scholar]
- Willison, R.S. Peach blotch. Phytopathology 1946, 36, 273–276. [Google Scholar]
- Kishi, K.; Takanashi, K.; Abiko, K. New virus diseases of peach, yellow mosaic, oil blotch and star mosaic. Bull. Hortic. Res. Stn. Jpn. Ser. A 1973, 12, 197–208. [Google Scholar]
- Desvignes, J.C. The virus diseases detected in greenhouse and field by the peach seedlings GF-305 indicator. Acta Hortic. 1976, 67, 315–323. [Google Scholar] [CrossRef]
- Desvignes, J.C. Peach latent mosaic and its relation to peach mosaic and peach yellow mosaic virus diseases. Acta Hortic. 1986, 193, 51–57. [Google Scholar] [CrossRef]
- Flores, R.; Llácer, G. Isolation of a viroid-like RNA associated with peach latent mosaic disease. Acta Hortic. 1988, 235, 325–332. [Google Scholar] [CrossRef]
- Rodio, M.E.; Delgado, S.; Flores, R.; Di Serio, F. Variants of Peach latent mosaic viroid inducing peach calico: Uneven distribution in infected plants and requirements of the insertion containing the pathogenicity determinant. J. Gen. Virol. 2006, 87, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Rodio, M.E.; Delgado, S.; De Stradis, A.; Gómez, M.D.; Flores, R.; Di Serio, F. A viroid RNA with a specific structural motif inhibits chloroplast development. Plant Cell 2007, 19, 3610–3626. [Google Scholar] [CrossRef] [PubMed]
- Navarro, B.; Gisel, A.; Rodio, M.E.; Delgado, S.; Flores, R.; Di Serio, F. Small RNAs containing the pathogenic determinant of a chloroplast-replicating viroid guide the degradation of a host mRNA as predicted by RNA silencing. Plant J. 2012, 70, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; He, Y.; Kang, Y.P.; Hong, N.; Farooq, A.B.U.; Wang, G.P.; Xu, W.X. Virulence determination and molecular features of peach latent mosaic viroid isolates derived from phenotypically different peach leaves: A nucleotide polymorphism in L11 contributes to symptom alteration. Virus Res. 2013, 177, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qi, S.; Tang, N.; Zhang, X.; Chen, S.; Zhu, P.; Ma, L.; Cheng, J.; Xu, Y.; Lu, M.; et al. Discovery of replicating circular RNAs by RNA-Seq and computational algorithms. PLoS Pathog. 2014, 10, e1004553. [Google Scholar] [CrossRef] [PubMed]
- Serra, P.; Messmer, A.; Sanderson, D.; James, D.; Flores, R. Apple hammerhead viroid-like RNA is a bona fide viroid: Autonomous replication and structural features support its inclusion as a new member in the genus Pelamoviroid. Virus Res. 2018, 249, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Messmer, A.; Sanderson, D.; Braun, G.; Serra, P.; Flores, R.; James, D. Molecular and phylogenetic identification of unique isolates of hammerhead viroid-like RNA from “Pacific Gala” apple (Malus domestica) in Canada. Can. J. Plant Pathol. 2017, 39, 342–353. [Google Scholar] [CrossRef]
- Barba, M.; Hadidi, A. Application of next-generation sequencing technologies to viroids. In Viroids and Satellites; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: London, UK, 2017; pp. 401–412. ISBN 9780128014981. [Google Scholar]
- Wu, Q.; Wang, Y.; Cao, M.; Pantaleo, V.; Burgyan, J.; Li, W.X.; Ding, S.W. Homology-independent discovery of replicating pathogenic circular RNAs by deep sequencing and a new computational algorithm. Proc. Natl. Acad. Sci. USA 2012, 109, 3938–3943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadidi, A.; Flores, R.; Candresse, T.; Barba, M. Next-generation sequencing and genome editing in plant virology. Front. Microbiol. 2016, 7, 1325. [Google Scholar] [CrossRef] [PubMed]

| 1. The viroid must be concomitant with the disease |
| 2. The viroid must be: -isolated from the diseased plant and/or generated ex novo, either in vitro (RNA transcription) or in vivo (agroinoculation) |
| -multiplied in the original and/or in an experimental host |
| -purified physico-chemically (i.e., by electrophoresis) |
| -identified for its intrinsic properties (i.e., circularity, size and sequence) |
| 3. When the purified viroid or the corresponding RNAs generated ex novo are inoculated into a healthy host plant, they must reproduce the disease |
| 4. The same viroid must be re-isolated from the inoculated natural and/or experimental host |
| Disease | Host | Viroid | Koch’s Postulates | Reference |
|---|---|---|---|---|
| Apple scar skin | Apple | Apple scar skin viroid | Yes | [33] |
| Dapple apple | Apple | Apple scar skin viroid | Yes | [34,35] |
| Japanese pear fruit dimple | Pear | Apple scar skin viroid | Yes | [36] |
| Apple fruit crinkle | Apple | Apple fruit crinkle viroids | Yes | [37] |
| Pear rusty skin | Pear | Apple scar skin viroid | No | [34,38] |
| Pear fruit crinkle | Pear | Apple scar skin viroid | No | [39] |
| Scarred, cracked, russeted pear fruit | Pear | Apple scar skin viroid/peach latent mosaic viroid | No | [40,41] |
| Apple dimple fruit | Apple | Apple dimple fruit viroid | Yes | [24] |
| Blister bark in cv. ‘Nero26’ | Apple | Apple fruit crinkle viroid | Yes | [42] |
| Pear blister canker in cv. ‘A20’ | Pear | Pear blister canker viroid | Yes | [43] |
| Plum dapple fruit | Plum | Hop stunt viroid | Yes | [44] |
| Peach dapple fruit | Peach | Hop stunt viroid | No | [45] |
| Peach latent mosaic | Peach | Peach latent mosaic viroid | Yes | [46] |
| Peach calico | Peach | Peach latent mosaic viroid | Yes | [47] |
| Peach yellow mosaic | Peach | Peach latent mosaic viroid | Yes | [48] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Serio, F.; Ambrós, S.; Sano, T.; Flores, R.; Navarro, B. Viroid Diseases in Pome and Stone Fruit Trees and Koch’s Postulates: A Critical Assessment. Viruses 2018, 10, 612. https://doi.org/10.3390/v10110612
Di Serio F, Ambrós S, Sano T, Flores R, Navarro B. Viroid Diseases in Pome and Stone Fruit Trees and Koch’s Postulates: A Critical Assessment. Viruses. 2018; 10(11):612. https://doi.org/10.3390/v10110612
Chicago/Turabian StyleDi Serio, Francesco, Silvia Ambrós, Teruo Sano, Ricardo Flores, and Beatriz Navarro. 2018. "Viroid Diseases in Pome and Stone Fruit Trees and Koch’s Postulates: A Critical Assessment" Viruses 10, no. 11: 612. https://doi.org/10.3390/v10110612