Identification of Ellagic Acid from Plant Rhodiola rosea L. as an Anti-Ebola Virus Entry Inhibitor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Generation of Pseudovirions
2.3. Preparation of TCM Extracts
2.4. High-Throughput Screen
2.5. Time-of-Addition Experiment
2.6. Infectious Virus Assays
3. Results
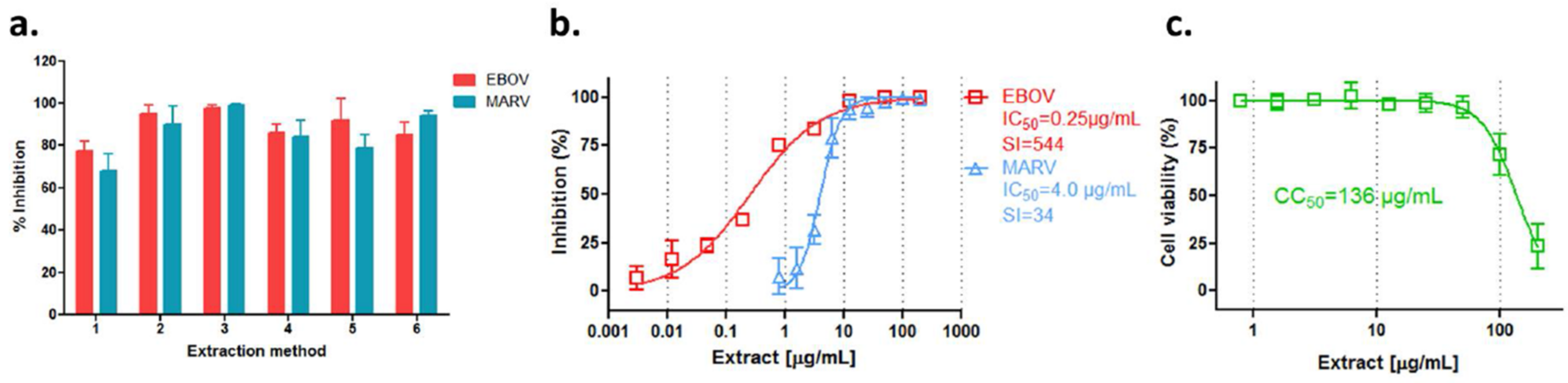
3.1. Extracts of Rhodiola rosea Specifically Block Entry of Ebola and Marburg Viruses
3.2. Anti-Filovirus Activities of the R. rosea Extracts by Different Extraction Methods
3.3. Ellagic Acid and Gallic Acid of R. rosea Block Ebola-GP Mediated Viral Entry
3.4. R. rosea Extract, Ellagic Acid and Gallic Acid Inhibit Infectious EBOV Infection
3.5. R. rosea Blocks EBOV Infection at a Late Stage of Virus Entry
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kuhn, J.H.; Becker, S.; Ebihara, H.; Geisbert, T.W.; Johnson, K.M.; Kawaoka, Y.; Lipkin, W.I.; Negredo, A.I.; Netesov, S.V.; Nichol, S.T.; et al. Proposal for a revised taxonomy of the family filoviridae: Classification, names of taxa and viruses, and virus abbreviations. Arch. Virol. 2010, 155, 2083–2103. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.M.; Cheng, H.; Lee, C.; Du, R.; Han, J.; Perez, J.; Peet, N.; Manicassamy, B.; Rong, L. Development of potential small molecule therapeutics for treatment of ebola virus. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Peng, T. Traditional Chinese herbal medicine as a source of molecules with antiviral activity. Antivir. Res. 2013, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, H.; Yan, H.; Wang, P.Z.; Rong, R.; Zhang, Y.Y.; Zhang, C.B.; Du, R.K.; Rong, L.J. A cell-based high-throughput protocol to screen entry inhibitors of highly pathogenic viruses with traditional Chinese medicines. J. Med. Virol. 2017, 89, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.H.; Chandran, K. Filovirus entry into cells—New insights. Curr. Opin. Virol. 2012, 2, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, B.; Wang, J.; Rumschlag, E.; Tymen, S.; Volchkova, V.; Volchkov, V.; Rong, L. Characterization of Marburg virus glycoprotein in viral entry. Virology 2007, 358, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Manicassamy, B.; Wang, J.; Jiang, H.; Rong, L. Comprehensive analysis of ebola virus GP1 in viral entry. J. Virol. 2005, 79, 4793–4805. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, A.; Wang, M.; Cheng, H.; Lear-Rooney, C.M.; Koning, K.; Rumschlag-Booms, E.; Varhegyi, E.; Olinger, G.; Rong, L. Role of EXT1 and glycosaminoglycans in the early stage of filovirus entry. J. Virol. 2015, 89, 5441–5449. [Google Scholar] [CrossRef] [PubMed]
- Salvador, B.; Sexton, N.R.; Carrion, R.; Nunneley, J.; Patterson, J.L.; Steffen, I.; Lu, K.; Muench, M.O.; Lembo, D.; Simmons, G. Filoviruses utilize glycosaminoglycans for their attachment to target cells. J. Virol. 2013, 87, 3295–3304. [Google Scholar] [CrossRef] [PubMed]
- Takada, A.; Fujioka, K.; Tsuiji, M.; Morikawa, A.; Higashi, N.; Ebihara, H.; Kobasa, D.; Feldmann, H.; Irimura, T.; Kawaoka, Y. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J. Virol. 2004, 78, 2943–2947. [Google Scholar] [CrossRef] [PubMed]
- Nanbo, A.; Imai, M.; Watanabe, S.; Noda, T.; Takahashi, K.; Neumann, G.; Halfmann, P.; Kawaoka, Y. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 2010, 6, e1001121. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.F.; Kolokoltsov, A.A.; Albrecht, T.; Davey, R.A. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010, 6, e1001110. [Google Scholar] [CrossRef] [PubMed]
- Brecher, M.; Schornberg, K.L.; Delos, S.E.; Fusco, M.L.; Saphire, E.O.; White, J.M. Cathepsin cleavage potentiates the ebola virus glycoprotein to undergo a subsequent fusion-relevant conformational change. J. Virol. 2012, 86, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Chandran, K.; Sullivan, N.J.; Felbor, U.; Whelan, S.P.; Cunningham, J.M. Endosomal proteolysis of the ebola virus glycoprotein is necessary for infection. Science 2005, 308, 1643–1645. [Google Scholar] [CrossRef] [PubMed]
- Schornberg, K.; Matsuyama, S.; Kabsch, K.; Delos, S.; Bouton, A.; White, J. Role of endosomal cathepsins in entry mediated by the ebola virus glycoprotein. J. Virol. 2006, 80, 4174–4178. [Google Scholar] [CrossRef] [PubMed]
- Carette, J.E.; Raaben, M.; Wong, A.C.; Herbert, A.S.; Obernosterer, G.; Mulherkar, N.; Kuehne, A.I.; Kranzusch, P.J.; Griffin, A.M.; Ruthel, G.; et al. Ebola virus entry requires the cholesterol transporter niemann-pick C1. Nature 2011, 477, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Cote, M.; Misasi, J.; Ren, T.; Bruchez, A.; Lee, K.; Filone, C.M.; Hensley, L.; Li, Q.; Ory, D.; Chandran, K.; et al. Small molecule inhibitors reveal niemann-pick C1 is essential for ebola virus infection. Nature 2011, 477, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Qian, H.W.; Zhou, X.H.; Wu, J.P.; Wan, T.; Cao, P.P.; Huang, W.Y.; Zhao, X.; Wang, X.D.; Wang, P.Y.; et al. Structural insights into the niemann-pick C1 (NPC1)-mediated cholesterol transfer and ebola infection. Cell 2016, 165, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, Y.; Song, J.; Qi, J.X.; Lu, G.W.; Yan, J.H.; Gao, G.F. Ebola viral glycoprotein bound to its endosomal receptor niemann-pick C1. Cell 2016, 164, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Johansen, L.M.; Brannan, J.M.; Delos, S.E.; Shoemaker, C.J.; Stossel, A.; Lear, C.; Hoffstrom, B.G.; DeWald, L.E.; Schornberg, K.L.; Scully, C.; et al. FDA-approved selective estrogen receptor modulators inhibit ebola virus infection. Sci. Transl. Med. 2013, 5, 190ra79. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Lear-Rooney, C.M.; Johansen, L.; Varhegyi, E.; Chen, Z.W.; Olinger, G.G.; Rong, L.J. Inhibition of ebola and marburg virus entry by g protein-coupled receptor antagonists. J. Virol. 2015, 89, 9932–9938. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Schafer, A.; Soloveva, V.; Gharaibeh, D.; Kenny, T.; Retterer, C.; Zamani, R.; Bavari, S.; Peet, N.P.; Rong, L.J. Identification of a coumarin-based antihistamine-like small molecule as an anti-filoviral entry inhibitor. Antivir. Res. 2017, 145, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Mills, D.M.; Mitchell, D.; Ndungo, E.; Williams, J.D.; Herbert, A.S.; Dye, J.M.; Moir, D.T.; Chandran, K.; Patterson, J.L.; et al. Novel small molecule entry inhibitors of ebola virus. J. Infect. Dis. 2015, 212 (Suppl. 2), S425–S434. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Canard, B.; Decroly, E. Filovirus proteins for antiviral drug discovery: Structure/function bases of the replication cycle. Antivir. Res. 2017, 141, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Anantpadma, M.; Kouznetsova, J.; Wang, H.; Huang, R.; Kolokoltsov, A.; Guha, R.; Lindstrom, A.R.; Shtanko, O.; Simeonov, A.; Maloney, D.J.; et al. Large-scale screening and identification of novel ebola virus and marburg virus entry inhibitors. Antimicrob. Agents Chemother. 2016, 60, 4471–4481. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, H.; Theriault, S.; Neumann, G.; Alimonti, J.B.; Geisbert, J.B.; Hensley, L.E.; Groseth, A.; Jones, S.M.; Geisbert, T.W.; Kawaoka, Y.; et al. In vitro and in vivo characterization of recombinant ebola viruses expressing enhanced green fluorescent protein. J. Infect. Dis. 2007, 196, S313–S322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ren, J.; Harlos, K.; Jones, D.M.; Zeltina, A.; Bowden, T.A.; Padilla-Parra, S.; Fry, E.E.; Stuart, D.I. Toremifene interacts with and destabilizes the ebola virus glycoprotein. Nature 2016, 535, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Walldorf, J.A.; Cloessner, E.A.; Hyde, T.B.; MacNeil, A.; Taskforce, C.D.C.E.E.V. Considerations for use of ebola vaccine during an emergency response. Vaccine 2017. [Google Scholar] [CrossRef] [PubMed]
- Kaletsky, R.L.; Simmons, G.; Bates, P. Proteolysis of the ebola virus glycoproteins enhances virus binding and infectivity. J. Virol. 2007, 81, 13378–13384. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, L.G.; Rollin, P.E. Release of cellular proteases into the acidic extracellular milieu exacerbates ebola virus-induced cell damage. Virology 2007, 358, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gnirss, K.; Kuhl, A.; Karsten, C.; Glowacka, I.; Bertram, S.; Kaup, F.; Hofmann, H.; Pohlmann, S. Cathepsins B and L activate ebola but not marburg virus glycoproteins for efficient entry into cell lines and macrophages independent of tmprss2 expression. Virology 2012, 424, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Wang, T.; Kaletsky, R.L.; Myers, M.C.; Purvis, J.E.; Jing, H.; Huryn, D.M.; Greenbaum, D.C.; Smith, A.B., 3rd; Bates, P.; et al. A small-molecule oxocarbazate inhibitor of human cathepsin l blocks severe acute respiratory syndrome and ebola pseudotype virus infection into human embryonic kidney 293T cells. Mol. Pharmacol. 2010, 78, 319–324. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, W.A.; Schulze, C.J.; Herbert, A.S.; Krause, T.B.; Wirchnianski, A.A.; Dye, J.M.; Chandran, K.; Bogyo, M. Cysteine cathepsin inhibitors as anti-ebola agents. ACS Infect. Dis. 2016, 2, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Nyakatura, E.K.; Frei, J.C.; Lai, J.R. Chemical and structural aspects of ebola virus entry inhibitors. ACS Infect. Dis. 2015, 1, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Vedantham, P.; Lu, K.; Agudelo, J.; Carrion, R., Jr.; Nunneley, J.W.; Barnard, D.; Pohlmann, S.; McKerrow, J.H.; Renslo, A.R.; et al. Protease inhibitors targeting coronavirus and filovirus entry. Antivir. Res. 2015, 116, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Hert, J.; Irwin, J.J.; Laggner, C.; Keiser, M.J.; Shoichet, B.K. Quantifying biogenic bias in screening libraries. Nat. Chem. Biol. 2009, 5, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, H.; Ratia, K.; Varhegyi, E.; Hendrickson, W.G.; Li, J.; Rong, L.J. A comparative high-throughput screening protocol to identify entry inhibitors of enveloped viruses. J. Biomol. Screen. 2014, 19, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ao, Z.; Bello, A.; Ran, X.; Liu, S.; Wigle, J.; Kobinger, G.; Yao, X. Characterization of the inhibitory effect of an extract of prunella vulgaris on ebola virus glycoprotein (GP)-mediated virus entry and infection. Antivir. Res. 2016, 127, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Kratz, J.M.; Andrighetti-Frohner, C.R.; Kolling, D.J.; Leal, P.C.; Cirne-Santos, C.C.; Yunes, R.A.; Nunes, R.J.; Trybala, E.; Bergstrom, T.; Frugulhetti, I.C.P.P.; et al. Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate. Mem. Inst. Oswaldo Cruz. 2008, 103, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Song, J.H.; Bhatt, L.R.; Baek, S.H. Anti-human rhinovirus activity of gallic acid possessing antioxidant capacity. Phytother. Res. 2010, 24, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, Y.; Kolokoltsov, A.A.; Chen, C.C.; Tidwell, M.W.; Bauta, W.E.; Klugbauer, N.; Grimm, C.; Wahl-Schott, C.; Biel, M.; Davey, R.A. Ebola virus. Two-pore channels control ebola virus host cell entry and are drug targets for disease treatment. Science 2015, 347, 995–998. [Google Scholar] [CrossRef] [PubMed]


| No. | Extraction Method |
|---|---|
| 1 | water decoction followed by 70% ethanol precipitation |
| 2 | water extraction |
| 3 | 70% ethanol ultrasonic extraction |
| 4 | water decoction |
| 5 | 70% ethanol reflux extraction |
| 6 | water decoction followed by 60% ethanol precipitation |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Q.; Du, R.; Anantpadma, M.; Schafer, A.; Hou, L.; Tian, J.; Davey, R.A.; Cheng, H.; Rong, L. Identification of Ellagic Acid from Plant Rhodiola rosea L. as an Anti-Ebola Virus Entry Inhibitor. Viruses 2018, 10, 152. https://doi.org/10.3390/v10040152
Cui Q, Du R, Anantpadma M, Schafer A, Hou L, Tian J, Davey RA, Cheng H, Rong L. Identification of Ellagic Acid from Plant Rhodiola rosea L. as an Anti-Ebola Virus Entry Inhibitor. Viruses. 2018; 10(4):152. https://doi.org/10.3390/v10040152
Chicago/Turabian StyleCui, Qinghua, Ruikun Du, Manu Anantpadma, Adam Schafer, Lin Hou, Jingzhen Tian, Robert A. Davey, Han Cheng, and Lijun Rong. 2018. "Identification of Ellagic Acid from Plant Rhodiola rosea L. as an Anti-Ebola Virus Entry Inhibitor" Viruses 10, no. 4: 152. https://doi.org/10.3390/v10040152
APA StyleCui, Q., Du, R., Anantpadma, M., Schafer, A., Hou, L., Tian, J., Davey, R. A., Cheng, H., & Rong, L. (2018). Identification of Ellagic Acid from Plant Rhodiola rosea L. as an Anti-Ebola Virus Entry Inhibitor. Viruses, 10(4), 152. https://doi.org/10.3390/v10040152






