First Diagnosed Case of Camelpox Virus in Israel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Samples and Antibodies
2.3. TEM Imaging
2.4. Infection of Chorioalanthoic Membrane & Vero Cells
2.5. Infection, Immunofluorescence Assay (IFA) and Cells Staining
2.6. Primer Design and Multiple Alignment Analysis
2.7. DNA Extraction and PCR Procedures
3. Results
3.1. Case Description and Clinical Signs
3.2. Diagnostic Transmission Electron Microscopy
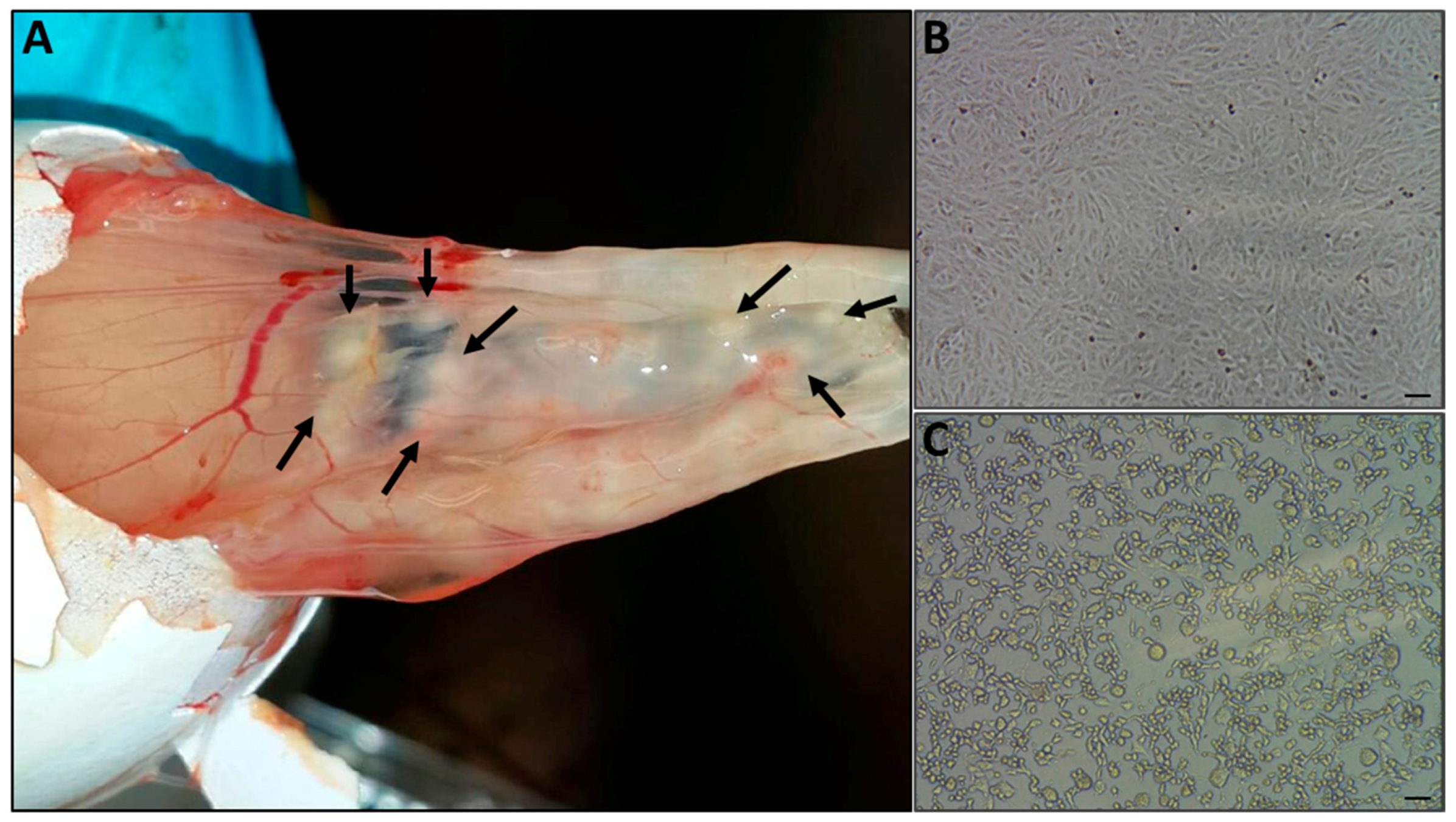
3.3. Pock Formation on CAM
3.4. CPE on Vero Cells
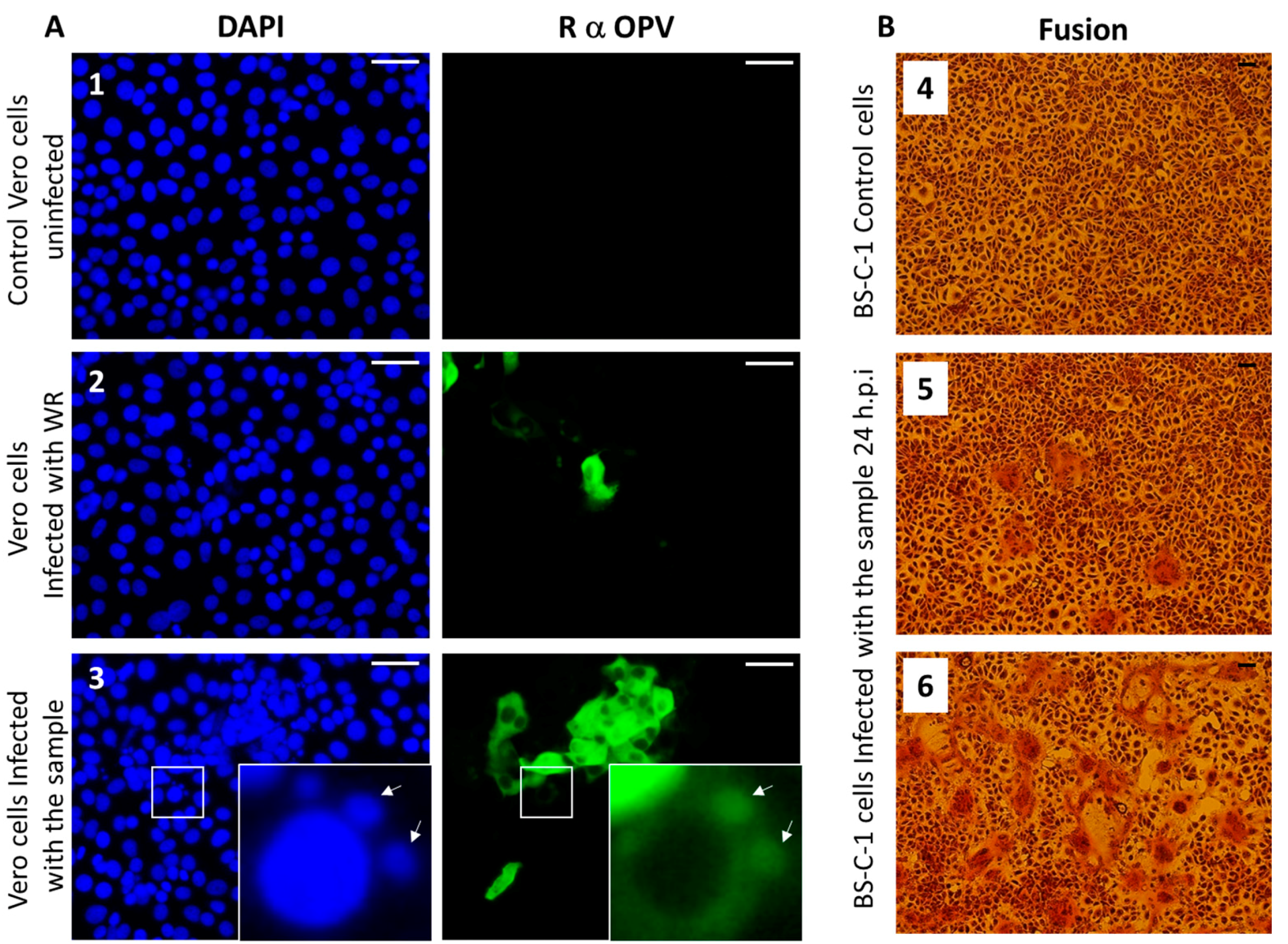
3.5. Immunofluorescence Assay
3.6. Syncytia Formation in Cultured Cells
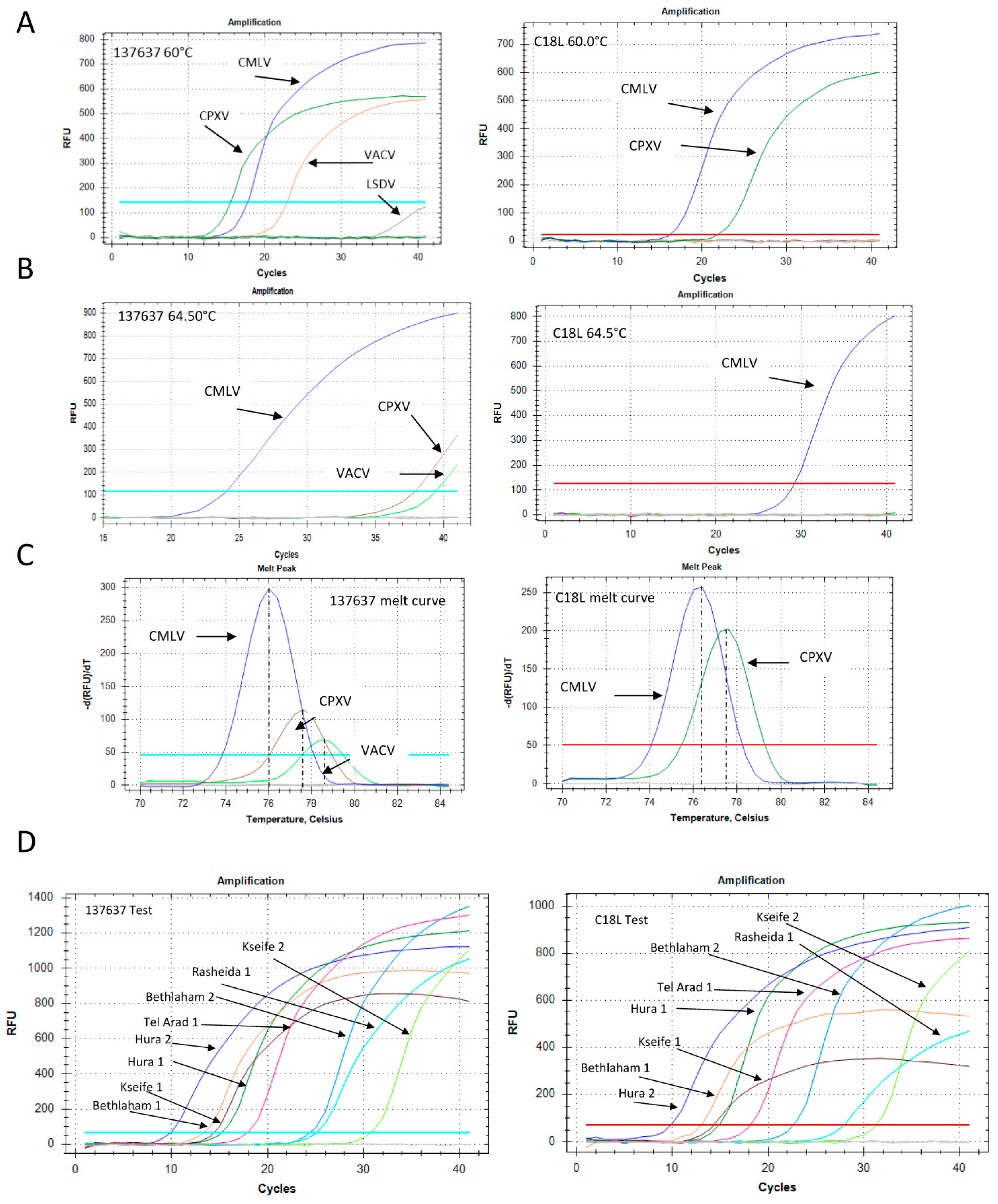
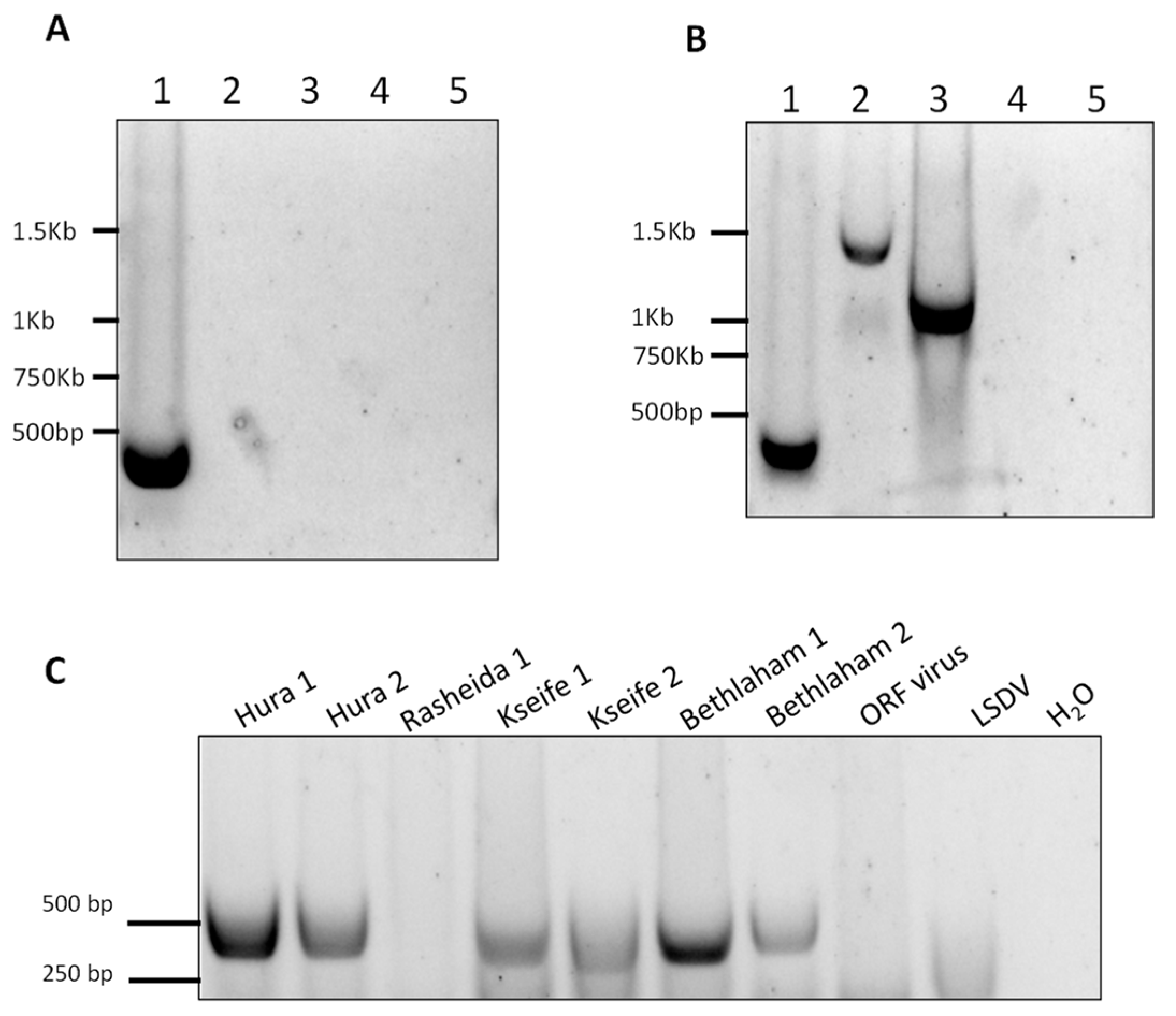
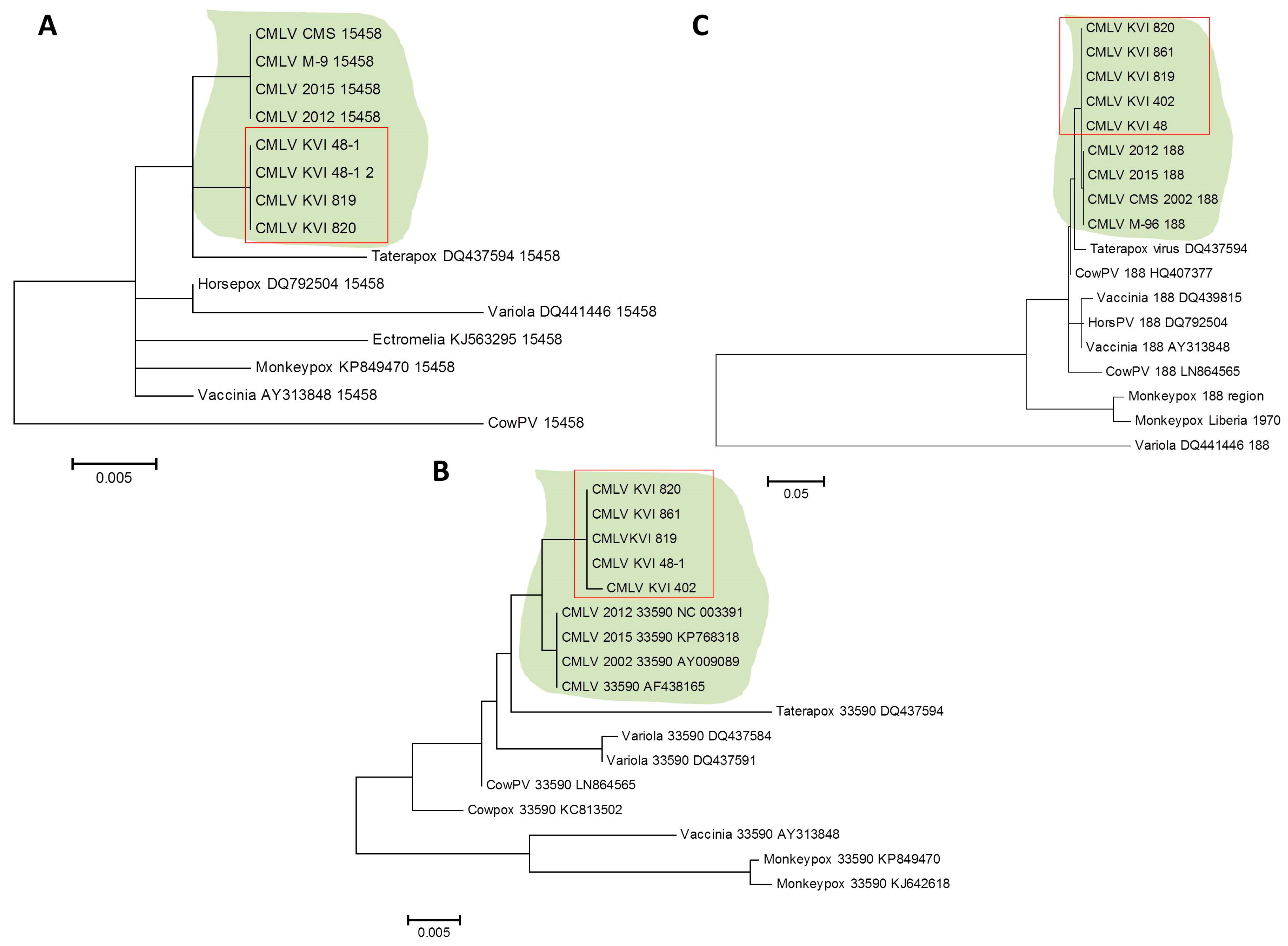
3.7. CMLV Identification Using PCR
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Breman, J.G.; Henderson, D.A. Diagnosis and management of smallpox. N. Engl. J. Med. 2002, 346, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, A.I.; Al-Busada, K.A.; El-Sabagh, I.M. Multiplex PCR for rapid diagnosis and differentiation of pox and pox-like diseases in dromedary camels. Virol. J. 2015, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.U.; Burk, R.D.; Chen, Z.; van Doorslaer, K.; zur Hausen, H.; de Villiers, E.M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 2010, 401, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bera, B.C.; Shanmugasundaram, K.; Barua, S.; Venkatesan, G.; Virmani, N.; Riyesh, T.; Gulati, B.R.; Bhanuprakash, V.; Vaid, R.K.; Kakker, N.K.; et al. Zoonotic cases of camelpox infection in India. Vet. Microbiol. 2011, 152, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, A.I.; Abdelazim, F. Human and dromedary camel infection with camelpox virus in Eastern Sudan. Vector Borne Zoonotic Dis. 2017, 17, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.S.; Kumar, S.; Mehta, S.C.; Narnaware, S.D.; Singh, R.; Tuteja, F.C. Camelpox: A brief review on its epidemiology, current status and challenges. Acta Trop. 2016, 158, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Duraffour, S.; Meyer, H.; Andrei, G.; Snoeck, R. Camelpox virus. Antivir. Res. 2011, 92, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Gubser, C.; Smith, G.L. The sequence of camelpox virus shows it is most closely related to variola virus, the cause of smallpox. J. Gen. Virol. 2002, 83, 855–872. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.L.; Peng, C.; McFadden, G.; Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014, 21, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Dashtseren, T.; Solovyev, B.V.; Varejka, F.; Khokhoo, A. Camel contagious ecthyma (pustular dermatitis). Acta Virol. 1984, 28, 122–127. [Google Scholar] [PubMed]
- Fleming, S.B.; Wise, L.M.; Mercer, A.A. Molecular genetic analysis of orf virus: A poxvirus that has adapted to skin. Viruses 2015, 7, 1505–1539. [Google Scholar] [CrossRef] [PubMed]
- Friederichs, S.; Krebs, S.; Blum, H.; Wolf, E.; Lang, H.; von Buttlar, H.; Buttner, M. Comparative and retrospective molecular analysis of parapoxvirus (PPV) isolates. Virus Res. 2014, 181, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.S.; Kumar, S.; Mehta, S.C.; Singh, R.; Nath, K.; Narnaware, S.D.; Tuteja, F.C. Molecular characterization of camelpox virus isolates from bikaner, India: Evidence of its endemicity. Acta Trop. 2017, 171, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Melamed, S.; Paran, N.; Katz, L.; Ben-Nathan, D.; Israely, T.; Schneider, P.; Levin, R.; Lustig, S. Tail scarification with vaccinia virus lister as a model for evaluation of smallpox vaccine potency in mice. Vaccine 2007, 25, 7743–7753. [Google Scholar] [PubMed]
- Paran, N.; Suezer, Y.; Lustig, S.; Israely, T.; Schwantes, A.; Melamed, S.; Katz, L.; Preuss, T.; Hanschmann, K.M.; Kalinke, U.; et al. Postexposure immunization with modified vaccinia virus ankara or conventional lister vaccine provides solid protection in a murine model of human smallpox. J. Infect. Dis. 2009, 199, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Achdout, H.; Lustig, S.; Israely, T.; Erez, N.; Politi, B.; Tamir, H.; Israeli, O.; Waner, T.; Melamed, S.; Paran, N. Induction, treatment and prevention of eczema vaccinatum in atopic dermatitis mouse models. Vaccine 2017, 35, 4245–4254. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Paran, N.; Maik-Rachline, G.; Politi, B.; Israely, T.; Schnider, P.; Fuchs, P.; Melamed, S.; Lustig, S. Induction of cell-cell fusion by ectromelia virus is not inhibited by its fusion inhibitory complex. Virol. J. 2009, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Moller, L.; Schunadel, L.; Nitsche, A.; Schwebke, I.; Hanisch, M.; Laue, M. Evaluation of virus inactivation by formaldehyde to enhance biosafety of diagnostic electron microscopy. Viruses 2015, 7, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Muscle: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Kent, W.J.; Smit, A.; Zhang, Z.; Baertsch, R.; Hardison, R.C.; Haussler, D.; Miller, W. Human-mouse alignments with blastz. Genome Res. 2003, 13, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. Mega6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V.; Bhanuprakash, V.; Hosamani, M.; Jayappa, K.D.; Venkatesan, G.; Chauhan, B.; Singh, R.K. A polymerase chain reaction strategy for the diagnosis of camelpox. J. Vet. Diagn. Investig. 2009, 21, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Poxvirus DNA replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a010199. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, V.; Venkatesan, G.; Bhanuprakash, V.; Singh, R.K. Camelpox, an emerging orthopox viral disease. Indian J. Virol. 2013, 24, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Oron, C.; Paran, N.; Keysary, A.; Israeli, O.; Yitzhaki, S.; Olshevsky, U. Establishment of cell-based reporter system for diagnosis of poxvirus infection. J. Virol. Methods 2010, 167, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hazelton, P.R.; Gelderblom, H.R. Electron microscopy for rapid diagnosis of infectious agents in emergent situations. Emerg. Infect. Dis. 2003, 9, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Wibbelt, G.; Tausch, S.H.; Dabrowski, P.W.; Kershaw, O.; Nitsche, A.; Schrick, L. Berlin squirrelpox virus, a new poxvirus in red squirrels, Berlin, Germany. Emerg. Infect. Dis. 2017, 23, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Gelaye, E.; Achenbach, J.E.; Ayelet, G.; Jenberie, S.; Yami, M.; Grabherr, R.; Loitsch, A.; Diallo, A.; Lamien, C.E. Genetic characterization of poxviruses in Camelus dromedarius in Ethiopia, 2011–2014. Antivir. Res. 2016, 134, 17–25. [Google Scholar] [CrossRef] [PubMed]


| Primer Name | Primer Sequence | Amplicon Length in Sequence No. NC-003391 |
|---|---|---|
| 15458 Fwd | 5′-CTATATCTATATGAGATGAC-3′ | 420 bp |
| 15877 Rev | 5′-GTTGGTAGTAGGGTACTCGTG-3′ | |
| CMLV 33590 F | 5′-TCTGGAAGTGGATATACATAG-3′ | 902 bp |
| CMLV 34492 R | 5′-GGGATAATCCAGAATTGATAATAGTGG-3′ | |
| CMLV 188946 F | 5′-GTATAATGTATGTAACCCGTAC-3′ | 1094 bp |
| CMLV 190040 R | 5′-TACATACCATTAATAATGCAAGC-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erster, O.; Melamed, S.; Paran, N.; Weiss, S.; Khinich, Y.; Gelman, B.; Solomony, A.; Laskar-Levy, O. First Diagnosed Case of Camelpox Virus in Israel. Viruses 2018, 10, 78. https://doi.org/10.3390/v10020078
Erster O, Melamed S, Paran N, Weiss S, Khinich Y, Gelman B, Solomony A, Laskar-Levy O. First Diagnosed Case of Camelpox Virus in Israel. Viruses. 2018; 10(2):78. https://doi.org/10.3390/v10020078
Chicago/Turabian StyleErster, Oran, Sharon Melamed, Nir Paran, Shay Weiss, Yevgeny Khinich, Boris Gelman, Aharon Solomony, and Orly Laskar-Levy. 2018. "First Diagnosed Case of Camelpox Virus in Israel" Viruses 10, no. 2: 78. https://doi.org/10.3390/v10020078




