Expression of TIM-3 on Plasmacytoid Dendritic Cells as a Predictive Biomarker of Decline in HIV-1 RNA Level during ART
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Patients
2.3. Patients PBMCs
2.4. In Vitro pDC Stimulation
2.5. Flow Cytometry Analysis
2.6. Statistical Analysis
3. Results
3.1. Persistent Dysfunction of pDCs from ART-Treated HIV-1-Infected Individuals after Sustained Suppression of HIV-1 RNA
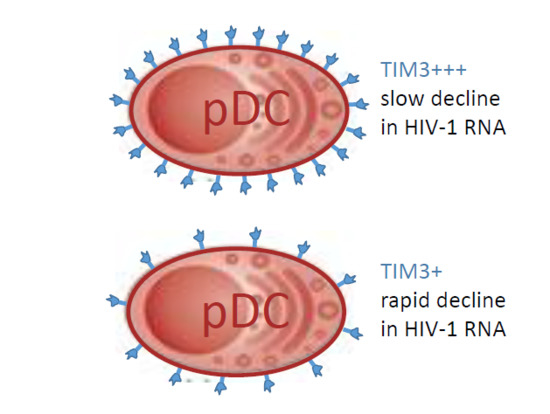
3.2. Decline in HIV-1 RNA Level after Initiation of ART Correlates with Expression of TIM-3 on pDCs
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lepelley, A.; Louis, S.; Sourisseau, M.; Law, H.K.; Pothlichet, J.; Schilte, C.; Chaperot, L.; Plumas, J.; Randall, R.E.; Si-Tahar, M.; et al. Innate sensing of HIV-infected cells. PLoS Pathog. 2011, 7, e1001284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bego, M.G.; Cong, L.; Mack, K.; Kirchhoff, F.; Cohen, E.A. Differential Control of BST2 Restriction and Plasmacytoid Dendritic Cell Antiviral Response by Antagonists Encoded by HIV-1 Group M and O Strains. J. Virol. 2016, 90, 10236–10246. [Google Scholar] [CrossRef] [PubMed]
- Leifer, C.A.; Medvedev, A.E. Molecular mechanisms of regulation of Toll-like receptor signaling. J. Leukoc. Biol. 2016, 100, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Liu, Y.J. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell 2013, 4, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, I.; Caux, C.; Hasan, U.; Bendriss-Vermare, N.; Olive, D. Impaired Toll-like receptor 7 and 9 signaling: From chronic viral infections to cancer. Trends Immunol. 2010, 31, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Heikenwalder, M.; Polymenidou, M.; Junt, T.; Sigurdson, C.; Wagner, H.; Akira, S.; Zinkernagel, R.; Aguzzi, A. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat. Med. 2004, 10, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, E.; Pollet, E.; Vu Manh, T.P.; Uze, G.; Dalod, M. Harnessing Mechanistic Knowledge on Beneficial Versus Deleterious IFN-I Effects to Design Innovative Immunotherapies Targeting Cytokine Activity to Specific Cell Types. Front. Immunol. 2014, 5, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centlivre, M.; Legrand, N.; Steingrover, R.; van der Sluis, R.; Grijsen, M.L.; Bakker, M.; Jurriaans, S.; Berkhout, B.; Paxton, W.A.; Prins, J.M.; et al. Altered dynamics and differential infection profiles of lymphoid and myeloid cell subsets during acute and chronic HIV-1 infection. J. Leukoc. Biol. 2011, 89, 785–795. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Borrow, P.; Tomaras, G.D.; Goonetilleke, N.; Haynes, B.F. The immune response during acute HIV-1 infection: Clues for vaccine development. Nat. Rev. Immunol. 2010, 10, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Cheng, M.; Nunoya, J.; Cheng, L.; Guo, H.; Yu, H.; Liu, Y.J.; Su, L.; Zhang, L. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS Pathog. 2014, 10, e1004291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X.; Lu, J.; Zhang, S.; Gu, L.; Fu, J.; Jin, L.; Li, H.; Zhao, M.; Zhang, J.; et al. B and T lymphocyte attenuator down-regulation by HIV-1 depends on type I interferon and contributes to T-cell hyperactivation. J. Infect. Dis. 2011, 203, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, L.; Zhao, J.; Li, G.; Zhang, L.; Chen, W.; Nie, W.; Reszka-Blanco, N.J.; Wang, F.S.; Su, L. Plasmacytoid dendritic cells promote HIV-1-induced group 3 innate lymphoid cell depletion. J. Clin. Investig. 2015, 125, 3692–3703. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Teque, F.; Patel, M.; Fujimura, S.H.; Schmidt, B.; Levy, J.A. Plasmacytoid dendritic cell number and responses to Toll-like receptor 7 and 9 agonists vary in HIV Type 1-infected individuals in relation to clinical state. AIDS Res. Hum. Retrovir. 2013, 29, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Soumelis, V.; Scott, I.; Gheyas, F.; Bouhour, D.; Cozon, G.; Cotte, L.; Huang, L.; Levy, J.A.; Liu, Y.J. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 2001, 98, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A.W.; Graham, D.R.; Shearer, G.M.; Herbeuval, J.P. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 17453–17458. [Google Scholar] [CrossRef] [PubMed]
- Benlahrech, A.; Yasmin, A.; Westrop, S.J.; Coleman, A.; Herasimtschuk, A.; Page, E.; Kelleher, P.; Gotch, F.; Imami, N.; Patterson, S. Dysregulated immunophenotypic attributes of plasmacytoid but not myeloid dendritic cells in HIV-1 infected individuals in the absence of highly active anti-retroviral therapy. Clin. Exp. Immunol. 2012, 170, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.A.; Clayton, K.L.; Mujib, S.; Zhang, H.; Rahman, A.K.; Liu, J.; Yue, F.Y.; Benko, E.; Kovacs, C.; Ostrowski, M.A. Tim-3 is a Marker of Plasmacytoid Dendritic Cell Dysfunction during HIV Infection and Is Associated with the Recruitment of IRF7 and p85 into Lysosomes and with the Submembrane Displacement of TLR9. J. Immunol. 2017, 198, 3181–3194. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Manches, O.; Wilen, C.; Gopal, R.; Huq, R.; Wu, V.; Sunseri, N.; Bhardwaj, N. CD4 Receptor is a Key Determinant of Divergent HIV-1 Sensing by Plasmacytoid Dendritic Cells. PLoS Pathog. 2016, 12, e1005553. [Google Scholar] [CrossRef] [PubMed]
- Dzionek, A.; Sohma, Y.; Nagafune, J.; Cella, M.; Colonna, M.; Facchetti, F.; Gunther, G.; Johnston, I.; Lanzavecchia, A.; Nagasaka, T.; et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 2001, 194, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, E.; Cicala, C.; Van Ryk, D.; Goode, D.J.; Macleod, K.; Arthos, J.; Fauci, A.S. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{α} secretion in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 2007, 104, 3396–3401. [Google Scholar] [CrossRef] [PubMed]
- Rajasuriar, R.; Wright, E.; Lewin, S.R. Impact of antiretroviral therapy (ART) timing on chronic immune activation/inflammation and end-organ damage. Curr. Opin. HIV AIDS 2015, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- French, M.A.; King, M.S.; Tschampa, J.M.; da Silva, B.A.; Landay, A.L. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J. Infect. Dis. 2009, 200, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W.; Martin, J.N.; Sinclair, E.; Bredt, B.; Hagos, E.; Lampiris, H.; Deeks, S.G. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 2003, 187, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.R.; Katzenstein, T.L.; Pedersen, B.K.; Gerstoft, J.; Ullum, H. Residual viraemia in HIV-1-infected patients with plasma viral load ≤20 copies/mL is associated with increased blood levels of soluble immune activation markers. Scand. J. Immunol. 2008, 68, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Hatano, H.; Jain, V.; Hunt, P.W.; Lee, T.H.; Sinclair, E.; Do, T.D.; Hoh, R.; Martin, J.N.; McCune, J.M.; Hecht, F.; et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J. Infect. Dis. 2013, 208, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Melzer, S.; Zachariae, S.; Bocsi, J.; Engel, C.; Loffler, M.; Tarnok, A. Reference intervals for leukocyte subsets in adults: Results from a population-based study using 10-color flow cytometry. Cytom. Part B 2015, 88, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Bisset, L.R.; Lung, T.L.; Kaelin, M.; Ludwig, E.; Dubs, R.W. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur. J. Haematol. 2004, 72, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Shahal-Zimra, Y.; Rotem, Z.; Chezar, J.; Shochat, T.; Ross, L.; Pickholtz, I.; Rabizadeh, E. Lymphocyte Subset Reference Ranges in Healthy Israeli Adults. Isr. Med. Assoc. J. 2016, 18, 739–743. [Google Scholar] [PubMed]
- Saidi, H.; Bras, M.; Formaglio, P.; Melki, M.T.; Charbit, B.; Herbeuval, J.P.; Gougeon, M.L. HMGB1 Is Involved in IFN-alpha Production and TRAIL Expression by HIV-1-Exposed Plasmacytoid Dendritic Cells: Impact of the Crosstalk with NK Cells. PLoS Pathog. 2016, 12, e1005407. [Google Scholar] [CrossRef] [PubMed]
- Herbeuval, J.P.; Hardy, A.W.; Boasso, A.; Anderson, S.A.; Dolan, M.J.; Dy, M.; Shearer, G.M. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: Role of type I IFN-producing plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 2005, 102, 13974–13979. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Bover, L.; Cho, M.; Wen, X.; Hanabuchi, S.; Bao, M.; Rosen, D.B.; Wang, Y.H.; Shaw, J.L.; Du, Q.; et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J. Exp. Med. 2009, 206, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhang, L.; Rosen, D.B.; Bover, L.; Watanabe, G.; Bao, M.; Lanier, L.L.; Liu, Y.J. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol. 2007, 5, e248. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, A.; Otero, K.; Czerkowicz, J.M.; Kerns, H.M.; Shapiro, R.I.; Ranger, A.M.; Otipoby, K.L.; Taylor, F.R.; Cameron, T.O.; Viney, J.L.; et al. Anti-BDCA2 monoclonal antibody inhibits plasmacytoid dendritic cell activation through Fc-dependent and Fc-independent mechanisms. EMBO Mol. Med. 2015, 7, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, I.; Janovec, V.; Stranska, R.; Bendriss-Vermare, N. Cross Talk between Inhibitory Immunoreceptor Tyrosine-Based Activation Motif-Signaling and Toll-Like Receptor Pathways in Macrophages and Dendritic Cells. Front. Immunol. 2017, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Aouar, B.; Kovarova, D.; Letard, S.; Font-Haro, A.; Florentin, J.; Weber, J.; Durantel, D.; Chaperot, L.; Plumas, J.; Trejbalova, K.; et al. Dual Role of the Tyrosine Kinase Syk in Regulation of Toll-Like Receptor Signaling in Plasmacytoid Dendritic Cells. PLoS ONE 2016, 11, e0156063. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Rosen, D.B.; Ito, T.; Bover, L.; Bao, M.; Watanabe, G.; Yao, Z.; Zhang, L.; Lanier, L.L.; Liu, Y.J. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J. Exp. Med. 2006, 203, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, Y.; Shi, B.; Zhang, X.; Wang, J.; Zhang, Z.; Shen, F.; Zhang, Q.; Sun, S.; Yuan, Z. HBsAg inhibits TLR9-mediated activation and IFN-alpha production in plasmacytoid dendritic cells. Mol. Immunol. 2009, 46, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Florentin, J.; Aouar, B.; Dental, C.; Thumann, C.; Firaguay, G.; Gondois-Rey, F.; Soumelis, V.; Baumert, T.F.; Nunes, J.A.; Olive, D.; et al. HCV glycoprotein E2 is a novel BDCA-2 ligand and acts as an inhibitor of IFN production by plasmacytoid dendritic cells. Blood 2012, 120, 4544–4551. [Google Scholar] [CrossRef] [PubMed]



| Subject No. 1 | Transmission 2 | Age | Diagnosis-Initiation of ART (Months) | Therapy Regimen 3 | CD4+ T Cells (Cell/mm3) 0-Month ART | CD4+ T Cells (Cell/mm3) 3-Month ART | HIV-1 RNA (Copies/mL) VL0-month 4 | HIV-1 RNA (Copies/mL) VL3-month 5 |
|---|---|---|---|---|---|---|---|---|
| 1 | MSM | 26 | 4 | ABC/3TC+RPV | 336 | 498 | 44,800 | 44 |
| 2 | MSM | 23 | 3 | ABC/3TC+EFV | 468 | 599 | 44,600 | <20 |
| 3 | MSM | 25 | 52 | ABC/3TC+DRV/r | 565 | 676 | 50,600 | 102 |
| 4 | MSM | 22 | 10 | TDF/FTC+EFV | 514 | 624 | 92,100 | 34 |
| 5 | MSM | 27 | 10 | ABC/3TC+DRV/r | 527 | 644 | 153,000 | 34 |
| 6 | Bi | 44 | 8 | ABC/3TC+EFV | 480 | 530 | 82,700 | 52 |
| 7 | MSM | 26 | 12 | TDF/FTC/RPV | 1023 | 1111 | 6150 | <20 |
| 8 | MSM | 27 | 5 | TDF/FTC+DRV/r | 315 | 438 | 144,000 | 850 |
| 9 | MSM | 38 | 15 | TDF/FTC/EVG/c | 521 | 598 | 27,300 | 34 |
| 10 | Bi | 49 | 62 | ABC/3TC+DRV/r | 379 | 619 | 119,000 | 67 |
| 11 | Bi | 48 | 6 | ABC/3TC+DRV/r | 372 | 501 | 20,200 | <0 |
| 12 | MSM | 29 | 4 | ABC/3TC+LPV/r | 267 | 546 | 83,900 | <20 |
| 13 | MSM | 31 | 1 | TDF/FTC/RPV | 402 | 528 | 47,300 | 81 |
| 14 | MSM | 37 | 13 | TDF/FTC/EVG/c | 634 | 782 | 99,700 | 61 |
| 15 | MSM | 36 | 11 | TDF/FTC+DRV/r | 503 | 597 | 180,000 | <20 |
| 16 | MSM | 28 | 32 | TDF/FTC+DRV/r | 206 | 217 | 98,100 | 135 |
| 17 | MSM | 24 | 14 | TDF/FTC/RPV | 377 | 414 | 35,400 | 73 |
| 18 | MSM | 19 | 4 | ABC/3TC+DRV/r | 418 | 418 | 109,000 | 391 |
| 19 | MSM | 26 | 21 | TDF/FTC/RPV | 534 | 540 | 43,900 | 28 |
| 20 | MSM | 44 | 4 | TDF/FTC+DTG | 538 | 585 | 39,900 | 166 |
| 21 | MSM | 28 | 25 | TDF/FTC+DTG | 384 | 404 | 13,400 | <20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Font-Haro, A.; Janovec, V.; Hofman, T.; Machala, L.; Jilich, D.; Melkova, Z.; Weber, J.; Trejbalova, K.; Hirsch, I. Expression of TIM-3 on Plasmacytoid Dendritic Cells as a Predictive Biomarker of Decline in HIV-1 RNA Level during ART. Viruses 2018, 10, 154. https://doi.org/10.3390/v10040154
Font-Haro A, Janovec V, Hofman T, Machala L, Jilich D, Melkova Z, Weber J, Trejbalova K, Hirsch I. Expression of TIM-3 on Plasmacytoid Dendritic Cells as a Predictive Biomarker of Decline in HIV-1 RNA Level during ART. Viruses. 2018; 10(4):154. https://doi.org/10.3390/v10040154
Chicago/Turabian StyleFont-Haro, Albert, Vaclav Janovec, Tomas Hofman, Ladislav Machala, David Jilich, Zora Melkova, Jan Weber, Katerina Trejbalova, and Ivan Hirsch. 2018. "Expression of TIM-3 on Plasmacytoid Dendritic Cells as a Predictive Biomarker of Decline in HIV-1 RNA Level during ART" Viruses 10, no. 4: 154. https://doi.org/10.3390/v10040154