Natural Products Isolated from Oriental Medicinal Herbs Inactivate Zika Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Emodin and Berberine
2.2. Cell Line
2.3. Virus Preparation
2.4. Cytotoxicity of Berberine and Emodin in Vero E6 Cells
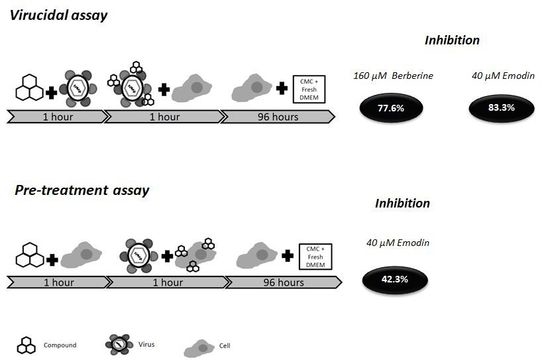
2.5. Virucidal Effects of Berberine and Emodin
2.6. Pre-Treatment of Vero E6 Cells with Berberine and Emodin
2.7. Selectivity Index (SI)
2.8. Analyses of Virions by Dynamic Light Scattering (DLS)
2.9. Statistical Analysis
3. Results
3.1. Berberine and Emodin Presented Low Citotoxicity in Vero Cells
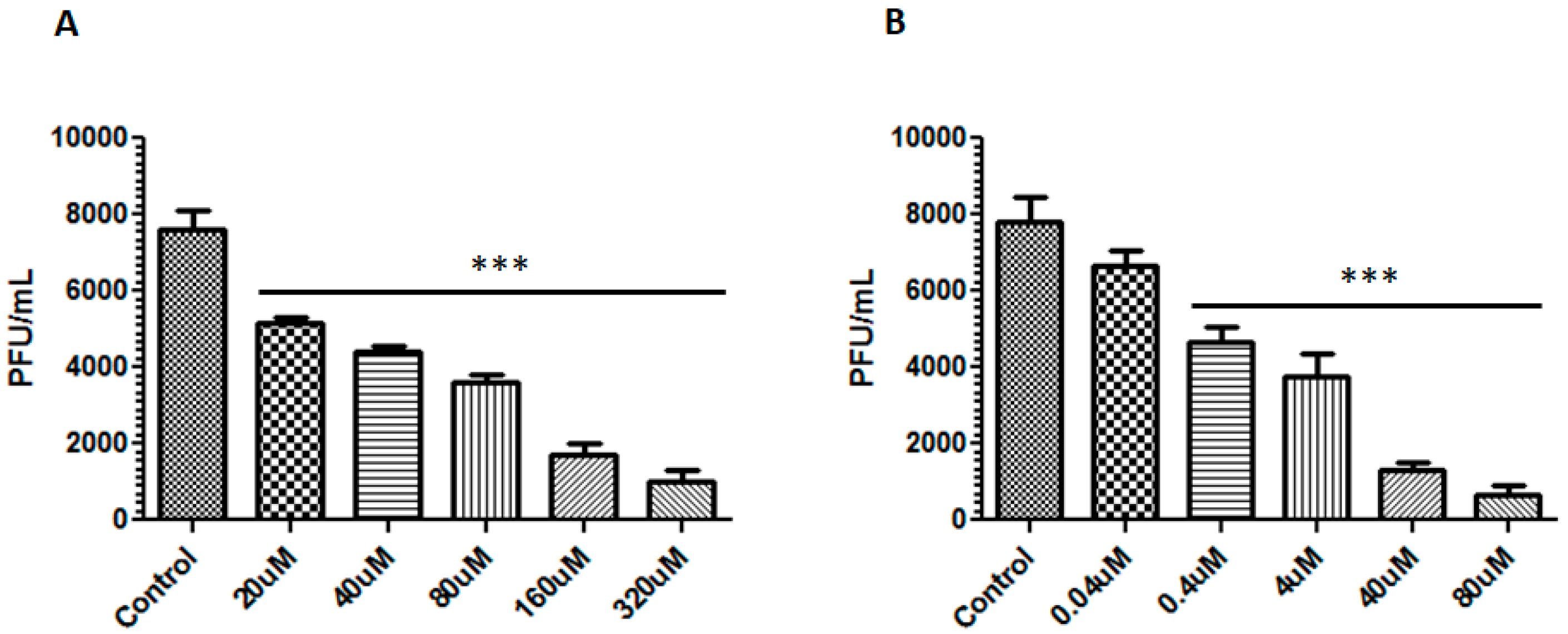
3.2. Berberine and Emodin Reduced the Infectivity of ZIKV in Vero Cells
3.3. Emodin Reduced ZIKV Entry in Vero Cells
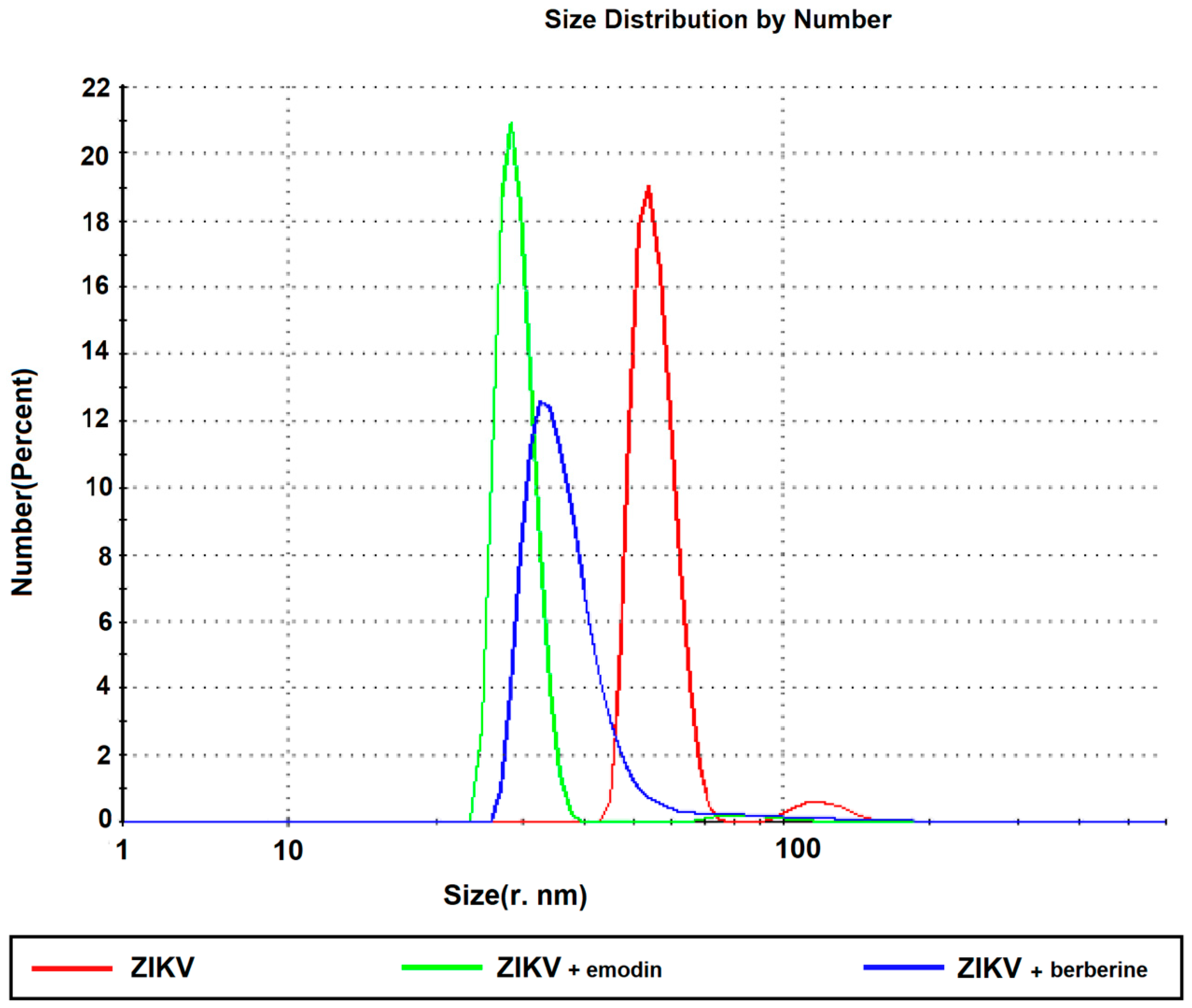
3.4. Berberine and Emodin Impair ZIKV Particles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- De Carvalho, N.S.; De Carvalho, B.F.; Fugaca, C.A.; Doris, B.; Biscaia, E.S. Zika virus infection during pregnancy and microcephaly occurrence: A review of literature and brazilian data. Braz. J. Infect. Dis. 2016, 20, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Garcez, P.P.; Loiola, E.C.; Madeiro da Costa, R.; Higa, L.M.; Trindade, P.; Delvecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastere, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-barre syndrome outbreak associated with zika virus infection in french polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Atkinson, B.; Hall, G.; Watson, R.J.; Bosworth, A.; Bonney, L.C.; Kitchen, S.; Hewson, R. A susceptible mouse model for zika virus infection. PLoS Negl. Trop. Dis. 2016, 10, e0004658. [Google Scholar] [CrossRef]
- Eyer, L.; Nencka, R.; Huvarova, I.; Palus, M.; Joao Alves, M.; Gould, E.A.; De Clercq, E.; Ruzek, D. Nucleoside inhibitors of zika virus. J. Infect. Dis. 2016, 214, 707–711. [Google Scholar] [CrossRef]
- Zmurko, J.; Marques, R.E.; Schols, D.; Verbeken, E.; Kaptein, S.J.; Neyts, J. The viral polymerase inhibitor 7-deaza-2′-c-methyladenosine is a potent inhibitor of in vitro zika virus replication and delays disease progression in a robust mouse infection model. PLoS Negl. Trop. Dis. 2016, 10, e0004695. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberis vulgaris and berberine: An update review. Phytother. Res. PTR 2016, 30, 1745–1764. [Google Scholar] [CrossRef]
- Hayashi, K.; Minoda, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg. Med. Chem. Lett. 2007, 17, 1562–1564. [Google Scholar] [CrossRef]
- Chin, L.W.; Cheng, Y.W.; Lin, S.S.; Lai, Y.Y.; Lin, L.Y.; Chou, M.Y.; Chou, M.C.; Yang, C.C. Anti-herpes simplex virus effects of berberine from coptidis rhizoma, a major component of a chinese herbal medicine, ching-wei-san. Arch. Virol. 2010, 155, 1933–1941. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.Q.; Kim, Y.J.; Wu, J.; Wang, Q.; Hao, Y. In vivo and in vitro antiviral effects of berberine on influenza virus. Chin. J. Integr. Med. 2011, 17, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.S.; Kaukinen, P.; Glasker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kummerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016, 126, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Zhao, H.P.; Zhao, Y.L.; Jin, C.; Liu, D.J.; Kong, W.J.; Fang, F.; Zhang, L.; Wang, H.J.; Xiao, X.H. Hepatotoxicity or hepatoprotection? Pattern recognition for the paradoxical effect of the Chinese herb Rheum palmatum L. In treating rat liver injury. PLoS ONE 2011, 6, e24498. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kao, L.; Lin, C.C. Comparison of the antioxidant and transmembrane permeative activities of the different polygonum cuspidatum extracts in phospholipid-based microemulsions. J. Agric. Food Chem. 2011, 59, 9135–9141. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.; Ullah, M.F.; Hadi, S.M. DNA degradation by aqueous extract of aloe vera in the presence of copper ions. Indian J. Biochem. Biophys. 2010, 47, 161–165. [Google Scholar] [PubMed]
- Yang, Y.C.; Lim, M.Y.; Lee, H.S. Emodin isolated from cassia obtusifolia (leguminosae) seed shows larvicidal activity against three mosquito species. J. Agric. Food Chem. 2003, 51, 7629–7631. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, N.; Zhong, Y.; Yang, Z.Q. Antiviral effect of emodin from rheum palmatum against coxsakievirus b5 and human respiratory syncytial virus in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2015, 35, 916–922. [Google Scholar] [CrossRef]
- Li, L.; Song, X.; Yin, Z.; Jia, R.; Li, Z.; Zhou, X.; Zou, Y.; Yin, L.; Yue, G.; Ye, G.; et al. The antibacterial activity and action mechanism of emodin from polygonum cuspidatum against haemophilus parasuis in vitro. Microbiol. Res. 2016, 186–187, 139–145. [Google Scholar] [CrossRef]
- Yiu, C.Y.; Chen, S.Y.; Yang, T.H.; Chang, C.J.; Yeh, D.B.; Chen, Y.J.; Lin, T.P. Inhibition of epstein-barr virus lytic cycle by an ethyl acetate subfraction separated from polygonum cuspidatum root and its major component, emodin. Molecules 2014, 19, 1258–1272. [Google Scholar] [CrossRef]
- Xiong, H.R.; Luo, J.; Hou, W.; Xiao, H.; Yang, Z.Q. The effect of emodin, an anthraquinone derivative extracted from the roots of rheum tanguticum, against herpes simplex virus in vitro and in vivo. J. Ethnopharmacol. 2011, 133, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.S.; Jia, X.L.; Song, P.; Cheng, Y.A.; Zhang, X.; Sun, M.Z.; Liu, E.Q. Inhibitory effect of emodin and astragalus polysaccharide on the replication of hbv. World J. Gastroenterol. 2009, 15, 5669–5673. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Wu, C.F.; Hsiao, N.W.; Chang, C.Y.; Li, S.W.; Wan, L.; Lin, Y.J.; Lin, W.Y. Aloe-emodin is an interferon-inducing agent with antiviral activity against japanese encephalitis virus and enterovirus 71. Int. J. Antimicrob. Agents 2008, 32, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Azevedo Rdo, S.; Kraemer, M.U.; Souza, R.; Cunha, M.S.; Hill, S.C.; Theze, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, H.; Ye, J. Traditional chinese medicine in treatment of metabolic syndrome. Endocr. Metab. Immune Disord. Drug Targets 2008, 8, 99–111. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Ni, J. Emodin: A review of its pharmacology, toxicity and pharmacokinetics. Phytother. Res. PTR 2016, 30, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Wang, K.; Yu, W.; Sun, B.; Schwarz, W. Emodin inhibits current through sars-associated coronavirus 3a protein. Antivir. Res. 2011, 90, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wei, F.; Chen, L.J.; Xiong, H.R.; Liu, Y.Y.; Luo, F.; Hou, W.; Xiao, H.; Yang, Z.Q. In vitro and in vivo studies of the inhibitory effects of emodin isolated from polygonum cuspidatum on coxsakievirus b(4). Molecules 2013, 18, 11842–11858. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, C.Y.; Ho, T.Y. Emodin is a novel alkaline nuclease inhibitor that suppresses herpes simplex virus type 1 yields in cell cultures. Br. J. Pharmacol. 2008, 155, 227–235. [Google Scholar] [CrossRef]
- Sydiskis, R.J.; Owen, D.G.; Lohr, J.L.; Rosler, K.H.; Blomster, R.N. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob. Agents Chemother. 1991, 35, 2463–2466. [Google Scholar] [CrossRef]
- Alves, D.S.; Perez-Fons, L.; Estepa, A.; Micol, V. Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem. Pharmacol. 2004, 68, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Alam, Z.; Al-Mahdi, Z.; Zhu, Y.; McKee, Z.; Parris, D.S.; Parikh, H.I.; Kellogg, G.E.; Kuchta, A.; McVoy, M.A. Anti-cytomegalovirus activity of the anthraquinone atanyl blue prl. Antivir. Res. 2015, 114, 86–95. [Google Scholar] [CrossRef]
- Li, S.W.; Yang, T.C.; Lai, C.C.; Huang, S.H.; Liao, J.M.; Wan, L.; Lin, Y.J.; Lin, C.W. Antiviral activity of aloe-emodin against influenza a virus via galectin-3 up-regulation. Eur. J. Pharmacol. 2014, 738, 125–132. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, A.R.; Duarte, E.L.; Stassen, H.; Lamy, M.T.; Coutinho, K. Experimental and theoretical studies of emodin interacting with a lipid bilayer of dmpc. Biophys. Rev. 2017, 9, 729–745. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Tsujita, T.; Katagiri, Y.; Yoshida, S.; Yamada, Y. Purification and characterization of s-adenosyl-l-methionine: Norcoclaurine 6-o-methyltransferase from cultured coptis japonica cells. Eur. J. Biochem. 1994, 225, 125–131. [Google Scholar] [CrossRef]
- Mischitelli, M.; Jemaa, M.; Almasry, M.; Faggio, C.; Lang, F. Triggering of erythrocyte cell membrane scrambling by emodin. Cell. Physiol. Biochem. 2016, 40, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.S.; Ma, J.Y.; Feng, R.; Ma, C.; Chen, W.J.; Sun, Y.P.; Fu, J.; Huang, M.; He, C.Y.; Shou, J.W.; et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of zika virus infection in human skin cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef]
- El Costa, H.; Gouilly, J.; Mansuy, J.M.; Chen, Q.; Levy, C.; Cartron, G.; Veas, F.; Al-Daccak, R.; Izopet, J.; Jabrane-Ferrat, N. Zika virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci. Rep. 2016, 6, 35296. [Google Scholar] [CrossRef]
- Chan, J.F.; Yip, C.C.; Tsang, J.O.; Tee, K.M.; Cai, J.P.; Chik, K.K.; Zhu, Z.; Chan, C.C.; Choi, G.K.; Sridhar, S.; et al. Differential cell line susceptibility to the emerging zika virus: Implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg. Microbes Infect. 2016, 5, e93. [Google Scholar] [CrossRef]
- Rad, S.Z.K.; Rameshrad, M.; Hosseinzadeh, H. Toxicology effects of berberis vulgaris (barberry) and its active constituent, berberine: A review. Iran. J. Basic Med. Sci. 2017, 20, 516–529. [Google Scholar] [PubMed]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Ahmed, T.; Gilani, A.U.; Abdollahi, M.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. Berberine and neurodegeneration: A review of literature. Pharmacol. Rep. PR 2015, 67, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Lin, Q.; Luo, G.-S.; Dai, Y.-Y. Solubility of berberine chloride in various solvents. J. Chem. Eng. Data 2006, 51, 2. [Google Scholar] [CrossRef]
- Duan, H.-G.; Wei, Y.-H.; Li, B.-X.; Qin, H.-Y.; Wu, X.-A. Improving the dissolution and oral bioavailability of the poorly water-soluble drug aloe-emodin by solid dispersion with polyethylene glycol 6000. Drug Dev. Res. 2009, 70, 7. [Google Scholar] [CrossRef]
- Battu, S.K.; Repka, M.A.; Maddineni, S.; Chittiboyina, A.G.; Avery, M.A.; Majumdar, S. Physicochemical characterization of berberine chloride: A perspective in the development of a solution dosage form for oral delivery. AAPS PharmSciTech 2010, 11, 1466–1475. [Google Scholar] [CrossRef]
- Shia, C.S.; Hou, Y.C.; Tsai, S.Y.; Huieh, P.H.; Leu, Y.L.; Chao, P.D. Differences in pharmacokinetics and ex vivo antioxidant activity following intravenous and oral administrations of emodin to rats. J. Pharm. Sci. 2010, 99, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Y.; Chen, M.; Wang, Y. Berberine hydrochloride: Anticancer activity and nanoparticulate delivery system. Int. J. Nanomed. 2011, 6, 1773–1777. [Google Scholar] [CrossRef]
- Pund, S.; Borade, G.; Rasve, G. Improvement of anti-inflammatory and anti-angiogenic activity of berberine by novel rapid dissolving nanoemulsifying technique. Phytomedicine 2014, 21, 307–314. [Google Scholar] [CrossRef]
- Zhu, J.X.; Tang, D.; Feng, L.; Zheng, Z.G.; Wang, R.S.; Wu, A.G.; Duan, T.T.; He, B.; Zhu, Q. Development of self-microemulsifying drug delivery system for oral bioavailability enhancement of berberine hydrochloride. Drug Dev. Ind. Pharm. 2013, 39, 499–506. [Google Scholar] [CrossRef]
- Xue, M.; Yang, M.X.; Zhang, W.; Li, X.M.; Gao, D.H.; Ou, Z.M.; Li, Z.P.; Liu, S.H.; Li, X.J.; Yang, S.Y. Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int. J. Nanomed. 2013, 8, 4677–4687. [Google Scholar] [CrossRef] [PubMed]
- Freag, M.S.; Elnaggar, Y.S.; Abdelmonsif, D.A.; Abdallah, O.Y. Stealth, biocompatible monoolein-based lyotropic liquid crystalline nanoparticles for enhanced aloe-emodin delivery to breast cancer cells: In vitro and in vivo studies. Int. J. Nanomed. 2016, 11, 4799–4818. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yin, X.; Lu, Y.; Shan, W.; Xiong, S. Formulation, antileukemia mechanism, pharmacokinetics, and biodistribution of a novel liposomal emodin. Int. J. Nanomed. 2012, 7, 2325–2337. [Google Scholar]
- Liu, H.; Gao, M.; Xu, H.; Guan, X.; Lv, L.; Deng, S.; Zhang, C.; Tian, Y. A promising emodin-loaded poly (lactic-co-glycolic acid)-d-alpha-tocopheryl polyethylene glycol 1000 succinate nanoparticles for liver cancer therapy. Pharm. Res. 2016, 33, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, L.; Sun, Y.; Zhu, Y.; Sun, Z.; An, T.; Li, Y.; Lin, Y.; Fan, D.; Wang, Q. Development of phenylboronic acid-functionalized nanoparticles for emodin delivery. J. Mater. Chem. B Mater. Biol. Med. 2015, 3, 3840–3847. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, M.N.; Braga, A.C.S.; Campos, G.R.F.; Souza, M.M.; Matos, R.P.A.d.; Lopes, T.Z.; Candido, N.M.; Lima, M.L.D.; Machado, F.C.; Andrade, S.T.Q.d.; et al. Natural Products Isolated from Oriental Medicinal Herbs Inactivate Zika Virus. Viruses 2019, 11, 49. https://doi.org/10.3390/v11010049
Batista MN, Braga ACS, Campos GRF, Souza MM, Matos RPAd, Lopes TZ, Candido NM, Lima MLD, Machado FC, Andrade STQd, et al. Natural Products Isolated from Oriental Medicinal Herbs Inactivate Zika Virus. Viruses. 2019; 11(1):49. https://doi.org/10.3390/v11010049
Chicago/Turabian StyleBatista, Mariana N., Ana Cláudia S. Braga, Guilherme Rodrigues Fernandes Campos, Marcos Michel Souza, Renata Prandini Adum de Matos, Tairine Zara Lopes, Natalia Maria Candido, Maria Leticia Duarte Lima, Francielly Cristina Machado, Stephane Tereza Queiroz de Andrade, and et al. 2019. "Natural Products Isolated from Oriental Medicinal Herbs Inactivate Zika Virus" Viruses 11, no. 1: 49. https://doi.org/10.3390/v11010049
APA StyleBatista, M. N., Braga, A. C. S., Campos, G. R. F., Souza, M. M., Matos, R. P. A. d., Lopes, T. Z., Candido, N. M., Lima, M. L. D., Machado, F. C., Andrade, S. T. Q. d., Bittar, C., Nogueira, M. L., Carneiro, B. M., Mariutti, R. B., Arni, R. K., Calmon, M. F., & Rahal, P. (2019). Natural Products Isolated from Oriental Medicinal Herbs Inactivate Zika Virus. Viruses, 11(1), 49. https://doi.org/10.3390/v11010049