Anti-Inflammatory Effect of Baicalein on Polyinosinic–Polycytidylic Acid-Induced RAW 264.7 Mouse Macrophages
Abstract
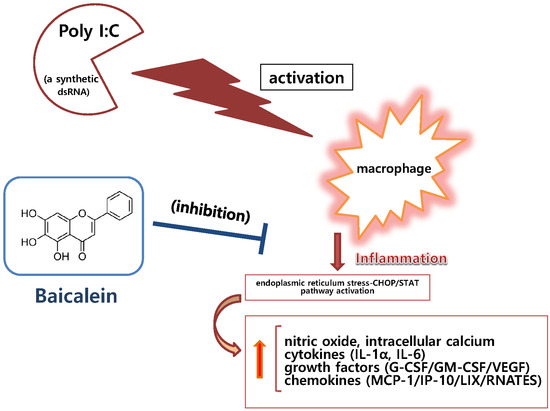
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. NO Assay
2.4. Intracellular Calcium Assay
2.5. Multiplex Bead-Based Cytokine Assay
2.6. RNA Isolation and Real Time RT-PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of Baicalein on NO Production and Intracellular Calcium Release
3.2. Effect of Baicalein on Cytokine Production
3.3. Effect of Baicalein on mRNA Expression of STAT1, STAT3, CHOP and Fas
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fan, G.; Zhang, Y.; Jiang, X.; Zhu, Y.; Wang, B.; Su, L.; Cao, W.; Zhang, H.; Gao, X. Anti-Inflammatory Activity of Baicalein in LPS-Stimulated RAW264. 7 Macrophages Via Estrogen Receptor and NF-κB-Dependent Pathways. Inflammation 2013, 36, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-Inflammatory Effect of Strawberry Extract Against LPS-Induced Stress in RAW 264.7 Macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.K.; Bae, J.S. Anti-Inflammatory Effects of Baicalin, Baicalein, and Wogonin in Vitro and in Vivo. Inflammation 2015, 38, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Yuk, S.; Lim, E.; Lee, J.Y.; Lee, Y.; Kim, Y.; Lee, T.H.; Park, S.K.; Bae, H.; Kim, H.M.; Ko, S. Antiinflammatory Effects of Epimedium Brevicornum Water Extract on lipopolysaccharide-activated RAW264. 7 Macrophages. Phytother. Res. 2010, 24, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Myristicin on RAW 264.7 Macrophages Stimulated with Polyinosinic-Polycytidylic Acid. Molecules 2011, 16, 7132–7142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, Y.; Kim, H.J.; Kim, Y.; Park, W. Immunostimulatory Effect of Laminarin on RAW 264.7 Mouse Macrophages. Molecules 2012, 17, 5404–5411. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, J.Y.; Han, H.S.; Kim, Y.J.; Kim, H.J.; Kim, Y.S.; Kim, H.M.; Ko, S.G.; An, H.J.; Lee, Y.J.; et al. Immunomodulatory Effects of Liriope platyphylla Water Extract on Lipopolysaccharide-Activated Mouse Macrophage. Nutrients 2012, 4, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effect of Wogonin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2015, 20, 6888–6900. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, W. Anti-Inflammatory Effect of Quercetin on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Molecules 2016, 21, 450. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Anti-Inflammatory Effects of Oroxylin A on RAW 264.7 Mouse Macrophages Induced with Polyinosinic-Polycytidylic Acid. Exp. Ther. Med. 2016, 12, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, W. Evidence-Guided Optimization of Herbal Formula Cheong-Hwa-Bo-Um-Tang using Multiplex Cytokine Profiling. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 72–80. [Google Scholar] [CrossRef]
- Hong, J.; Yang, G.; Kim, Y.B.; Eom, S.H.; Lew, J.; Kang, H. Anti-Inflammatory Activity of Cinnamon Water Extract in Vivo and in Vitro LPS-Induced Models. BMC Complement. Altern. Med. 2012, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-Candida Activity of Brazilian Medicinal Plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, L.; Zhou, H.; Zeng, L.; Chen, T.; Chen, Q.; Zhou, B.; Wang, Y.; Chen, Q.; Hu, P.; et al. Radix isatidis Polysaccharides Inhibit Influenza A Virus and Influenza A Virus-Induced Inflammation via Suppression of Host TLR3 Signaling In Vitro. Molecules 2017, 22, 116. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. New Therapeutic Aspects of Flavones: The Anticancer Properties of Scutellaria and its Main Active Constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Fu, T.; Gong, W.; Dunlop, N.; Kung, H.; Yan, Y.; Kang, J.; Wang, J.M. The Flavonoid Baicalin Exhibits Anti-Inflammatory Activity by Binding to Chemokines. Immunopharmacology 2000, 49, 295–306. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of Double-Stranded RNA and Activation of NF-κB by Toll-Like Receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Suárez, B.B.; Garrido, G.; Delgado, R.; Bosch, F.; Rabí, M.d.C. A Mangifera indica L. Extract could be used to Treat Neuropathic Pain and Implication of Mangiferin. Molecules 2010, 15, 9035–9045. [Google Scholar] [CrossRef] [PubMed]
- Serafini, N.; Dahdah, A.; Barbet, G.; Demion, M.; Attout, T.; Gautier, G.; Arcos-Fajardo, M.; Souchet, H.; Jouvin, M.H.; Vrtovsnik, F.; et al. The TRPM4 Channel Controls Monocyte and Macrophage, but Not Neutrophil, Function for Survival in Sepsis. J. Immunol. 2012, 189, 3689–3699. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Seimon, T.; Timmins, J.; Li, G.; Lim, W. Macrophage Apoptosis in Advanced Atherosclerosis. Ann. N. Y. Acad. Sci. 2009, 1173, E40–E45. [Google Scholar] [CrossRef] [PubMed]
- Stout, B.A.; Melendez, K.; Seagrave, J.; Holtzman, M.J.; Wilson, B.; Xiang, J.; Tesfaigzi, Y. STAT1 Activation Causes Translocation of Bax to the Endoplasmic Reticulum during the Resolution of Airway Mucous Cell Hyperplasia by IFN-Gamma. J. Immunol. 2007, 178, 8107–8116. [Google Scholar] [CrossRef] [PubMed]
- Timmins, J.M.; Ozcan, L.; Seimon, T.A.; Li, G.; Malagelada, C.; Backs, J.; Backs, T.; Bassel-Duby, R.; Olson, E.N.; Anderson, M.E.; et al. Calcium/calmodulin-Dependent Protein Kinase II Links ER Stress with Fas and Mitochondrial Apoptosis Pathways. J. Clin. Investig. 2009, 119, 2925–2941. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Choi, J.; Choi, H.; Cho, S.; Kim, H.; Jo, E.; Park, J.; Song, C. Endoplasmic Reticulum Stress Pathway-Mediated Apoptosis in Macrophages Contributes to the Survival of Mycobacterium tuberculosis. PLoS ONE 2011, 6, e28531. [Google Scholar] [CrossRef] [PubMed]





| Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| STAT1 | TGAGATGTCCCGGATAGTGG | CGCCAGAGAGAAATTCGTGT-3 |
| STAT3 | GTCTGCAGAGTTCAAGCACCT | TCCTCAGTCACGATCAAGGAG |
| CHOP | CGCTGTTTTCCCTTGCTG | TCCTCATACCAGGCTTCCA |
| Fas | CGCTGTTTTCCCTTGCTG | CCTTGAGTATGAACTCTTAACTGTGAG |
| TBP | GGGGAGCTGTGATGTGAAGT | CCAGGAAATAATTCTGGCTCA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-J.; Kim, H.-J.; Lee, J.Y.; Kim, D.-H.; Kang, M.S.; Park, W. Anti-Inflammatory Effect of Baicalein on Polyinosinic–Polycytidylic Acid-Induced RAW 264.7 Mouse Macrophages. Viruses 2018, 10, 224. https://doi.org/10.3390/v10050224
Kim Y-J, Kim H-J, Lee JY, Kim D-H, Kang MS, Park W. Anti-Inflammatory Effect of Baicalein on Polyinosinic–Polycytidylic Acid-Induced RAW 264.7 Mouse Macrophages. Viruses. 2018; 10(5):224. https://doi.org/10.3390/v10050224
Chicago/Turabian StyleKim, Young-Jin, Hyun-Ju Kim, Ji Young Lee, Do-Hoon Kim, Mi Suk Kang, and Wansu Park. 2018. "Anti-Inflammatory Effect of Baicalein on Polyinosinic–Polycytidylic Acid-Induced RAW 264.7 Mouse Macrophages" Viruses 10, no. 5: 224. https://doi.org/10.3390/v10050224