Protection against Congenital CMV Infection Conferred by MVA-Vectored Subunit Vaccines Extends to a Second Pregnancy after Maternal Challenge with a Heterologous, Novel Strain Variant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Guinea Pigs
2.2. Cells and Virus
2.3. Generation of Vaccine Constructs, Experimental Design, and Pregnancy Challenge
2.4. Statistical Analyses
3. Results
3.1. Dams Previously Immunized with 22122 Strain-Specific MVA Vaccines Were Challenged with 22122 GPCMV in Their First Pregnancies
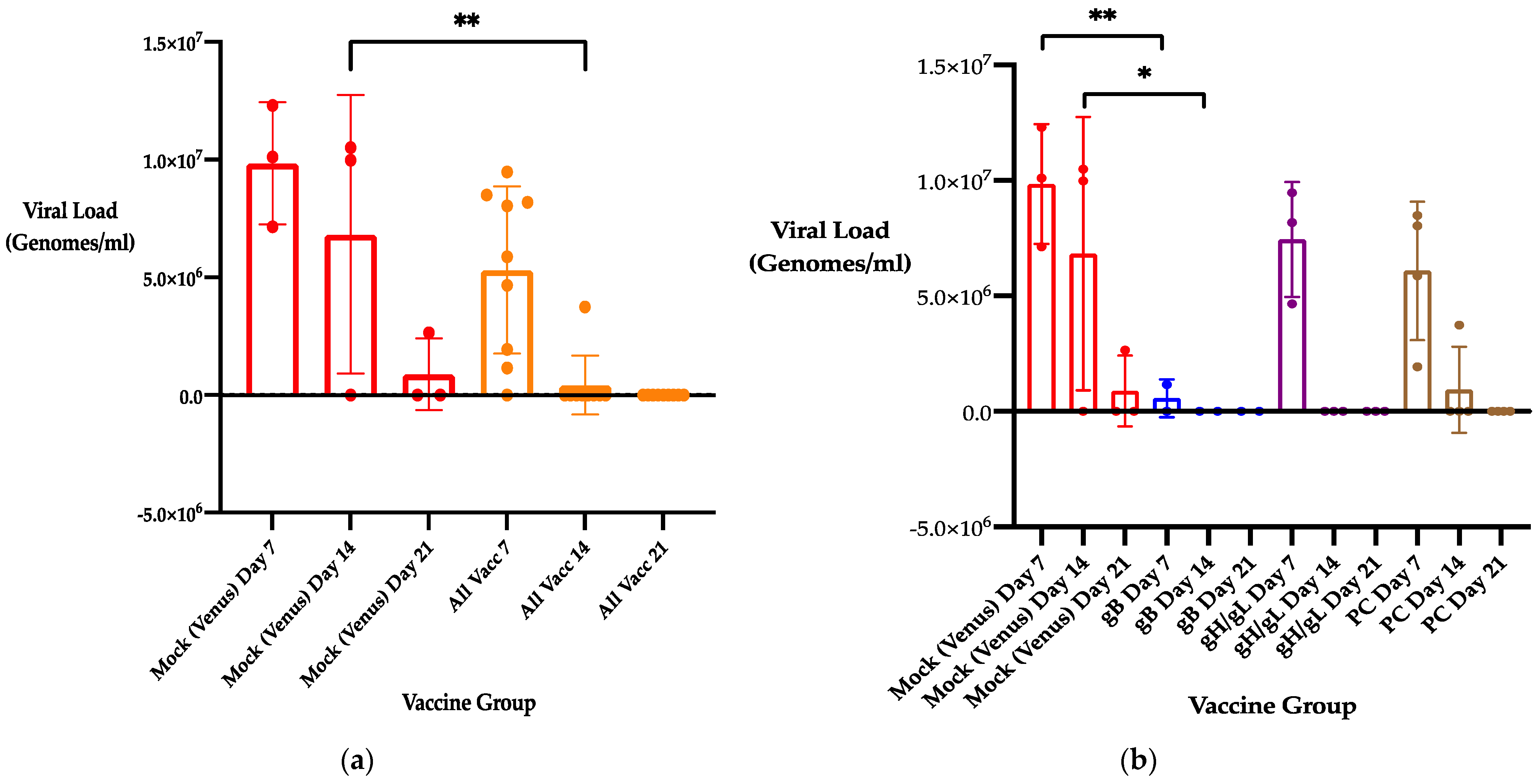
3.2. Previous Immunization with 22122 Subunit MVA Vaccines Reduces Maternal DNAemia Following Second Trimester Heterologous CIDMTR Challenge in a Second Pregnancy
3.3. Previous Immunization with 22122 Subunit MVA Vaccines Improves Pregnancy Outcomes (Pup Weights and Pup Survival) following Heterologous Challenge with CIDMTR Virus
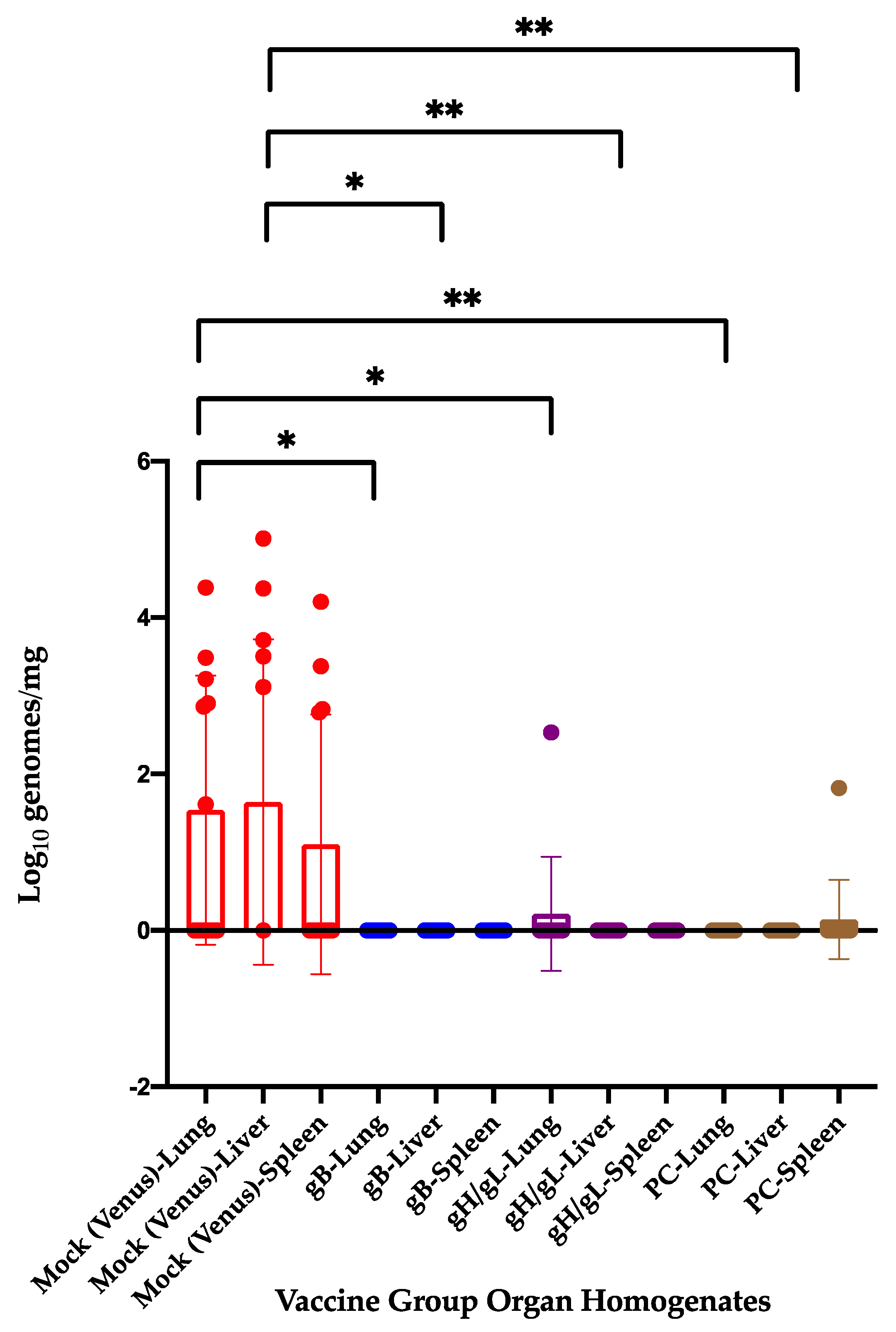
3.4. Previous Immunization with 22122-based Subunit MVA Vaccines Reduces Congenital GPCMV Transmission and Pup Viral Load from a Heterologous Strain following Viral Challenge in a Subsequent Pregnancy
4. Discussion
5. Conclusions
- The CIDMTR strain of GPCMV, a “clinical isolate” of the virus, is pathogenic in the guinea pig vertical transmission model.
- Animals immunized with MVA-vectored glycoprotein subunit vaccines based on the prototypical 22122 strain are protected against maternal viremia after challenge with a virulent, in vivo-passaged workpool of CIDMTR virus in a second pregnancy.
- The magnitude and duration of maternal CIDMTR DNAemia was noted to be statistically reduced in dams previously immunized with MVA/gB vaccine.
- Upon CIDMTR virus challenge in a second pregnancy, pups born to dams previously vaccinated with 22122 MVA/PC vaccine had statistically enhanced birth weights.
- All glycoprotein vaccine strategies protected against pup mortality and cCMV transmission in this reinfection model compared to the vector-only control.
- Based on its isolation from the same commercial vivarium, its biological/in vivo characterization, and its absolute identity at the sequence level for glycoprotein sequences annotated to date, the TAMYC strain of GPCMV is, pending additional sequence analyses/clarification, apparently identical to the CIDMTR strain. This independent confirmation of the novel CIDMTR strain should help inform and direct studies of cross-strain protection engendered by vaccines for HCMV.
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ORF | Glycoprotein | Percentage Identity |
|---|---|---|
| GP55 | Glycoprotein B | 99.8% |
| GP115 | Glycoprotein L | 99.2% |
| GP133 | PC; Homolog of UL131 | 92.1% |
| GP131 | PC; Homolog of UL130 | 89.5% |
| GP75 | Glycoprotein H | 84.4% |
| GP74 | Glycoprotein O | 81.1% |
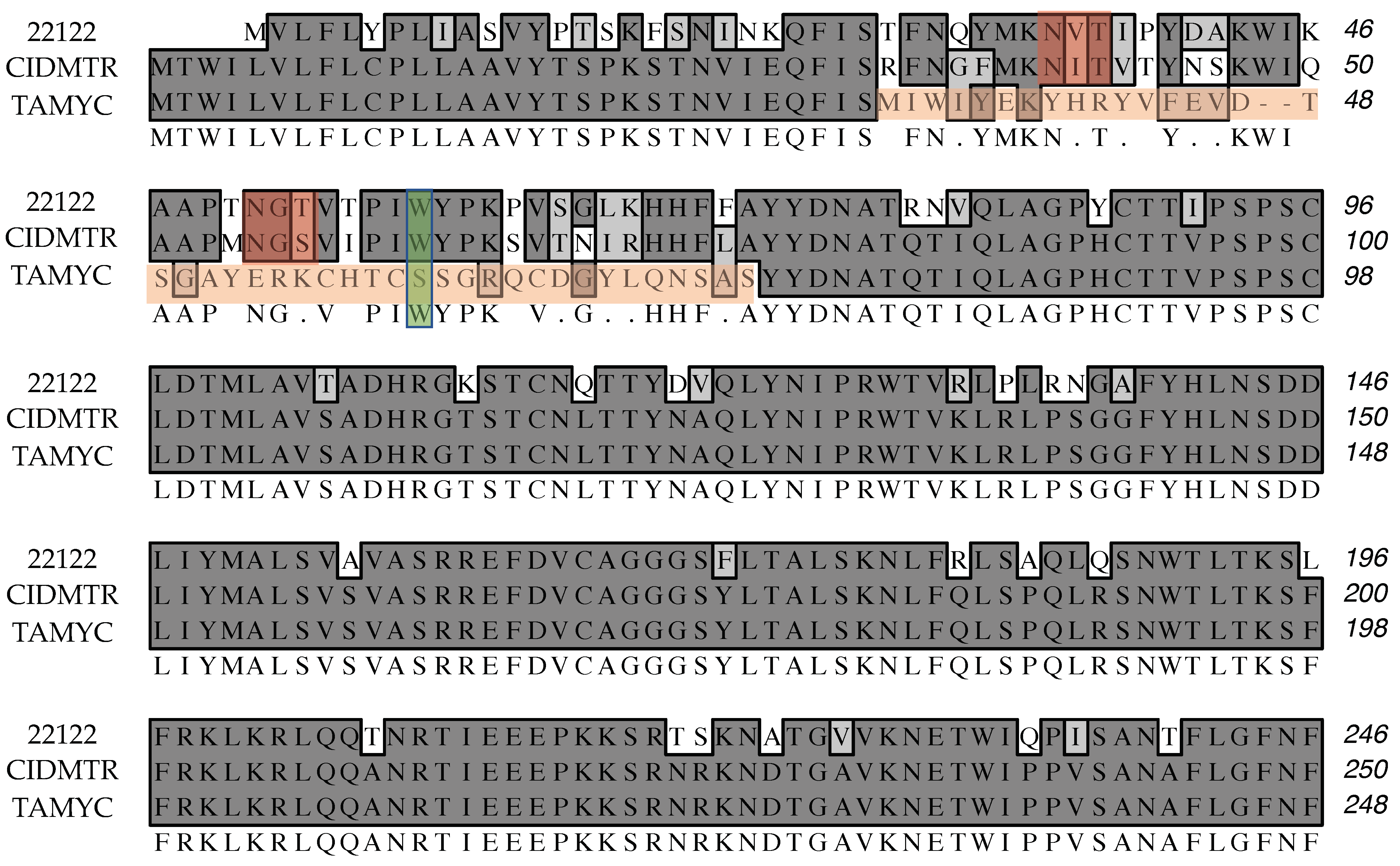

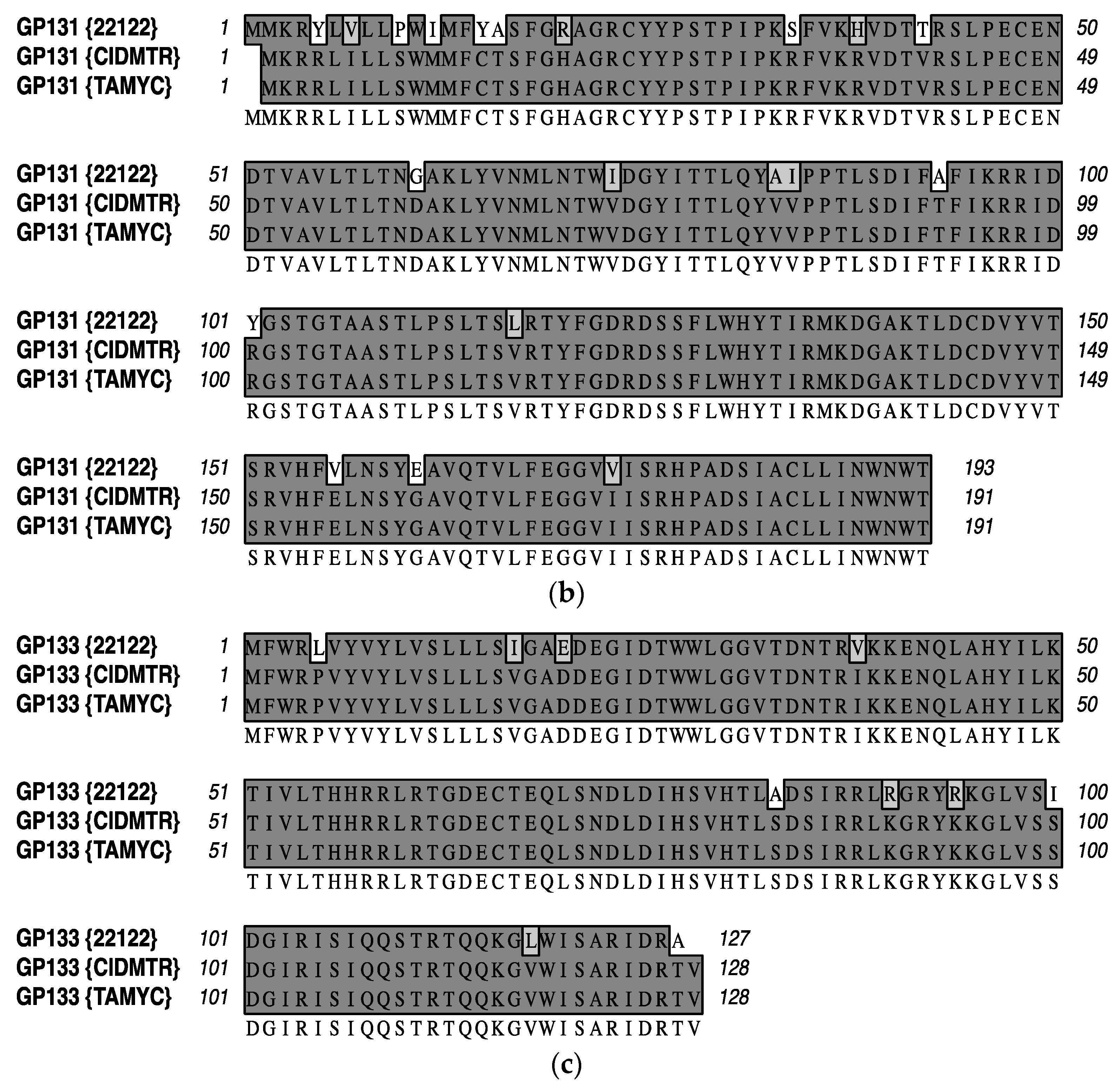
References
- Britt, W. Controversies in the natural history of congenital human cytomegalovirus infection: The paradox of infection and disease in offspring of women with immunity prior to pregnancy. Med. Microbiol. Immunol. 2015, 204, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Permar, S.R.; Schleiss, M.R.; Plotkin, S.A. Advancing Our Understanding of Protective Maternal Immunity as a Guide for Development of Vaccines To Reduce Congenital Cytomegalovirus Infections. J. Virol. 2018, 92, e00030-18. [Google Scholar] [CrossRef] [Green Version]
- Britt, W.J. Congenital Human Cytomegalovirus Infection and the Enigma of Maternal Immunity. J. Virol. 2017, 91, e02392-16. [Google Scholar] [CrossRef] [Green Version]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutre, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Yamamoto, A.Y.; Castellucci, R.A.; Aragon, D.C.; Mussi-Pinhata, M.M. Early high CMV seroprevalence in pregnant women from a population with a high rate of congenital infection. Epidemiol. Infect. 2013, 141, 2187–2191. [Google Scholar] [CrossRef]
- Yamamoto, A.Y.; Anastasio, A.R.T.; Massuda, E.T.; Isaac, M.L.; Manfredi, A.K.S.; Cavalcante, J.M.S.; Carnevale-Silva, A.; Fowler, K.B.; Boppana, S.B.; Britt, W.J.; et al. Contribution of Congenital Cytomegalovirus Infection to Permanent Hearing Loss in a Highly Seropositive Population: The Brazilian Cytomegalovirus Hearing and Maternal Secondary Infection Study. Clin. Infect. Dis. 2020, 70, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Mussi-Pinhata, M.M.; Yamamoto, A.Y.; Aragon, D.C.; Duarte, G.; Fowler, K.B.; Boppana, S.; Britt, W.J. Seroconversion for Cytomegalovirus Infection During Pregnancy and Fetal Infection in a Highly Seropositive Population: “The BraCHS Study“. J. Infect. Dis. 2018, 218, 1200–1204. [Google Scholar] [CrossRef] [Green Version]
- Mussi-Pinhata, M.M.; Yamamoto, A.Y.; Moura Brito, R.M.; de Lima Isaac, M.; de Carvalho e Oliveira, P.F.; Boppana, S.; Britt, W.J. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin. Infect. Dis. 2009, 49, 522–528. [Google Scholar] [CrossRef]
- Boeckh, M.; Geballe, A.P. Cytomegalovirus: Pathogen, paradigm, and puzzle. J. Clin. Investig. 2011, 121, 1673–1680. [Google Scholar] [CrossRef]
- Renzette, N.; Gibson, L.; Bhattacharjee, B.; Fisher, D.; Schleiss, M.R.; Jensen, J.D.; Kowalik, T.F. Rapid intrahost evolution of human cytomegalovirus is shaped by demography and positive selection. PLoS Genet. 2013, 9, e1003735. [Google Scholar] [CrossRef] [Green Version]
- Renzette, N.; Pokalyuk, C.; Gibson, L.; Bhattacharjee, B.; Schleiss, M.R.; Hamprecht, K.; Yamamoto, A.Y.; Mussi-Pinhata, M.M.; Britt, W.J.; Jensen, J.D.; et al. Limits and patterns of cytomegalovirus genomic diversity in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E4120–E4128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arav-Boger, R. Strain Variation and Disease Severity in Congenital Cytomegalovirus Infection: In Search of a Viral Marker. Infect. Dis. Clin. N. Am. 2015, 29, 401–414. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Valencia, S.M.; Pfeifer, S.P.; Jensen, J.D.; Kowalik, T.F.; Permar, S.R. Common Polymorphisms in the Glycoproteins of Human Cytomegalovirus and Associated Strain-Specific Immunity. Viruses 2021, 13, 1106. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Bialek, S.; Cannon, M.J. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin. Infect. Dis. 2011, 52, e11–e13. [Google Scholar] [CrossRef]
- Manicklal, S.; Emery, V.C.; Lazzarotto, T.; Boppana, S.B.; Gupta, R.K. The ”silent” global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 2013, 26, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.M.; McWhorter, A.R.; Masters, L.L.; Shellam, G.R.; Redwood, A.J. Laboratory strains of murine cytomegalovirus are genetically similar to but phenotypically distinct from wild strains of virus. J. Virol. 2008, 82, 6689–6696. [Google Scholar] [CrossRef] [Green Version]
- Bialas, K.M.; Tanaka, T.; Tran, D.; Varner, V.; Cisneros De La Rosa, E.; Chiuppesi, F.; Wussow, F.; Kattenhorn, L.; Macri, S.; Kunz, E.L.; et al. Maternal CD4+ T cells protect against severe congenital cytomegalovirus disease in a novel nonhuman primate model of placental cytomegalovirus transmission. Proc. Natl. Acad. Sci. USA 2015, 112, 13645–13650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, C.; Fruh, K. Rhesus CMV: An emerging animal model for human CMV. Med. Microbiol. Immunol. 2008, 197, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Lisnic, B.; Tomac, J.; Cekinovic, D.; Jonjic, S.; Juranic Lisnic, V. Rodent Models of Congenital Cytomegalovirus Infection; Methods in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2244, pp. 365–401. [Google Scholar] [CrossRef]
- Schleiss, M.R.; Hernandez-Alvarado, N.; Ramaraj, T.; Crow, J.A. Genome Sequence of a Novel, Newly Identified Isolate of Guinea Pig Cytomegalovirus, the CIDMTR Strain. Genome Announc. 2013, 1, e01052-13. [Google Scholar] [CrossRef] [Green Version]
- Schleiss, M.R.; McAllister, S.; Armien, A.G.; Hernandez-Alvarado, N.; Fernandez-Alarcon, C.; Zabeli, J.C.; Ramaraj, T.; Crow, J.A.; McVoy, M.A. Molecular and biological characterization of a new isolate of guinea pig cytomegalovirus. Viruses 2014, 6, 448–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crumpler, M.M.; Choi, K.Y.; McVoy, M.A.; Schleiss, M.R. A live guinea pig cytomegalovirus vaccine deleted of three putative immune evasion genes is highly attenuated but remains immunogenic in a vaccine/challenge model of congenital cytomegalovirus infection. Vaccine 2009, 27, 4209–4218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, H.; Wussow, F.; Fernandez-Alarcon, C.; Bierle, C.; Nguyen, J.; Diamond, D.J.; Schleiss, M.R. MVA-Vectored Pentameric Complex (PC) and gB Vaccines Improve Pregnancy Outcome after Guinea Pig CMV Challenge, but Only gB Vaccine Reduces Vertical Transmission. Vaccines 2019, 7, 182. [Google Scholar] [CrossRef] [Green Version]
- Schleiss, M.R.; Bierle, C.J.; Swanson, E.C.; McVoy, M.A.; Wang, J.B.; Al-Mahdi, Z.; Geballe, A.P. Vaccination with a Live Attenuated Cytomegalovirus Devoid of a Protein Kinase R Inhibitory Gene Results in Reduced Maternal Viremia and Improved Pregnancy Outcome in a Guinea Pig Congenital Infection Model. J. Virol. 2015, 89, 9727–9738. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Tamburro, K.; Dittmer, D.; Cui, X.; McVoy, M.A.; Hernandez-Alvarado, N.; Schleiss, M.R. Complete genome sequence of pathogenic Guinea pig cytomegalovirus from salivary gland homogenates of infected animals. Genome Announc. 2013, 1, e0005413. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.S.; Vera Cruz, D.; Su, M.; Xie, G.; Vandergrift, N.; Pass, R.F.; Forman, M.; Diener-West, M.; Koelle, K.; Arav-Boger, R.; et al. Intrahost Dynamics of Human Cytomegalovirus Variants Acquired by Seronegative Glycoprotein B Vaccinees. J. Virol. 2019, 93, e01695-18. [Google Scholar] [CrossRef] [Green Version]
- Hartley, J.W.; Rowe, W.P.; Huebner, R.J. Serial propagation of the guinea pig salivary gland virus in tissue culture. Proc. Soc. Exp. Biol. Med. 1957, 96, 281–285. [Google Scholar] [CrossRef]
- Middelkamp, J.N.; Patrizi, G.; Reed, C.A. Light and electron microscopic studies of the guinea pig cytomegalovirus. J. Ultrastruct. Res. 1967, 18, 85–101. [Google Scholar] [CrossRef]
- Choi, Y.C.; Hsiung, G.D. Cytomegalovirus infection in guinea pigs. II. Transplacental and horizontal transmission. J. Infect. Dis. 1978, 138, 197–202. [Google Scholar] [CrossRef]
- Nozawa, N.; Yamamoto, Y.; Fukui, Y.; Katano, H.; Tsutsui, Y.; Sato, Y.; Yamada, S.; Inami, Y.; Nakamura, K.; Yokoi, M.; et al. Identification of a 1.6 kb genome locus of guinea pig cytomegalovirus required for efficient viral growth in animals but not in cell culture. Virology 2008, 379, 45–54. [Google Scholar] [CrossRef] [Green Version]
- McVoy, M.A.; Wang, J.B.; Dittmer, D.P.; Bierle, C.J.; Swanson, E.C.; Fernandez-Alarcon, C.; Hernandez-Alvarado, N.; Zabeli, J.C.; Schleiss, M.R. Repair of a Mutation Disrupting the Guinea Pig Cytomegalovirus Pentameric Complex Acquired during Fibroblast Passage Restores Pathogenesis in Immune-Suppressed Guinea Pigs and in the Context of Congenital Infection. J. Virol. 2016, 90, 7715–7727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.Y.; El-Hamdi, N.S.; McGregor, A. Convalescent Immunity to Guinea Pig Cytomegalovirus Induces Limited Cross Strain Protection against Re-Infection but High-Level Protection against Congenital Disease. Int. J. Mol. Sci. 2020, 21, 5997. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, C.; Abdellatif, M.E.A.; Laib Sampaio, K.; Walther, P.; Sinzger, C. Importance of Highly Conserved Peptide Sites of Human Cytomegalovirus gO for Formation of the gH/gL/gO Complex. J. Virol. 2016, 91, e01339-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Vaccine Group | Animals Bred for 2nd Pregnancy | Previous Pup Mortality Post-22122 Challenge | Previous GPCMV Transmission Rate |
|---|---|---|---|
| Venus (Vector) | 3 | 4/4 (100%) | 3/3 (100%) 1 |
| MVA-gB | 2 | 0/4 (0%) | 0/4 (0%) |
| MVA-gH/gL | 3 | 0/6 (0%) | 0/3 (0%) 1 |
| MVA-PC | 4 | 0/10 (0%) | 0/3 (0%) 1 |
| All Vaccine Groups | 9 | 0/20 (0%) | 0/10 |
| Vaccine Group | Live Pups | Dead Pups | Mortality Rate |
|---|---|---|---|
| Venus (Vector) | 6 | 6 | 50% |
| MVA-gB | 9 | 0 | 0% |
| MVA-gH/gL | 11 | 2 | 15% |
| MVA-PC | 12 | 1 | 14% |
| All Vaccine Groups | 32 | 3 | 8.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Alarcón, C.; Buchholz, G.; Contreras, H.; Wussow, F.; Nguyen, J.; Diamond, D.J.; Schleiss, M.R. Protection against Congenital CMV Infection Conferred by MVA-Vectored Subunit Vaccines Extends to a Second Pregnancy after Maternal Challenge with a Heterologous, Novel Strain Variant. Viruses 2021, 13, 2551. https://doi.org/10.3390/v13122551
Fernández-Alarcón C, Buchholz G, Contreras H, Wussow F, Nguyen J, Diamond DJ, Schleiss MR. Protection against Congenital CMV Infection Conferred by MVA-Vectored Subunit Vaccines Extends to a Second Pregnancy after Maternal Challenge with a Heterologous, Novel Strain Variant. Viruses. 2021; 13(12):2551. https://doi.org/10.3390/v13122551
Chicago/Turabian StyleFernández-Alarcón, Claudia, Grace Buchholz, Heidi Contreras, Felix Wussow, Jenny Nguyen, Don J. Diamond, and Mark R. Schleiss. 2021. "Protection against Congenital CMV Infection Conferred by MVA-Vectored Subunit Vaccines Extends to a Second Pregnancy after Maternal Challenge with a Heterologous, Novel Strain Variant" Viruses 13, no. 12: 2551. https://doi.org/10.3390/v13122551






