Mosquitoes as Suitable Vectors for Alphaviruses
Abstract
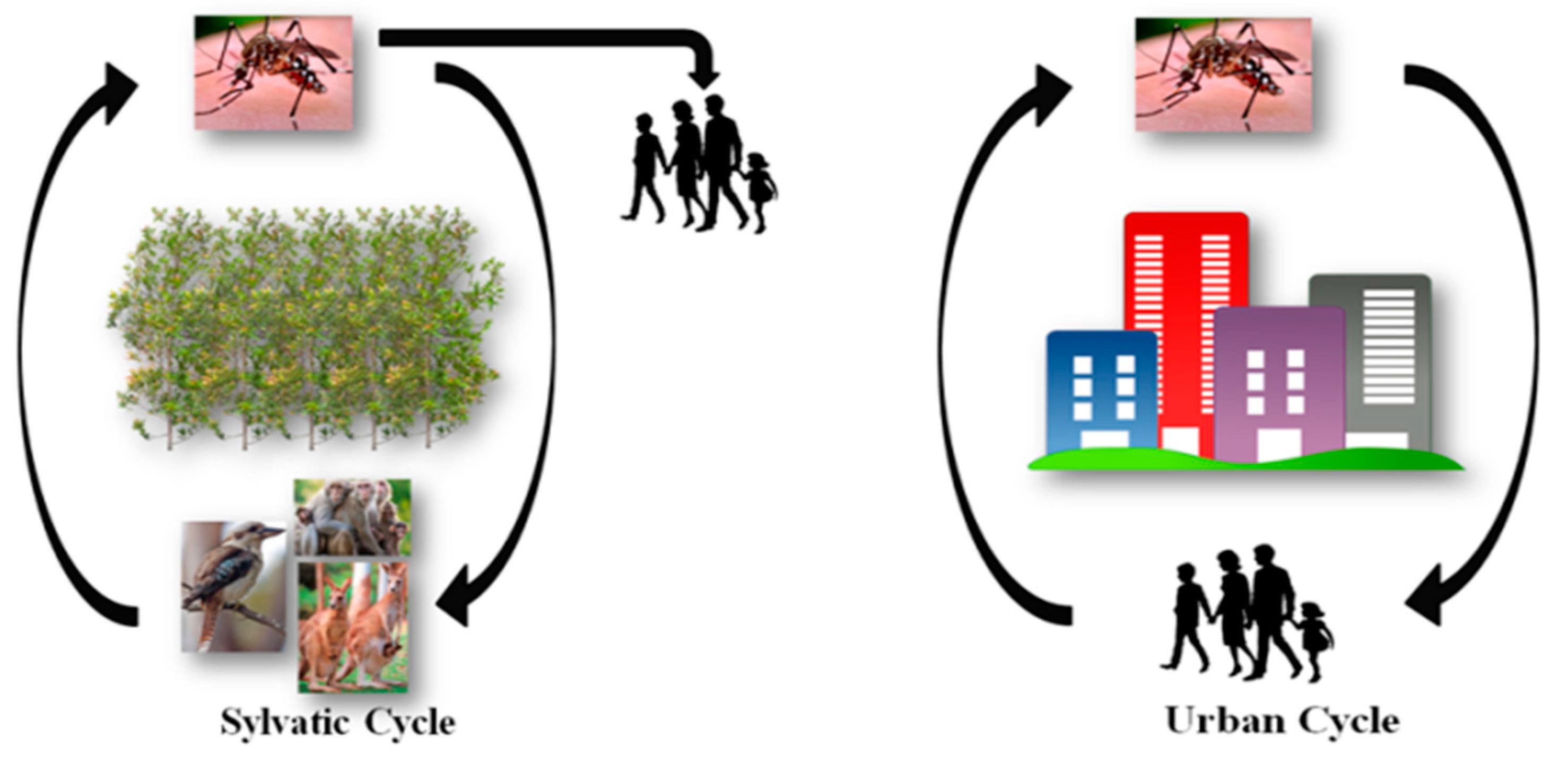
:1. Alphavirus Transmission and the Mosquito Life Cycle
2. Factors of Vector Competence
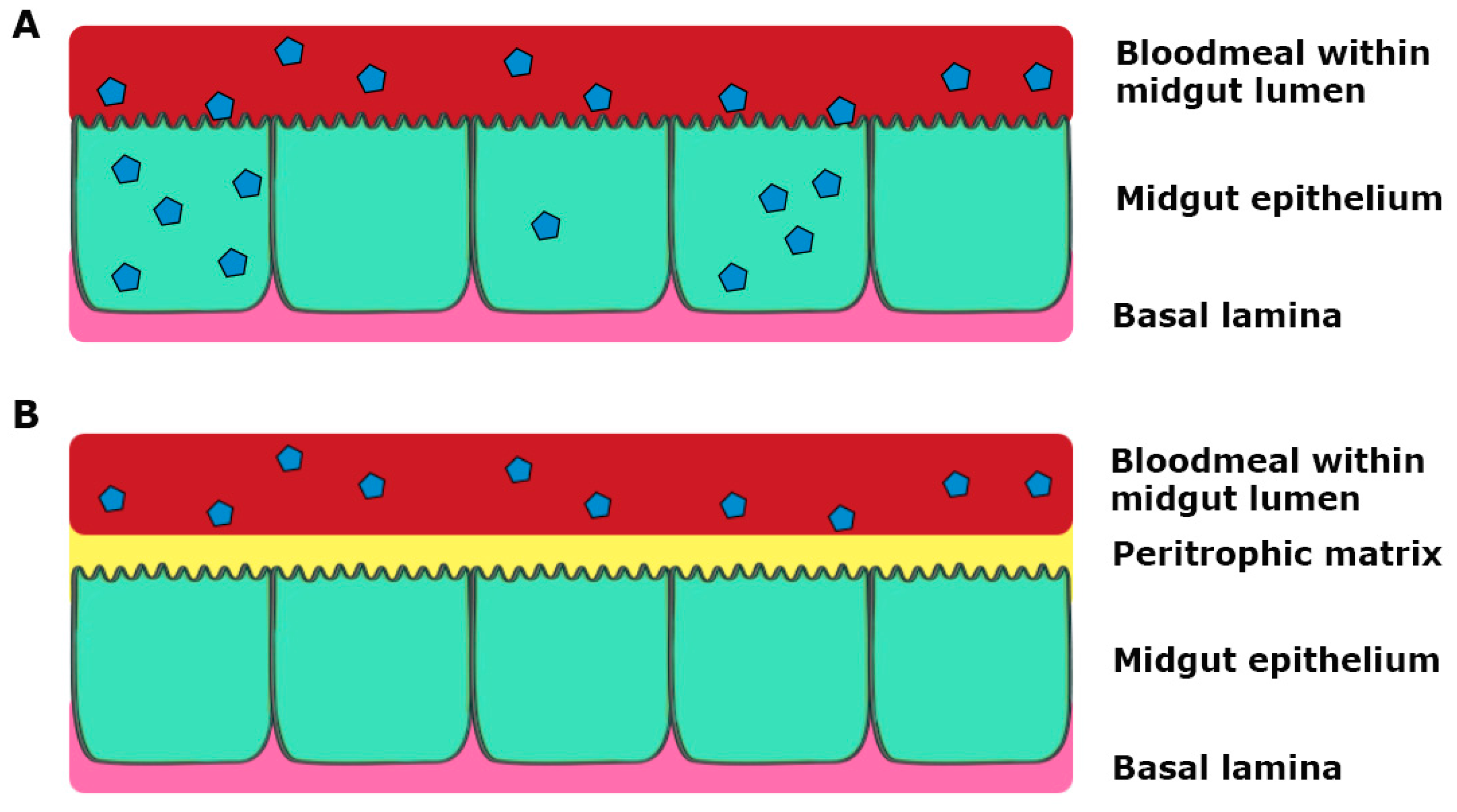
3. Tissue Barriers to Infection in the Mosquito Vector
4. The Roles of Parasites in Alphavirus Infection
5. Mechanisms of Viral Adaption to Vector
5.1. Effect of Different Viral Strains (or Mutations) on Vector Competency
5.2. Effect of Temperature on Alphaviral Vector Competence
5.3. Co-Infection with Other Viruses
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weaver, S.C.; Barrett, A.D.T. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Speers, D.J.; Mackenzie, J.S. The viruses of Australia and the risk to tourists. Travel Med. Infect. Dis. 2011, 9, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Thiberville, S.-D.; Moyen, N.; Dupuis-Maguiraga, L.; Nougairede, A.; Gould, E.A.; Roques, P.; de Lamballerie, X. Chikungunya fever: Epidemiology, clinical syndrome, pathogenesis and therapy. Antivir. Res. 2013, 99, 345–370. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Moutailler, S.; Pages, F.; Jarjaval, F.; Failloux, A.B. Introduction of Aedes albopictus in Gabon: What consequences for dengue and chikungunya transmission? Trop. Med. Int. Health 2008, 13, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Staples, J.E.; Breiman, R.F.; Powers, A.M. Chikungunya fever: An epidemiological review of a re-emerging infectious disease. Clin. Infect. Dis. 2009, 49, 942–948. [Google Scholar] [CrossRef] [PubMed]
- WHO Media Centre: Japanese Encephalitis. Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs386/en/ (accessed on 8 October 2017).
- Naish, S.; Hu, W.; Mengersen, K.; Tong, S. Spatio-temporal patterns of Barmah Forest virus disease in Queensland, Australia. PLoS ONE 2011, 6, e25688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harley, D.; Ritchie, S.; Bain, C.; Sleigh, A.C. Risks for Ross River virus disease in tropical Australia. Int. J. Epidemiol. 2005, 34, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Tokachil, N.; Yusoff, N.; Saaid, A.; Appandi, N.; Harun, F. Effect of water availability in opening containers of breeding site on Aedes aegypti life cycle. AIP Conf. Proc. 2017, 1905, 050043. [Google Scholar]
- Ng, L.C.; Lam, S.; Teo, D. Epidemiology of dengue and chikungunya viruses and their potential impact on the blood supply. ISBT Sci. Ser. 2009, 4, 357–367. [Google Scholar] [CrossRef]
- Life Cycle. Available online: http://www.mosquito.org/life-cycle (accessed on 8 October 2017).
- Bennett, K.E.; Flick, D.; Fleming, K.H.; Jochim, R.; Beaty, B.J.; Black, W.C.T. Quantitative trait loci that control dengue-2 virus dissemination in the mosquito Aedes aegypti. Genetics 2005, 170, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Lounibos, L.P.; Kramer, L.D. Invasiveness of Aedes aegypti and Aedes albopictus and Vectorial Capacity for Chikungunya Virus. J. Infect. Dis. 2016, 214, S453–S458. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Lobo, J.M.; Christofferson, R.C.; Mores, C.N. Investigations of Koutango Virus Infectivity and Dissemination Dynamics in Aedes aegypti Mosquitoes. Environ. Health Insights 2014, 8, 9–13. [Google Scholar] [PubMed]
- Hardy, J.L.; Houk, E.J.; Kramer, L.D.; Reeves, W.C. Intrinsic Factors Affecting Vector Competence of Mosquitoes for Arboviruses. Annu. Rev. Entomol. 1983, 28, 229–262. [Google Scholar] [CrossRef] [PubMed]
- Black, W.C.T.; Bennett, K.E.; Gorrochotegui-Escalante, N.; Barillas-Mury, C.V.; Fernandez-Salas, I.; de Lourdes Munoz, M.; Farfan-Ale, J.A.; Olson, K.E.; Beaty, B.J. Flavivirus susceptibility in Aedes aegypti. Arch. Med. Res. 2002, 33, 379–388. [Google Scholar] [CrossRef]
- Richards, S.L.; Anderson, S.L.; Lord, C.C.; Smartt, C.T.; Tabachnick, W.J. Relationships between infection, dissemination, and transmission of West Nile virus RNA in Culex pipiens quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Battle, K.E.; Hay, S.I.; Barker, C.M.; Scott, T.W.; McKenzie, F.E. Ross, Macdonald, and a Theory for the Dynamics and Control of Mosquito-Transmitted Pathogens. PLoS Pathog. 2012, 8, e1002588. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Godfray, H.C.J.; Tatem, A.J.; Gething, P.W.; Cohen, J.M.; McKenzie, F.E.; Perkins, T.A.; Reiner, R.C.; Tusting, L.S.; Sinka, M.E.; et al. Vectorial capacity and vector control: Reconsidering sensitivity to parameters for malaria elimination. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Ciota, A.T. Dissecting vectorial capacity for mosquito-borne viruses. Curr. Opin. Virol. 2015, 15, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, C.; Behura, S.K.; Debruyn, B.; Lovin, D.D.; Harker, B.W.; Gomez-Machorro, C.; Mori, A.; Romero-Severson, J.; Severson, D.W. Comparative expression profiles of midgut genes in dengue virus refractory and susceptible Aedes aegypti across critical period for virus infection. PLoS ONE 2012, 7, e47350. [Google Scholar] [CrossRef] [PubMed]
- Zieler, H.; Garon, C.F.; Fischer, E.R.; Shahabuddin, M. A tubular network associated with the brush-border surface of the Aedes aegypti midgut: Implications for pathogen transmission by mosquitoes. J. Exp. Biol. 2000, 203, 1599–1611. [Google Scholar] [PubMed]
- Dong, S.; Balaraman, V.; Kantor, A.M.; Lin, J.; Grant, D.G.; Held, N.L.; Franz, A.W.E. Chikungunya virus dissemination from the midgut of Aedes aegypti is associated with temporal basal lamina degradation during bloodmeal digestion. PLoS Negl. Trop. Dis. 2017, 11, e0005976. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.W.E.; Kantor, A.M.; Passarelli, A.L.; Clem, R.J. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanley, K.A.; Nelson, J.T.; Schirtzinger, E.E.; Whitehead, S.S.; Hanson, C.T. Superior infectivity for mosquito vectors contributes to competitive displacement among strains of dengue virus. BMC Ecol. 2008, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.I.; Richardson, J.H.; Sanchez-Vargas, I.; Olson, K.E.; Beaty, B.J. Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- King, J.G.; Hillyer, J.F. Infection-Induced Interaction between the Mosquito Circulatory and Immune Systems. PLoS Pathog. 2012, 8, e1003058. [Google Scholar] [CrossRef] [PubMed]
- Forrester, N.L.; Coffey, L.L.; Weaver, S.C. Arboviral bottlenecks and challenges to maintaining diversity and fitness during mosquito transmission. Viruses 2014, 6, 3991–4004. [Google Scholar] [CrossRef] [PubMed]
- Carrington, L.B.; Simmons, C.P. Human to mosquito transmission of dengue viruses. Front. Immunol. 2014, 5, 290. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Ramirez, J.L.; Dimopoulos, G. The Aedes aegypti Toll Pathway Controls Dengue Virus Infection. PLoS Pathog. 2008, 4, e1000098. [Google Scholar] [CrossRef] [PubMed]
- Romoser, W.S.; Turell, M.J.; Lerdthusnee, K.; Neira, M.; Dohm, D.; Ludwig, G.; Wasieloski, L. Pathogenesis of Rift Valley fever virus in mosquitoes—Tracheal conduits & the basal lamina as an extra-cellular barrier. Arch. Virol. Suppl. 2005, 19, 89–100. [Google Scholar]
- Tchankouo-Nguetcheu, S.; Bourguet, E.; Lenormand, P.; Rousselle, J.-C.; Namane, A.; Choumet, V. Infection by chikungunya virus modulates the expression of several proteins in Aedes aegypti salivary glands. Parasites Vectors 2012, 5, 264. [Google Scholar] [CrossRef] [PubMed]
- Ciano, K.; Saredy, J.; Bowers, D. Heparan Sulfate Proteoglycan: An Arbovirus Attachment Factor Integral to Mosquito Salivary Gland Ducts. Viruses 2014, 6, 5182–5197. [Google Scholar] [CrossRef] [PubMed]
- Dubrulle, M.; Mousson, L.; Moutailler, S.; Vazeille, M.; Failloux, A.B. Chikungunya virus and Aedes mosquitoes: Saliva is infectious as soon as two days after oral infection. PLoS ONE 2009, 4, e5895. [Google Scholar] [CrossRef] [PubMed]
- Pingen, M.; Bryden, S.R.; Pondeville, E.; Schnettler, E.; Kohl, A.; Merits, A.; Fazakerley, J.K.; Graham, G.J.; McKimmie, C.S. Host Inflammatory Response to Mosquito Bites Enhances the Severity of Arbovirus Infection. Immunity 2016, 44, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Pingen, M.; Schmid, M.A.; Harris, E.; McKimmie, C.S. Mosquito Biting Modulates Skin Response to Virus Infection. Trends Parasitol. 2017, 33, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Johansson, M.A. The incubation periods of Dengue viruses. PLoS ONE 2012, 7, e50972. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Kantor, A.M.; Lin, J.; Passarelli, A.L.; Clem, R.J.; Franz, A.W.E. Infection pattern and transmission potential of chikungunya virus in two New World laboratory-adapted Aedes aegypti strains. Sci. Rep. 2016, 6, 60185. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.A.; Turell, M.J. Brugia malayi microfilariae transport alphaviruses across the mosquito midgut. PLoS ONE 2017, 12, e0172309. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.A.; Turell, M.J. Dual host infections: Enhanced infectivity of eastern equine encephalitis virus to Aedes mosquitoes mediated by Brugia microfilariae. Am. J. Trop. Med. Hyg. 1996, 54, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.A.; Trpis, M.; Turell, M.J. Brugia malayi Microfilariae (Nematoda: Filaridae) Enhance the Infectivity of Venezuelan Equine Encephalitis Virus to Aedes Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 1999, 36, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.A.; Vythilingam, I.; Lim, Y.A.L.; Zabari, N.Z.A.M.; Lee, H.L. Detection of Wolbachia in Aedes albopictus and Their Effects on Chikungunya Virus. Am. J. Trop. Med. Hyg. 2017, 96, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Wong, P.J.; Li, M.I.; Yang, H.; Ng, L.C.; O’Neill, S.L. wMel limits zika and chikungunya virus infection in a Singapore Wolbachia-introgressed Ae. aegypti strain, wMel-Sg. PLoS Negl. Trop. Dis. 2017, 11, e0005496. [Google Scholar] [CrossRef] [PubMed]
- Amuzu, H.E.; McGraw, E.A. Wolbachia-Based Dengue Virus Inhibition Is Not Tissue-Specific in Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0005145. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Walker, E.C.; Yepes, A.U.; Velez, I.D.; Christensen, B.M.; Osorio, J.E. The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004677. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.J.; Isern, S.; Michael, S.F.; Corley, R.B.; Connor, J.H.; Frydman, H.M. Variable Inhibition of Zika Virus Replication by Different Wolbachia Strains in Mosquito Cell Cultures. J. Virol. 2017, 91, e00339-17. [Google Scholar] [CrossRef] [PubMed]
- Rainey, S.M.; Martinez, J.; McFarlane, M.; Juneja, P.; Sarkies, P.; Lulla, A.; Schnettler, E.; Varjak, M.; Merits, A.; Miska, E.A.; et al. Wolbachia Blocks Viral Genome Replication Early in Infection without a Transcriptional Response by the Endosymbiont or Host Small RNA Pathways. PLoS Pathog. 2016, 12, e1005536. [Google Scholar] [CrossRef] [PubMed]
- Mousson, L.; Zouache, K.; Arias-Goeta, C.; Raquin, V.; Mavingui, P.; Failloux, A.-B. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl. Trop. Dis. 2012, 6, e1989. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Bian, G.; Pan, X.; Xi, Z. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl. Trop. Dis. 2012, 6, e1754. [Google Scholar] [CrossRef] [PubMed]
- Terradas, G.; McGraw, E.A. Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Curr. Opin. Insect Sci. 2017, 22, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Audsley, M.D.; Ye, Y.H.; McGraw, E.A. The microbiome composition of Aedes aegypti is not critical for Wolbachia-mediated inhibition of dengue virus. PLoS Negl. Trop. Dis. 2017, 11, e0005426. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Ramirez, J.L.; Rooney, A.P.; Kim, C.-H. Comparative analysis of gut microbiota of mosquito communities in central Illinois. PLoS Negl. Trop. Dis. 2017, 11, e0005377. [Google Scholar] [CrossRef] [PubMed]
- Angleró-Rodríguez, Y.I.; Talyuli, O.A.; Blumberg, B.J.; Kang, S.; Demby, C.; Shields, A.; Carlson, J.; Jupatanakul, N.; Dimopoulos, G. An Aedes aegypti-associated fungus increases susceptibility to dengue virus by modulating gut trypsin activity. eLife 2017, 6, e28844. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.L.; Short, S.M.; Bahia, A.C.; Saraiva, R.G.; Dong, Y.; Kang, S.; Tripathi, A.; Mlambo, G.; Dimopoulos, G. Chromobacterium Csp_P Reduces Malaria and Dengue Infection in Vector Mosquitoes and Has Entomopathogenic and In Vitro Anti-pathogen Activities. PLoS Pathog. 2014, 10, e1004398. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T.; Kramer, L.D. Insights into arbovirus evolution and adaptation from experimental studies. Viruses 2010, 2, 2594–2617. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Rourke, M.F.; Holmes, E.C.; Aaskov, J.G. Persistence of multiple genetic lineages within intrahost populations of Ross River virus. J. Virol. 2011, 85, 5674–5678. [Google Scholar] [CrossRef] [PubMed]
- Van Slyke, G.A.; Ciota, A.T.; Willsey, G.G.; Jaeger, J.; Shi, P.Y.; Kramer, L.D. Point mutations in the West Nile virus (Flaviviridae; Flavivirus) RNA-dependent RNA polymerase alter viral fitness in a host-dependent manner in vitro and in vivo. Virology 2012, 427, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Vigerust, D.J.; Shepherd, V.L. Virus glycosylation: Role in virulence and immune interactions. Trends Microbiol. 2007, 15, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Davey, N.E.; Travé, G.; Gibson, T.J. How viruses hijack cell regulation. Trends Biochem. Sci. 2011, 36, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Davey, N.E. SLiMDisc: Short, linear motif discovery, correcting for common evolutionary descent. Nucleic Acids Res. 2006, 34, 3546–3554. [Google Scholar] [CrossRef] [PubMed]
- Davey, N.E.; Shields, D.C.; Edwards, R.J. Masking residues using context-specific evolutionary conservation significantly improves short linear motif discovery. Bioinformatics 2009, 25, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Diella, F. Understanding eukaryotic linear motifs and their role in cell signaling and regulation. Front. Biosci. 2008, 13, 6580–6603. [Google Scholar] [CrossRef] [PubMed]
- Neduva, V.; Russell, R.B. Linear motifs: Evolutionary interaction switches. FEBS Lett. 2005, 579, 3342–3345. [Google Scholar] [CrossRef] [PubMed]
- De Lamballerie, X.; Leroy, E.; Charrel, R.N.; Ttsetsarkin, K.; Higgs, S.; Gould, E.A. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: A sign of things to come? Virol. J. 2008, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Schuffenecker, I.; Iteman, I.; Michault, A.; Murri, S.; Frangeul, L.; Vaney, M.-C.; Lavenir, R.; Pardigon, N.; Reynes, J.-M.; Pettinelli, F.; et al. Genome Microevolution of Chikungunya Viruses Causing the Indian Ocean Outbreak. PLoS Med. 2006, 3, e263. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A Single Mutation in Chikungunya Virus Affects Vector Specificity and Epidemic Potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Moutailler, S.; Coudrier, D.; Rousseaux, C.; Khun, H.; Huerre, M.; Thiria, J.; Dehecq, J.-S.; Fontenille, D.; Schuffenecker, I.; et al. Two Chikungunya Isolates from the Outbreak of La Reunion (Indian Ocean) Exhibit Different Patterns of Infection in the Mosquito, Aedes albopictus. PLoS ONE 2007, 2, e1168. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, O.; Albert, M.L. Biology and pathogenesis of chikungunya virus. Nat. Rev. Microbiol. 2010, 8, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Vega-Rúa, A.; Zouache, K.; Girod, R.; Failloux, A.-B.; Lourenço-de-Oliveira, R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. J. Virol. 2014, 88, 6294–6306. [Google Scholar] [CrossRef] [PubMed]
- Arias-Goeta, C.; Mousson, L.; Rougeon, F.; Failloux, A.B. Dissemination and transmission of the E1-226V variant of chikungunya virus in Aedes albopictus are controlled at the midgut barrier level. PLoS ONE 2013, 8, e57548. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Javelle, E.; Oliver, M.; Leparc Goffart, I.; Marimoutou, C. Chikungunya virus infection. Curr. Infect. Dis. Rep. 2011, 13, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; McGee, C.E.; Higgs, S. Chikungunya virus adaptation to Aedes albopictus mosquitoes does not correlate with acquisition of cholesterol dependence or decreased pH threshold for fusion reaction. Virol. J. 2011, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Chen, R.; Leal, G.; Forrester, N.; Higgs, S.; Huang, J.; Weaver, S.C. Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes. Proc. Natl. Acad. Sci. USA. 2011, 108, 7872–7877. [Google Scholar] [CrossRef] [PubMed]
- Coffey, L.; Failloux, A.-B.; Weaver, S. Chikungunya Virus–Vector Interactions. Viruses 2014, 6, 4628–4663. [Google Scholar] [CrossRef] [PubMed]
- Perera-Lecoin, M.; Luplertlop, N.; Surasombatpattana, P.; Liégeois, F.; Hamel, R.; Thongrungkiat, S.; Vargas, R.E.M.; Yssel, H.; Missé, D. Dengue and Chikungunya Coinfection—The Emergence of an Underestimated Threat. In Current Topics in Chikungunya; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Niyas, K.P.; Abraham, R.; Unnikrishnan, R.; Mathew, T.; Nair, S.; Manakkadan, A.; Issac, A.; Sreekumar, E. Molecular characterization of Chikungunya virus isolates from clinical samples and adult Aedes albopictus mosquitoes emerged from larvae from Kerala, South India. Virol. J. 2010, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Weaver, S.C. Sequential Adaptive Mutations Enhance Efficient Vector Switching by Chikungunya Virus and Its Epidemic Emergence. PLoS Pathog. 2011, 7, e1002412. [Google Scholar] [CrossRef] [PubMed]
- Stapleford, K.A.; Coffey, L.L.; Lay, S.; Bordería, A.V.; Duong, V.; Isakov, O.; Rozen-Gagnon, K.; Arias-Goeta, C.; Blanc, H.; Beaucourt, S.; et al. Emergence and Transmission of Arbovirus Evolutionary Intermediates with Epidemic Potential. Cell Host Microbe 2014, 15, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Moutailler, S.; Barré, H.; Vazeille, M.; Failloux, A.-B. Recently introduced Aedes albopictus in Corsica is competent to Chikungunya virus and in a lesser extent to dengue virus. Trop. Med. Int. Health 2009, 14, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Rohani, A.; Potiwat, R.; Zamree, I.; Lee, H.L. Refractoriness of Aedes aegypti (Linnaeus) to dual infection with dengue and chikungunya virus. Southeast Asian J. Trop. Med. Public Health 2009, 40, 443–448. [Google Scholar] [PubMed]
- Boyd, A.M.; Kay, B.H. Experimental Infection and Transmission of Barmah Forest Virus by Aedes vigilax (Diptera: Culicidae). J. Med. Entomol. 1999, 36, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.M.; Kay, B.H. Vector Competence of Aedes notoscriptus (Diptera: Culicidae) for Ross River Virus in Queensland, Australia. J. Med. Entomol. 1998, 35, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.M.; Kay, B.H. Vector Competence of Aedes notoscriptus (Diptera: Culicidae) for Barmah Forest Virus and of Aedes aegypti (Diptera: Culicidae) for Dengue 1–4 Viruses in Queensland, Australia. J. Med. Entomol. 1999, 36, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Saxton-Shaw, K.D.; Ledermann, J.P.; Borland, E.M.; Stovall, J.L.; Mossel, E.C.; Singh, A.J.; Wilusz, J.; Powers, A.M. O’nyong nyong Virus Molecular Determinants of Unique Vector Specificity Reside in Non-Structural Protein. PLoS Negl. Trop. Dis. 2013, 7, e1931. [Google Scholar] [CrossRef] [PubMed]
- Kääriäinen, L.; Ahola, T. Functions of alphavirus nonstructural proteins in RNA replication. In Progress in Nucleic Acid Research and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 71, pp. 187–222. [Google Scholar]
- Tuittila, M. Amino acid mutations in the replicase protein nsP3 of Semliki Forest virus cumulatively affect neurovirulence. J. Gen. Virol. 2003, 84, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Aaskov, J.; Jones, A.; Choi, W.; Lowry, K.; Stewart, E. Lineage replacement accompanying duplication and rapid fixation of an RNA element in the nsP3 gene in a species of alphavirus. Virology 2011, 410, 353–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lastarza, M.W.; Grakoui, A.; Rice, C.M. Deletion and Duplication Mutations in the C-Terminal Nonconserved Region of Sindbis Virus nsP3: Effects on Phosphorylation and on Virus Replication in Vertebrate and Invertebrate Cells. Virology 1994, 202, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Neuvonen, M.; Kazlauskas, A.; Martikainen, M.; Hinkkanen, A.; Ahola, T.; Saksela, K. SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication. PLoS Pathog. 2011, 7, e1002383. [Google Scholar] [CrossRef] [PubMed]
- Adelman, Z.N.; Anderson, M.A.E.; Wiley, M.R.; Murreddu, M.G.; Samuel, G.H.; Morazzani, E.M.; Myles, K.M. Cooler temperatures destabilize RNA interference and increase susceptibility of disease vector mosquitoes to viral infection. PLoS Negl. Trop. Dis. 2013, 7, e2239. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.C. The Effect of Various Temperatures in Modifying the Extrinsic Incubation Period of the Yellow Fever Virus in Aëdes aegypti. Am. J. Epidemiol. 1932, 16, 163–176. [Google Scholar] [CrossRef]
- Hurlbut, H.S. The Effect of Environmental Temperature Upon the Transmission of St. Louis Encephalitis Virusby Culex pipiens Quinquefasciatus. J. Med. Entomol. 1973, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, W.J. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J. Exp. Biol. 2010, 213, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, C.J.; Reiskind, M.H.; Pesko, K.N.; Greene, K.E.; Lounibos, L.P. Larval environmental temperature and the susceptibility of Aedes albopictus Skuse (Diptera: Culicidae) to Chikungunya virus. Vector Borne Zoonotic Dis. 2010, 10, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Turell, M.J. Effect of environmental temperature on the vector competence of Aedes taeniorhynchus for Rift Valley fever and Venezuelan equine encephalitis viruses. Am. J. Trop. Med. Hyg. 1993, 49, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Kay, B.H.; Jennings, C.D. Enhancement or Modulation of the Vector Competence of Ochlerotatus vigilax (Diptera: Culicidae) for Ross River Virus by Temperature. J. Med. Entomol. 2002, 39, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Hardy, J.L.; Presser, S.B. Effect of temperature of extrinsic incubation on the vector competence of Culex tarsalis for western equine encephalomyelitis virus. Am. J. Trop. Med. Hyg. 1983, 32, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Hardy, J.L.; Presser, S.B. Characterization of Modulation of Western Equine Encephalomyelitis Virus by Culex tarsalis (Diptera: Culicidae) Maintained at 32 °C Following Parenteral Infection. J. Med. Entomol. 1998, 35, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.L.; Turell, M.J.; Shroyer, D.A.; Rosen, L.; Presser, S.B.; Kramer, L.D. Effect of Rearing Temperature on Transovarial Transmission of St. Louis Encephalitis Virus in Mosquitoes. Am. J. Trop. Med. Hyg. 1980, 29, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Blackshear, M., Jr.; Montgomery, A. Temperature and density-dependent effects of larval environment on Aedes aegypti competence for an alphavirus. J. Vector Ecol. 2012, 37, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.D.; Chin, P.; Cane, R.P.; Kauffman, E.B.; Mackereth, G. Vector competence of New Zealand mosquitoes for selected arboviruses. Am. J. Trop. Med. Hyg. 2011, 85, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.C.; Hardy, J.L.; Reisen, W.K.; Milby, M.M. Potential Effect of Global Warming on Mosquito-Borne Arboviruses. J. Med. Entomol. 1994, 31, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Karpf, A.R.; Lenches, E.; Strauss, E.G.; Strauss, J.H.; Brown, D.T. Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. J. Virol. 1997, 71, 7119–7123. [Google Scholar] [PubMed]
- Stollar, V.; Shenk, T.E. Homologous viral interference in Aedes albopictus cultures chronically infected with Sindbis virus. J. Virol. 1973, 11, 592–595. [Google Scholar] [PubMed]
- Eaton, B.T. Heterologous interference in Aedes albopictus cells infected with alphaviruses. J. Virol. 1979, 30, 45–55. [Google Scholar] [PubMed]
- Igarashi, A.; Koo, R.; Stollar, V. Evolution and properties of Aedes albopictus cell cultures persistently infected with sindbis virus. Virology 1977, 82, 69–83. [Google Scholar] [CrossRef]
- Miller, M.L.; Brown, D.T. The distribution of Sindbis virus proteins in mosquito cells as determined by immunofluorescence and immunoelectron microscopy. J. Gen. Virol. 1993, 74, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Sarver, N.; Stollar, V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology 1977, 80, 390–400. [Google Scholar] [CrossRef]
- Tooker, P.; Kennedy, S.I. Semliki Forest virus multiplication in clones of Aedes albopictus cells. J. Virol. 1981, 37, 589–600. [Google Scholar] [PubMed]
- Rückert, C.; Weger-Lucarelli, J.; Garcia-Luna, S.M.; Young, M.C.; Byas, A.D.; Murrieta, R.A.; Fauver, J.R.; Ebel, G.D. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat. Commun. 2017, 8, 15412. [Google Scholar] [CrossRef] [PubMed]
- Göertz, G.P.; Vogels, C.B.F.; Geertsema, C.; Koenraadt, C.J.M.; Pijlman, G.P. Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti. PLoS Negl. Trop. Dis. 2017, 11, e0005654. [Google Scholar] [CrossRef] [PubMed]
- Vazeille, M.; Mousson, L.; Martin, E.; Failloux, A.-B. Orally Co-Infected Aedes albopictus from La Reunion Island, Indian Ocean, Can Deliver Both Dengue and Chikungunya Infectious Viral Particles in Their Saliva. PLoS Negl. Trop. Dis. 2010, 4, e706. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.M.; Walker, L.R.; Abdel-Raouf, U.M.; Desouky, S.A.; Montasser, A.K.M.; Akula, S.M. Beyond RGD: Virus interactions with integrins. Arch. Virol. 2015, 160, 2669–2681. [Google Scholar] [CrossRef] [PubMed]
- Mathur, K.; Anand, A.; Dubey, S.K.; Sanan-Mishra, N.; Bhatnagar, R.K.; Sunil, S. Analysis of chikungunya virus proteins reveals that non-structural proteins nsP2 and nsP3 exhibit RNA interference (RNAi) suppressor activity. Sci. Rep. 2016, 6, 632. [Google Scholar] [CrossRef] [PubMed]
- Nuckols, J.T.; Huang, Y.J.S.; Higgs, S.; Miller, A.L.; Pyles, R.B.; Spratt, H.M.; Horne, K.M.; Vanlandingham, D.L. Evaluation of Simultaneous Transmission of Chikungunya Virus and Dengue Virus Type 2 in Infected Aedes aegypti and Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Potiwat, R.; Komalamisra, N.; Thavara, U.; Tawatsin, A.; Siriyasatien, P. Competitive suppression between chikungunya and dengue virus in Aedes albopictus c6/36 cell line. Southeast Asian J. Trop. Med. Public Health 2011, 42, 1388–1394. [Google Scholar] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, E.X.Y.; Lee, W.S.; Madzokere, E.T.; Herrero, L.J. Mosquitoes as Suitable Vectors for Alphaviruses. Viruses 2018, 10, 84. https://doi.org/10.3390/v10020084
Lim EXY, Lee WS, Madzokere ET, Herrero LJ. Mosquitoes as Suitable Vectors for Alphaviruses. Viruses. 2018; 10(2):84. https://doi.org/10.3390/v10020084
Chicago/Turabian StyleLim, Elisa X. Y., Wai Suet Lee, Eugene T. Madzokere, and Lara J. Herrero. 2018. "Mosquitoes as Suitable Vectors for Alphaviruses" Viruses 10, no. 2: 84. https://doi.org/10.3390/v10020084





