Protective Immunity Induced by DNA Vaccination against Ranavirus Infection in Chinese Giant Salamander Andrias davidianus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chinese Giant Salamanders, Cells, and Virus
2.3. Plasmid Construction
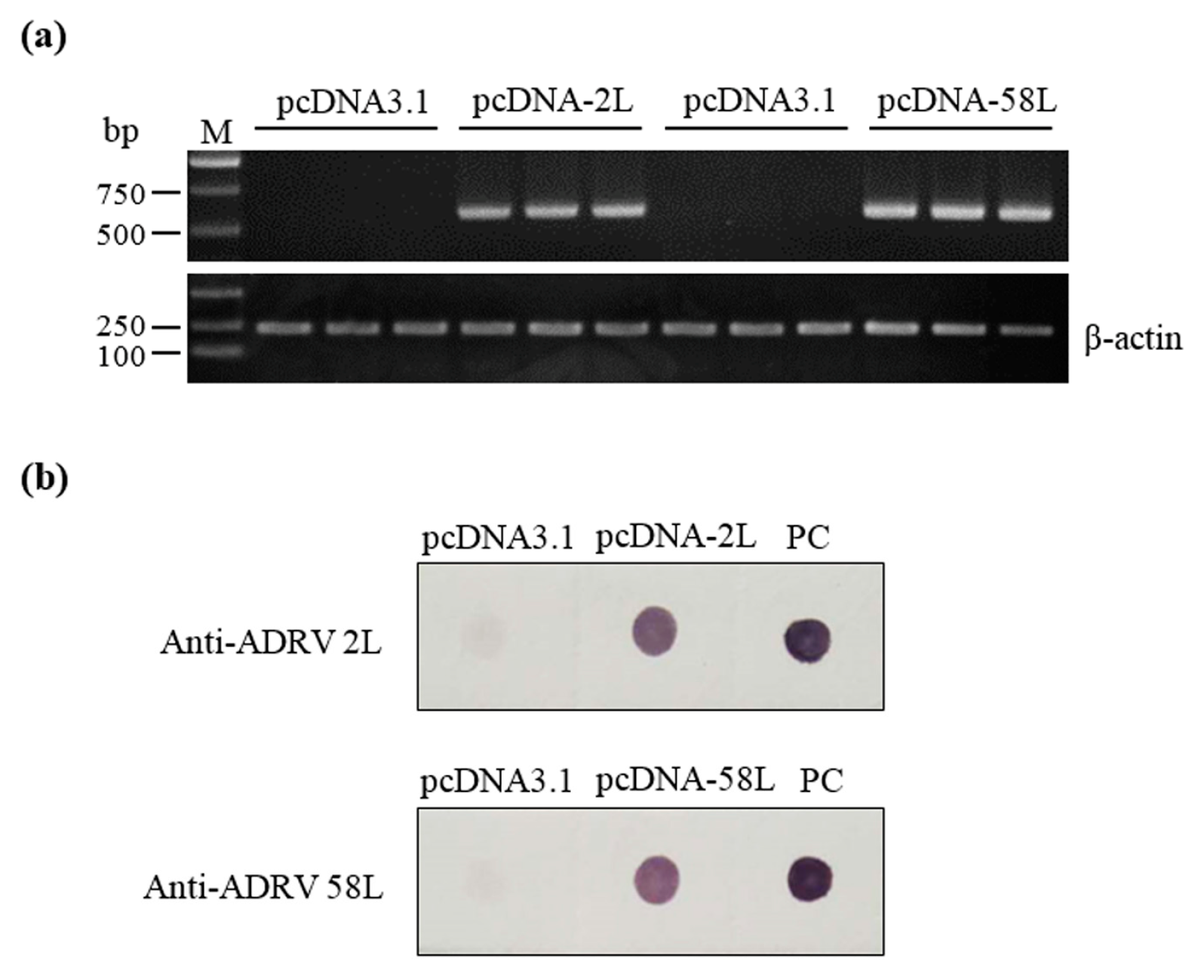
2.4. Detection of Expression of Vaccine Plasmids in EPC Cells
2.5. Vaccination and Challenge Experiment
2.6. Detection of Expression of Vaccine Plasmids in Chinese Giant Salamanders
2.7. Antibody Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Quantitative Real-Time PCR (qRT-PCR) Analysis of Expression of Immune Genes
2.9. Determination of ADRV Viral Load by qRT-PCR
2.10. Statistical Analysis
3. Results
3.1. Expression of Plasmid Constructs In Vitro and In Vivo
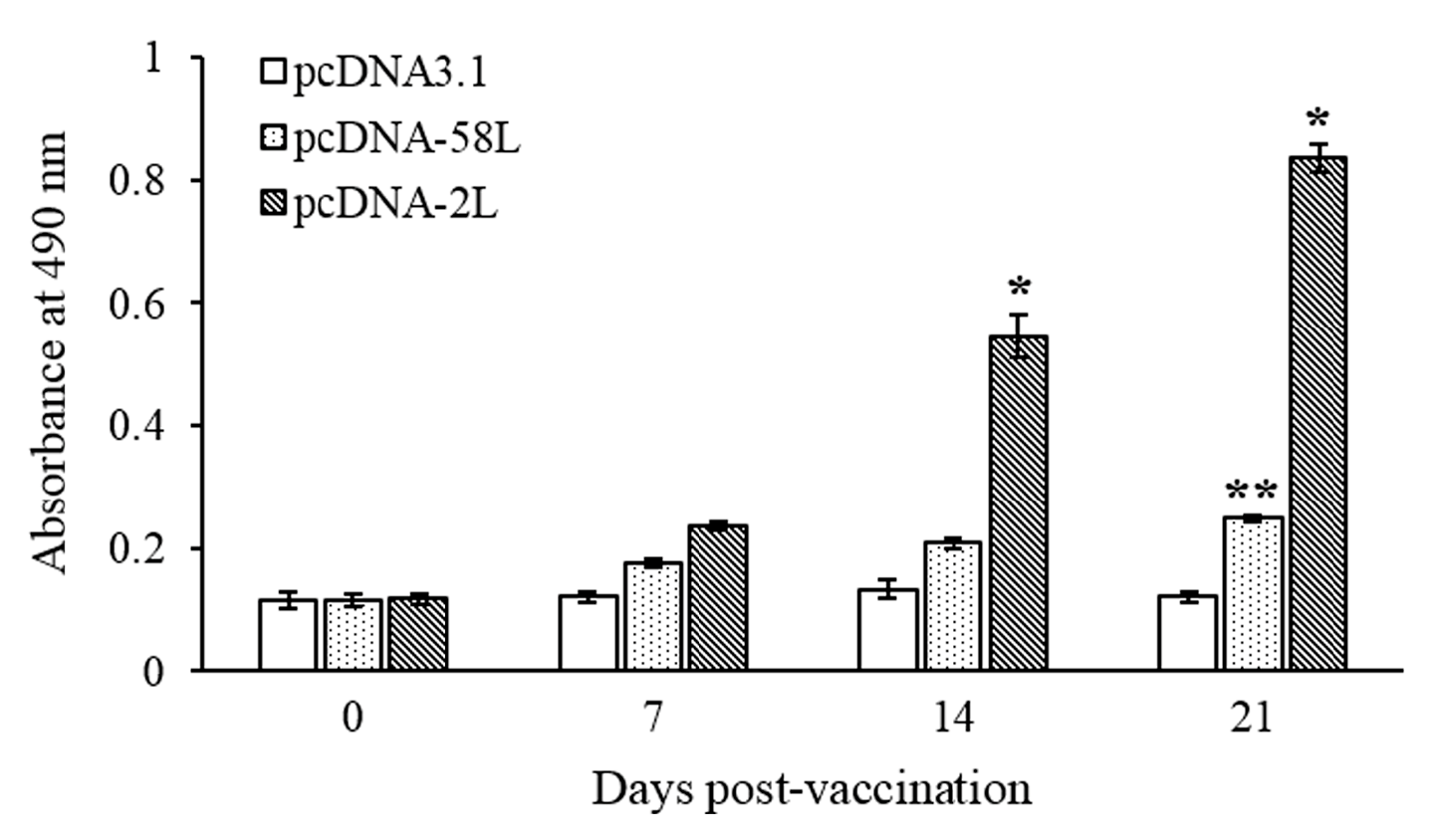
3.2. Specific Serum Antibody Response
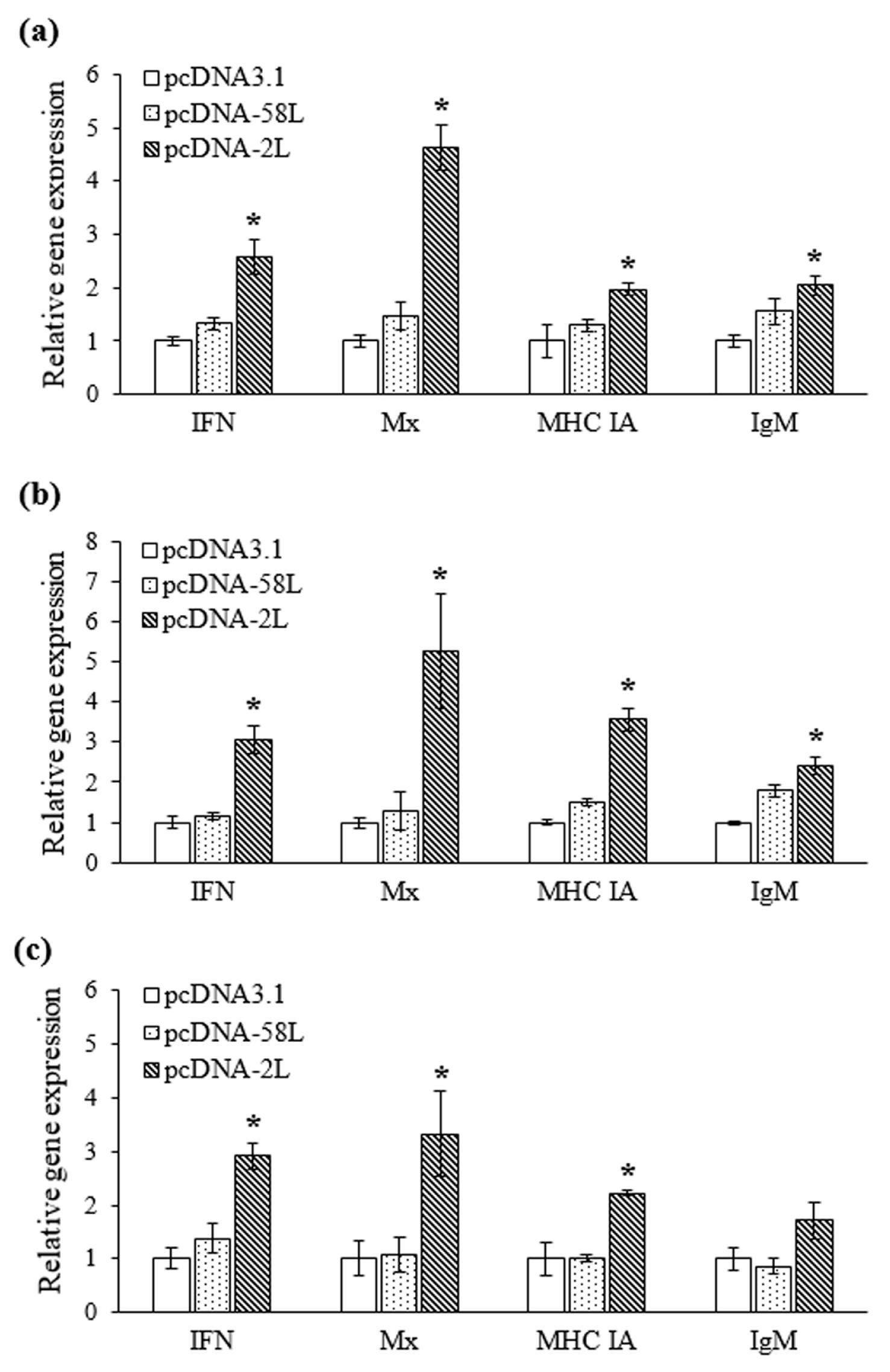
3.3. Expression of Immune-Related Genes
3.4. Protection of DNA Vaccination
3.5. Detection of ADRV Viral Load
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinchar, V.G.; Hick, P.; Ince, I.A.; Jancovich, J.K.; Marschang, R.; Qin, Q.W.; Subramaniam, K.; Waltzek, T.B.; Whittington, R.; Williams, T.; et al. ICTV virus taxonomy profile: Iridoviridae. J. Gen. Virol. 2017, 98, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Gray, M.; Storfer, A. Ecopathology of ranaviruses infecting amphibians. Viruses 2011, 3, 2351–2373. [Google Scholar] [CrossRef]
- Chinchar, V.G.; Waltzek, T.B. Ranaviruses: Not just for frogs. PLoS Pathog. 2014, 10, e1003850. [Google Scholar] [CrossRef] [PubMed]
- Pricea, S.J.; Arielc, E.; Maclainec, A.; Rosab, G.M.; Graye, M.J.; Brunnerf, J.L.; Garnerb, T.W. From fish to frogs and beyond: Impact and host range of emergent ranaviruses. Virology 2017, 511, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, W.; Wang, Q.J.; Zhao, H.; Zhang, H.X.; Marcec, R.M.; Willard, S.T.; Kouba, A.J. Reintroduction and post-release survival of a living fossil: The Chinese giant salamander. PLoS ONE 2016, 11, e0156715. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, X.; Yang, C.; An, J.; Qin, J.; Song, F.; Zeng, W. Iridovirus infection in Chinese giant salamanders, China, 2010. Emerg. Infect. Dis. 2011, 17, 2388–2389. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, K.Y.; Zhou, Z.Y.; Li, C.W.; Wang, J.; He, M.; Yin, Z.Q.; Lai, W.M. First report of a ranavirus associated with morbidity and mortality in farmed Chinese giant salamanders (Andrias davidianus). J. Comp. Pathol. 2011, 145, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Gui, J.F. Virus genomes and virus-host interactions in aquaculture animals. Sci. China Life Sci. 2015, 58, 156–169. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Gui, J.F.; Gao, X.C.; Pei, C.; Hong, Y.J.; Zhang, Q.Y. Genome architecture changes and major gene variations of Andrias davidianus ranavirus (ADRV). Vet. Res. 2013, 44, 101. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Z.Y.; Wang, J.; Yuan, J.D.; Liao, X.Y.; Gui, J.F.; Zhang, Q.Y. Extensive diversification of MHC in Chinese giant salamanders Andrias davidianus (Anda-MHC) reveals novel splice variants. Dev. Comp. Immunol. 2014, 42, 311–322. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Z.Y.; Wang, J.; Yuan, J.D.; Liao, X.Y.; Gui, J.F.; Zhang, Q.Y. Thymus cDNA library survey uncovers novel features of immune molecules in Chinese giant salamander Andrias davidianus. Dev. Comp. Immunol. 2014, 46, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.C.; Chen, Z.Y.; Yuan, J.D.; Zhang, Q.Y. Morphological changes in amphibian and fish cell lines infected with Andrias davidianus ranavirus. J. Comp. Pathol. 2015, 152, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Fan, Y.; Zhou, Y.; Liu, W.; Ma, J.; Meng, Y.; Xie, C.; Zeng, L. Characterization of Chinese giant salamander iridovirus tissue tropism and inflammatory response after infection. Dis. Aquat. Organ. 2015, 114, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.D.; Chen, Z.Y.; Huang, X.; Gao, X.C.; Zhang, Q.Y. Establishment of three cell lines from Chinese giant salamander and their sensitivities to the wild-type and recombinant ranavirus. Vet. Res. 2015, 46, 58. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, L.Q.; Wang, Y.X.; Shen, J.; Pan, C.Y.; Meng, Y.; Yang, C.M.; Ji, H.; Dong, W.Z. Autophagy and apoptosis induced by Chinese giant salamander (Andrias davidianus) iridovirus (CGSIV). Vet. Microbiol. 2016, 195, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, J.; Ma, J.; LaPatra, S.E.; Meng, Y.; Fan, Y.; Zhou, Y.; Yang, X.; Zeng, L. Immunological responses and protection in Chinese giant salamander Andrias davidianus immunized with inactivated iridovirus. Vet. Microbiol. 2014, 174, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fan, Y.; LaPatra, S.E.; Ma, J.; Xu, J.; Meng, Y.; Jiang, N.; Zeng, L. Protective immunity of a Pichia pastoris expressed recombinant iridovirus major capsid protein in the Chinese giant salamander, Andrias davidianus. Vaccine 2015, 33, 5662–5669. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, X.; Han, Y.; Jia, Q.; Gao, H. Vaccination with recombinant baculovirus expressing ranavirus major capsid protein induces protective immunity in Chinese giant salamander, Andrias davidianus. Viruses 2017, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Hølvold, L.B.; Myhr, A.I.; Dalmo, R.A. Strategies and hurdles using DNA vaccines to fish. Vet. Res. 2014, 45, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caipang, C.M.; Takano, T.; Hirono, I.; Aoki, T. Genetic vaccines protect red seabream, Pagrus major, upon challenge with red seabream iridovirus (RSIV). Fish Shellfish Immunol. 2006, 21, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Ou-yang, Z.; Wang, P.; Huang, Y.; Huang, X.; Wan, Q.; Zhou, S.; Wei, J.; Zhou, Y.; Qin, Q. Selection and identification of Singapore grouper iridovirus vaccine candidate antigens using bioinformatics and DNA vaccination. Vet. Immunol. Immunopathol. 2012, 149, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Li, N.; Lin, Q.; Guo, H.; Zhang, D.; Liu, L.; Wu, S. Protective immunity against infectious spleen and kidney necrosis virus induced by immunization with DNA plasmid containing mcp gene in Chinese perch Siniperca chuatsi. Fish Shellfish Immunol. 2014, 40, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.P.; Dong, C.F.; Weng, S.P.; Zhang, J.; Zhang, Y.; He, J.G. Antigenic identification of virion structural proteins from infectious spleen and kidney necrosis virus. Fish Shellfish Immunol. 2011, 31, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, S.; Xia, L.; Huang, X.; Huang, Y.; Cao, J.; Qin, Q. Characterization of the VP39 envelope protein from Singapore grouper iridovirus. Can. J. Microbiol. 2015, 61, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ke, F.; Lei, X.Y.; Zhu, R.; Zhang, Q.Y. Viral envelope protein 53R genehighly specific silencing and iridovirus resistance in fish cells by amiRNA. PLoS ONE 2010, 5, e10308. [Google Scholar]
- He, L.B.; Gao, X.C.; Ke, F.; Zhang, Q.Y. A conditional lethal mutation in Rana grylio virus ORF 53R resulted in a marked reduction in virion formation. Virus Res. 2013, 177, 194–200. [Google Scholar] [CrossRef] [PubMed]
- He, L.B.; Ke, F.; Wang, J.; Gao, X.C.; Zhang, Q.Y. Rana grylio virus (RGV) envelope protein 2L: Subcellular localization and essential roles in virus infectivity revealed by conditional lethal mutant. J. Gen. Virol. 2014, 95, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Lei, X.Y.; Zhang, Q.Y. The antiviral defense mechanisms in mandarin fish induced by DNA vaccination against a rhabdovirus. Vet. Microbiol. 2012, 157, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Amend, D.F. Potency testing of fish vaccines. International Sympo-sium on Fish Biologics: Serodiagnostics and Vaccines. Dev. Biol. Stand. 1981, 49, 447–454. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt. Method 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Koubourli, D.V.; Wendel, E.S.; Yaparla, A.; Ghaul, J.R.; Grayfer, L. Immune roles of amphibian (Xenopus laevis) tadpole granulocytes during Frog Virus 3 ranavirus infections. Dev. Comp. Immunol. 2017, 72, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Duffus, A.L.J.; Waltzek, T.B.; Stöhr, A.C.; Allender, M.C.; Gotesman, M.; Whittington, R.J.; Hick, P.; Hines, M.K.; Marschang, R.E. Distribution and host range of ranaviruses. In Ranaviruses: Lethal Pathogens of Ectothermic Vertebrates; Gray, M.J., Chinchar, V.G., Eds.; Springer: New York, NY, USA, 2015; pp. 9–59. [Google Scholar]
- Robert, J.; Jancovich, J.K. Recombinant ranaviruses for studying evolution of host-pathogen interactions in ectothermic vertebrates. Viruses 2016, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Chinchar, V.G.; Waltzek, T.B.; Subramaniam, K. Ranaviruses and other members of the family Iridoviridae: Their place in the virosphere. Virology 2017, 511, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Whitley, D.S.; Yu, K.; Sample, R.C.; Sinning, A.; Henegar, J.; Norcross, E.; Chinchar, V.G. Frog virus 3 ORF 53R, a putative myristoylated membrane protein, is essential for virus replication in vitro. Virology 2010, 405, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.R. Antibodies, viruses and vaccines. Nat. Rev. Immunol. 2002, 2, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Maniero, G.D.; Morales, H.; Gantress, J.; Robert, J. Generation of a long-lasting, protective, and neutralizing antibody response to the ranavirus FV3 by the frog Xenopus. Dev. Comp. Immunol. 2006, 30, 649–657. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Haller, O.; Staeheli, P.; Schwemmle, M.; Kochs, G. Mx GTPases: Dynamin-like antiviral machines of innate immunity. Trends Microbiol. 2015, 23, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ma, J.; Fan, Y.; Meng, Y.; Xu, J.; Zhou, Y.; Liu, W.; Zeng, X.; Zeng, L. Identification of type I IFN in Chinese giant salamander (Andrias davidianus) and the response to an iridovirus infection. Mol. Immunol. 2015, 65, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Dalmo, R.A. DNA vaccines for fish: Review and perspectives on correlates of protection. J. Fish Dis. 2018, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van de Weijer, M.L.; Luteijn, R.D.; Wiertz, E.J. Viral immune evasion: Lessons in MHC class I antigen presentation. Semin. Immunol. 2015, 27, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Robert, J. Antiviral immunity in amphibians. Viruses 2011, 3, 2065–2086. [Google Scholar] [CrossRef] [PubMed]
- Didinger, C.; Eimes, J.A.; Lillie, M.; Waldman, B. Multiple major histocompatibility complex class I genes in Asian anurans: Ontogeny and phylogeny. Dev. Comp. Immunol. 2017, 70, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Barbosa-Solomieu, V.; Chinchar, V.G. A decade of advances iniridovirus research. Adv. Virus Res. 2005, 65, 173–248. [Google Scholar] [PubMed]
- Jung, M.H.; Jung, S.J.; Vinay, T.N.; Nikapitiya, C.; Kim, J.O.; Lee, J.H.; Lee, J.; Oh, M.J. Effects of water temperature on mortality in Megalocytivirus-infected rock bream Oplegnathus fasciatus (Temminck et Schlegel) and development of protective immunity. J. Fish Dis. 2015, 38, 729–737. [Google Scholar] [CrossRef] [PubMed]

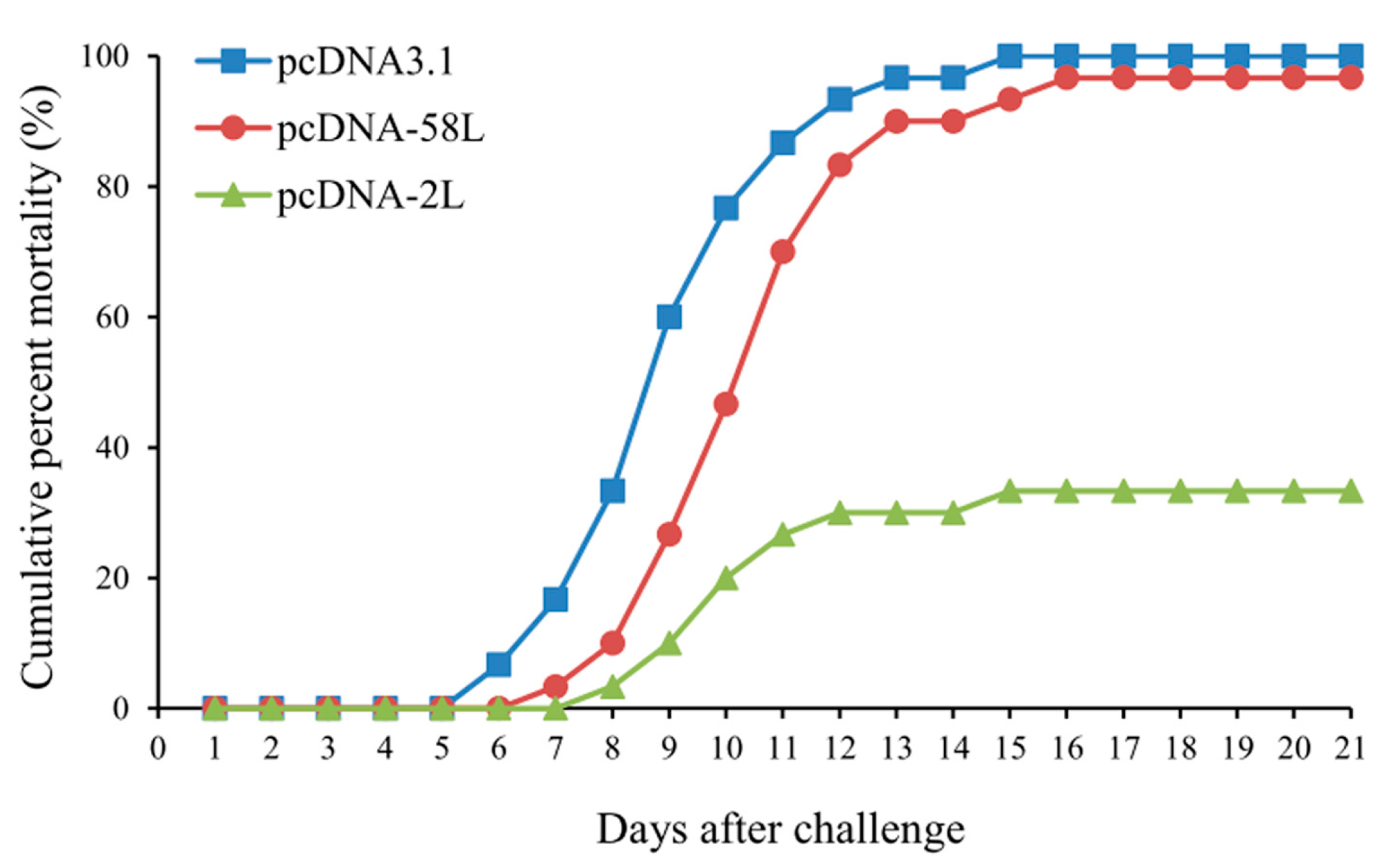
| Vaccinated Groups | Cumulative Mortality (Death/Total) | RPS a |
|---|---|---|
| pcDNA-2L | 33.3% (10/30) | 66.7 * |
| pcDNA-58L | 96.7% (29/30) | 3.3 |
| pcDNA3.1 | 100% (30/30) | - |
| Vaccinated Groups | No. of Animal a | Animal Status Death (D) or Survival (S) | Viral Load b |
|---|---|---|---|
| pcDNA-2L | A1 | S | 1.00 × 101 |
| A2 | S | 8.36 × 101 | |
| A3 | S | 1.71 × 101 | |
| A4 | S | 1.21 × 102 | |
| A5 | S | 1.83 × 102 | |
| A6 | S | 1.03 × 103 | |
| A7 | S | 1.32 × 101 | |
| A8 | S | 4.44 × 101 | |
| A9 | S | 3.28 × 102 | |
| A10 | S | 6.09 × 101 | |
| pcDNA-58L | B1 | S | 1.75 × 103 |
| pcDNA 3.1 | C1 | D | 6.26 × 107 |
| C2 | D | 1.08 × 108 | |
| C3 | D | 1.89 × 107 | |
| C4 | D | 1.75 × 107 | |
| C5 | D | 1.54 × 108 | |
| C6 | D | 1.66 × 107 | |
| C7 | D | 1.42 × 107 | |
| C8 | D | 1.91 × 107 | |
| C9 | D | 2.55 × 107 | |
| C10 | D | 5.37 × 107 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-Y.; Li, T.; Gao, X.-C.; Wang, C.-F.; Zhang, Q.-Y. Protective Immunity Induced by DNA Vaccination against Ranavirus Infection in Chinese Giant Salamander Andrias davidianus. Viruses 2018, 10, 52. https://doi.org/10.3390/v10020052
Chen Z-Y, Li T, Gao X-C, Wang C-F, Zhang Q-Y. Protective Immunity Induced by DNA Vaccination against Ranavirus Infection in Chinese Giant Salamander Andrias davidianus. Viruses. 2018; 10(2):52. https://doi.org/10.3390/v10020052
Chicago/Turabian StyleChen, Zhong-Yuan, Tao Li, Xiao-Chan Gao, Chen-Fei Wang, and Qi-Ya Zhang. 2018. "Protective Immunity Induced by DNA Vaccination against Ranavirus Infection in Chinese Giant Salamander Andrias davidianus" Viruses 10, no. 2: 52. https://doi.org/10.3390/v10020052




