A20 Enhances the Expression of the Proto-Oncogene C-Myc by Downregulating TRAF6 Ubiquitination after ALV-A Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Plasmids
2.2. Overexpression and shRNA Interference, Plasmid Construction, and Expression Analysis
2.3. RNA Extraction and Semi-Quantitative PCR
2.4. Co-Immunoprecipitation Analysis
2.5. Ubiquitination Assay
2.6. Western Blotting
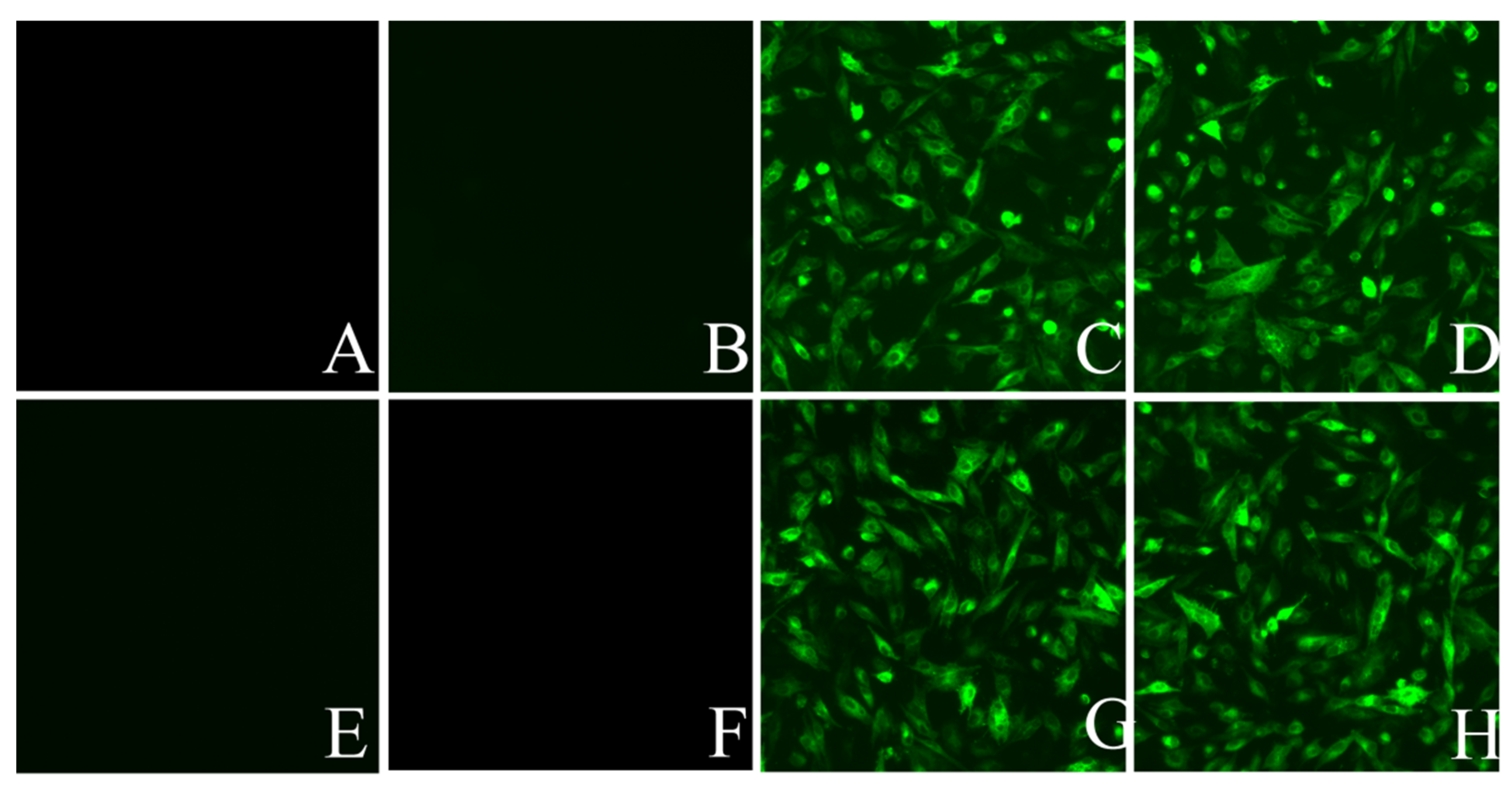
2.7. Indirect Immunofluorescence Assay (IFA)
2.8. Statistical Analysis
3. Results
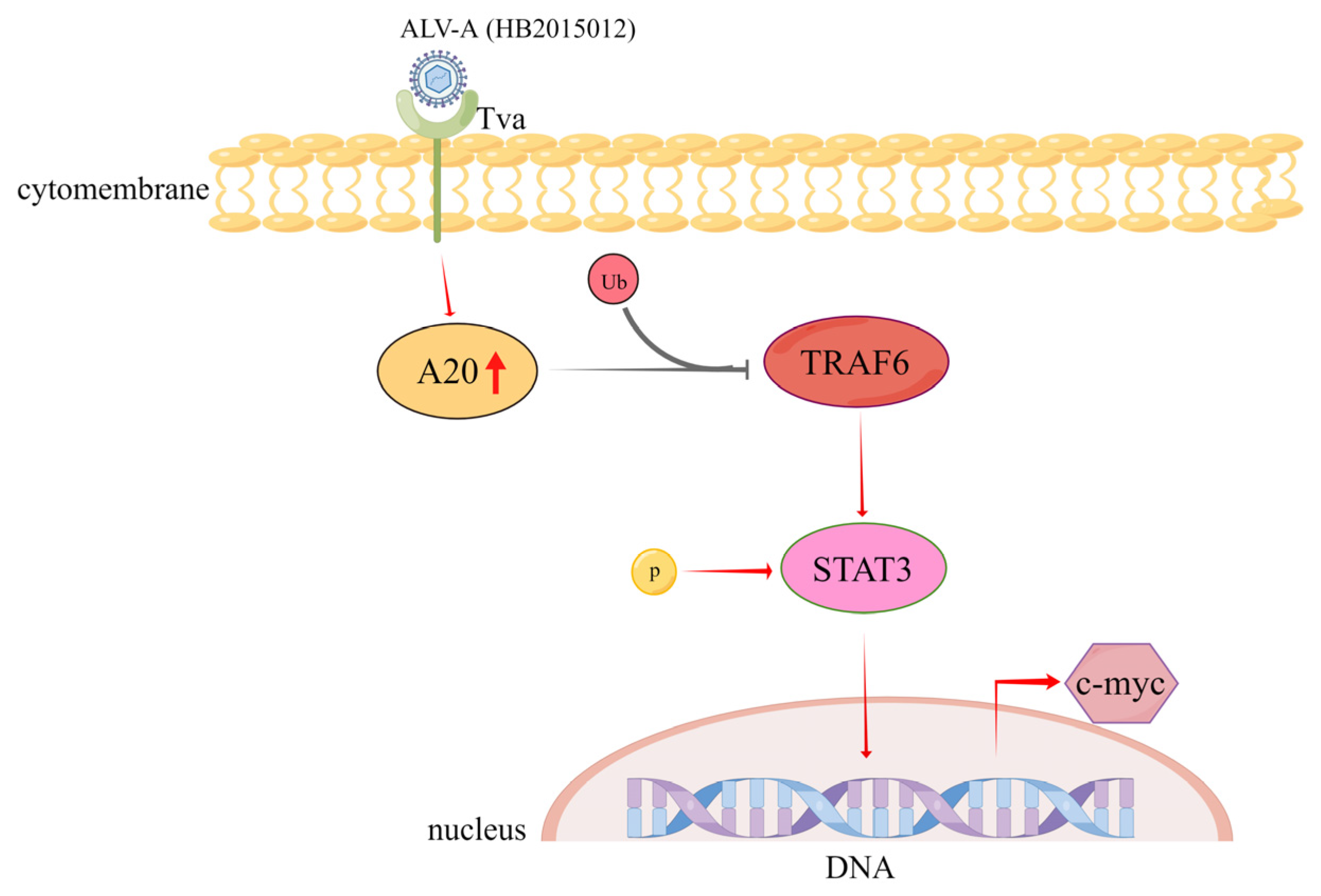
3.1. ALV-A Upregulates the Expression of A20
3.2. Overexpression and Knockdown of A20 Could Influence ALV-A Virus Replication
3.3. ALV-A Inhibits Ubiquitination of TRAF6 via A20
3.4. A20 Affects the Phosphorylation of STAT3 via TRAF6
3.5. STAT3 Promotes the Expression of C-Myc
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Qu, Y.; Liu, L.; Niu, Y.; Qu, Y.; Li, N.; Sun, W.; Lv, C.; Wang, P.; Zhang, G.; Liu, S. Viral proliferation and expression of tumor-related gene in different chicken embryo fibroblasts infected with different tumorigenic phenotypes of avian leukosis virus subgroup. J. Poult. Sci. 2016, 95, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Gu, Y.; Chen, X.; Li, T.; Gao, Y.; Wang, X.; Fang, C.; Fang, S.; Yang, Y. Identification and characterization of a novel natural recombinant avian leucosis virus from Chinese indigenous chicken flock. Virus Genes 2019, 55, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhou, D.; Xue, J.; Zhou, J.; Zhang, Y.; Zheng, G.; Yuan, S.; Yao, Y.; Cheng, Z. Interplay between CTHRC1 and the SU protein of avian leukosis virus subgroup J (ALV-J) facilitates viral replication. Virus Res. 2019, 264, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, P.; Cui, Z.-Z. Identification of a new subgroup of avian leukosis virus isolated from Chinese indigenous chicken breeds. Chin. J. Virol. 2012, 28, 609–614. [Google Scholar]
- Cui, Z.; Guo, H.; Sun, S. Prevalence Situation and Prevention and Control of Avian Leukosis. Chin. J. Vet. Drug 2009, 43, 37–41. [Google Scholar]
- Cui, Z. Technical scheme on prevention and control of avian Leucosis in breeding chicken farm. China Poultry 2015, 37, 1–7. [Google Scholar] [CrossRef]
- Payne, L.N.; Nair, V. The long view: 40 years of avian leukosis research. Avian Pathol. 2012, 41, 11–19. [Google Scholar] [CrossRef]
- Martens, A.; Van Loo, G. A20 at the Crossroads of Cell Death, Inflammation, and Autoimmunity. Cold Spring Harb. Perspect. Biol. 2019, 12, a036418. [Google Scholar] [CrossRef]
- Malynn, B.A.; Ma, A. A20: A multifunctional tool for regulating immunity and preventing disease. Cell. Immunol. 2019, 340, 103914. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Chung, J.Y.; Lamothe, B.; Rajashankar, K.; Lu, M.; Lo, Y.-C.; Lam, A.Y.; Darnay, B.G.; Wu, H. Molecular Basis for the Unique Deubiquitinating Activity of the NF-κB Inhibitor A20. J. Mol. Biol. 2008, 376, 526–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komander, D.; Barford, D. Structure of the A20 OTU domain and mechanistic insights into deubiquitination. Biochem. J. 2007, 409, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Bosanac, I.; Wertz, I.E.; Pan, B.; Yu, C.; Kusam, S.; Lam, C.; Phu, L.; Phung, Q.; Maurer, B.; Arnott, D.; et al. Ubiquitin Binding to A20 ZnF4 Is Required for Modulation of NF-κB Signaling. Mol. Cell 2010, 40, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Wertz, I.E.; O’Rourke, K.M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 2004, 430, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Ciccacci, C.; Latini, A.; Perricone, C.; Conigliaro, P.; Colafrancesco, S.; Ceccarelli, F.; Priori, R.; Conti, F.; Perricone, R.; Novelli, G.; et al. TNFAIP3 Gene Polymorphisms in Three Common Autoimmune Diseases: Systemic Lupus Erythematosus, Rheumatoid Arthritis, and Primary Sjogren Syndrome—Association with Disease Susceptibility and Clinical Phenotypes in Italian Patients. J. Immunol. Res. 2019, 2019, 6728694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westra, H.-J.; Martínez-Bonet, M.; Onengut-Gumuscu, S.; Lee, A.; Luo, Y.; Teslovich, N.; Worthington, J.; Martin, J.; Huizinga, T.; Klareskog, L.; et al. Fine-mapping and functional studies highlight potential causal variants for rheumatoid arthritis and type 1 diabetes. Nat. Genet. 2018, 50, 1366–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reek, J.V.D.; Coenen, M.; Arias, M.V.D.L.; Zweegers, J.; Rodijk-Olthuis, D.; Schalkwijk, J.; Vermeulen, S.; Joosten, I.; van de Kerkhof, P.; Seyger, M.; et al. Polymorphisms in CD84, IL12B and TNFAIP3 are associated with response to biologics in patients with psoriasis. Br. J. Dermatol. 2017, 176, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Schuijs, M.J.; Willart, M.A.; Vergote, K.; Gras, D.; Deswarte, K.; Ege, M.J.; Madeira, F.B.; Beyaert, R.; van Loo, G.; Bracher, F.; et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 2015, 349, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Baldassano, R.; Zhang, H.; Qu, H.-Q.; Imielinski, M.; Kugathasan, S.; Annese, V.; Dubinsky, M.; Rotter, J.I.; Russell, R.K.; et al. Comparative genetic analysis of inflammatory bowel disease and type 1 diabetes implicates multiple loci with opposite effects. Hum. Mol. Genet. 2010, 19, 2059–2067. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Wei, W.; Xiao, W.; Al-Saleem, E.D.; Nejati, R.; Chen, L.; Yin, J.; Fabrizio, J.; Petrus, M.N.; Waldmann, T.A.; et al. Essential role of the linear ubiquitin chain assembly complex and TAK1 kinase in A20 mutant Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 2020, 117, 28980–28991. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.A.; Küppers, R. NF-κB deregulation in Hodgkin lymphoma. Semin. Cancer Biol. 2016, 39, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Krappmann, D.; Vincendeau, M. Mechanisms of NF-κB deregulation in lymphoid malignancies. Semin. Cancer Biol. 2016, 39, 3–14. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Cai, Y.; Liu, R.; Lu, M.; Li, T.; Fu, Y.; Guo, M.; Huang, H.; Ou, Y.; et al. A20 targets PFKL and glycolysis to inhibit the progression of hepatocellular carcinoma. Cell Death Dis. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, D.; Zhang, X.; Han, S.; Yang, Y.; Ma, J.; Meng, D. A20 enhances the radiosensitivity of hepatocellular carcinoma cells to 60Co-γ ionizing radiation. Oncotarget 2017, 8, 93103–93116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Hu, L.; Luo, Z.; Zhang, J.; Zhang, C.; Qiu, B.; Dong, L.; Tan, Y.; Ding, J.; Tang, S.; et al. A20 suppresses hepatocellular carcinoma proliferation and metastasis through inhibition of Twist1 expression. Mol. Cancer 2015, 14, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Wang, X.; Wang, J.; Wang, X.; Zhou, H.; Zhang, L. The dual roles of A20 in cancer. Cancer Lett. 2021, 511, 26–35. [Google Scholar] [CrossRef]
- Lee, E.; Ouzounova, M.; Piranlioglu, R.; Ma, M.T.; Guzel, M.; Marasco, D.; Chadli, A.; Gestwicki, J.E.; Cowell, J.K.; Wicha, M.S.; et al. The pleiotropic effects of TNFα in breast cancer subtypes is regulated by TNFAIP3/A20. Oncogene 2018, 38, 469–482. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jung, S.M.; Yang, K.-M.; Bae, E.; Ahn, S.G.; Park, J.S.; Seo, D.; Kim, M.; Ha, J.; Lee, J.; et al. A20 promotes metastasis of aggressive basal-like breast cancers through multi-monoubiquitylation of Snail1. Nature 2017, 19, 1260–1273. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, L.; Guo, B.; Yuan, S.; Nie, J.; Wei, Y. Mechanism of Zinc Relieves Inflammatory Response Mediated by Zinc Finger Protein A20 in Chicken Intestinal Epithelial Cells. Chin. J. Animal Nutr. 2019, 31, 2254–2266. [Google Scholar]
- Moon, H.; Park, H.; Ro, S.W. c-Myc-driven Hepatocarcinogenesis. Anticancer Res. 2021, 41, 4937–4946. [Google Scholar] [CrossRef]
- Ye, L.; Pan, J.; Liang, M.; Pasha, M.A.; Shen, X.; D’Souza, S.S.; Fung, I.T.H.; Wang, Y.; Patel, G.; Tang, D.D.; et al. A critical role for c-Myc in group 2 innate lymphoid cell activation. Allergy 2019, 75, 841–852. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Q.; Zhang, L.; Zhang, Q.; Hu, L.; Li, C.; Wang, S.; Li, J.; Zhang, Y.; Yu, H.; et al. Encephalomyocarditis Virus 3C Protease Relieves TRAF Family Member-associated NF-κB Activator (TANK) Inhibitory Effect on TRAF6-mediated NF-κB Signaling through Cleavage of TANK. J. Biol. Chem. 2015, 290, 27618–27632. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Cai, B.; Ma, M.; Luo, W.; Zhang, Z.; Zhang, X.; Nie, Q. ALDH1A1 Inhibits Chicken Preadipocytes’ Proliferation and Differentiation via the PPARγ Pathway In Vitro and In Vivo. Int. J. Mol. Sci. 2020, 21, 3150. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Mizushima, S.-I.; Azuma, S.; Kobayashi, N.; Tojo, T.; Suzuki, K.; Aizawa, S.; Watanabe, T.; Mosialos, G.; Kieff, E.; et al. Identification of TRAF6, a Novel Tumor Necrosis Factor Receptor-associated Factor Protein That Mediates Signaling from an Amino-terminal Domain of the CD40 Cytoplasmic Region. J. Biol. Chem. 1996, 271, 28745–28748. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Xiong, J.; Takeuchi, M.; Kurama, T.; Goeddel, D.V. TRAF6 is a signal transducer for interleukin-1. Nature 1996, 383, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, X.-X.; Wang, J.-R.; Yang, T.-Y.; Li, X.-M.; He, X.-S.; Li, Y.; Ye, W.-L.; Wu, Y.; Gan, W.-J.; et al. TRAF6 inhibits colorectal cancer metastasis through regulating selective autophagic CTNNB1/β-catenin degradation and is targeted for GSK3B/GSK3β-mediated phosphorylation and degradation. Autophagy 2019, 15, 1506–1522. [Google Scholar] [CrossRef]
- Mo, G.; Fu, H.; Hu, B.; Zhang, Q.; Xian, M.; Zhang, Z.; Lin, L.; Shi, M.; Nie, Q.; Zhang, X. SOCS3 Promotes ALV-J Virus Replication via Inhibiting JAK2/STAT3 Phosphorylation During Infection. Front. Cell. Infect. Microbiol. 2021, 11, 748795. [Google Scholar] [CrossRef]
- Seffens, A.; Herrera, A.; Tegla, C.; Buus, T.B.; Hymes, K.B.; Ødum, N.; Geskin, L.J.; Koralov, S.B. STAT3 Dysregulation in Mature T and NK Cell Lymphomas. Cancers 2019, 11, 1711. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Liu, L.; Li, T.; Fang, S.; Yang, Y.; Gu, Y. Preliminary report on natural cases of mixed avian leukemia induced by ALV-A in laying hens with myeloma and lymphoma. China Poultry 2017, 39, 63–66. [Google Scholar] [CrossRef]
- Fadly, A.M. Avian Retroviruses. Veter. Clin. North Am. Food Anim. Pract. 1997, 13, 71–85. [Google Scholar] [CrossRef]
- Mooney, E.; Sahingur, S. The Ubiquitin System and A20: Implications in Health and Disease. J. Dent. Res. 2020, 100, 10–20. [Google Scholar] [CrossRef]
- Yang, M.; Jin, M.; Li, K.; Liu, H.; Yang, X.; Zhang, X.; Zhang, B.; Gong, A.; Bie, Q. TRAF6 Promotes Gastric Cancer Cell Self-Renewal, Proliferation, and Migration. Stem Cells Int. 2020, 2020, 3296192. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Jiang, M.; Tai, G. Mechanism by which TRAF6 Participates in the Immune Regulation of Autoimmune Diseases and Cancer. BioMed Res. Int. 2020, 2020, 4607197. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Liang, C.; Hua, J.; Zhang, B.; Liu, J.; Zhang, Y.; Wei, M.; Yu, X.; Xu, J.; Shi, S. A miR-146a-5p/TRAF6/NF-kB p65 axis regulates pancreatic cancer chemoresistance: Functional validation and clinical significance. Theranostics 2020, 10, 3967–3979. [Google Scholar] [CrossRef]
- Rong, Y.; Wang, D.; Wu, W.; Jin, D.; Kuang, T.; Ni, X.; Zhang, L.; Lou, W. TRAF6 is over-expressed in pancreatic cancer and promotes the tumorigenicity of pancreatic cancer cells. Med. Oncol. 2014, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Li, L.; Yang, S.; Wang, D.; Zhou, S.; Chen, X.; Tang, J. Regulatory role of tumor necrosis factor receptor-associated factor 6 in breast cancer by activating the protein kinase B/glycogen synthase kinase 3β signaling pathway. Mol. Med. Rep. 2017, 16, 2269–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Li, Y.-C.; Wu, L.; Yu, G.-T.; Zhang, W.-F.; Huang, C.-F.; Sun, Z.-J. TRAF6 regulates tumour metastasis through EMT and CSC phenotypes in head and neck squamous cell carcinoma. J. Cell. Mol. Med. 2018, 22, 1337–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.-X.; Zhao, W.; Shi, Y.; Li, Y.-N.; Zhang, L.-S.; Zhang, H.-Q.; Wang, D. Retinoic acid amide inhibits JAK/STAT pathway in lung cancer which leads to apoptosis. Tumor Biol. 2015, 36, 8671–8678. [Google Scholar] [CrossRef] [PubMed]
- Kowshik, J.; Baba, A.B.; Giri, H.; Reddy, G.D.; Dixit, M.; Nagini, S. Astaxanthin Inhibits JAK/STAT-3 Signaling to Abrogate Cell Proliferation, Invasion and Angiogenesis in a Hamster Model of Oral Cancer. PLoS ONE 2014, 9, e109114. [Google Scholar] [CrossRef] [PubMed]
- Tošić, I.; Frank, D.A. STAT3 as a mediator of oncogenic cellular metabolism: Pathogenic and therapeutic implications. Neoplasia 2021, 23, 1167–1178. [Google Scholar] [CrossRef]
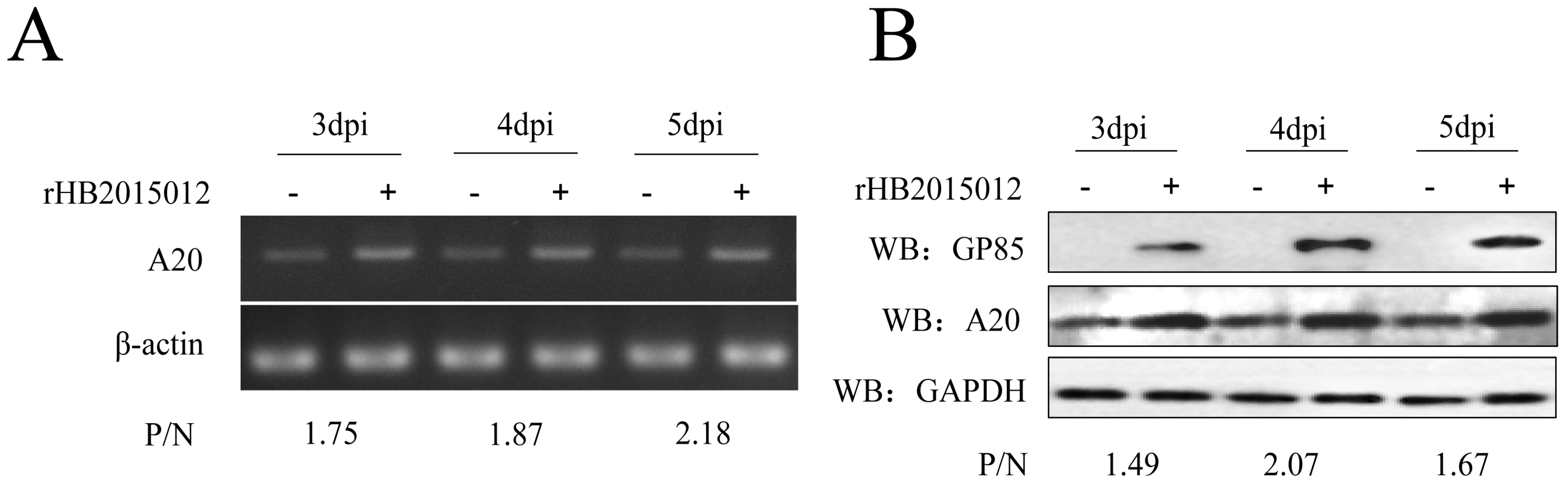
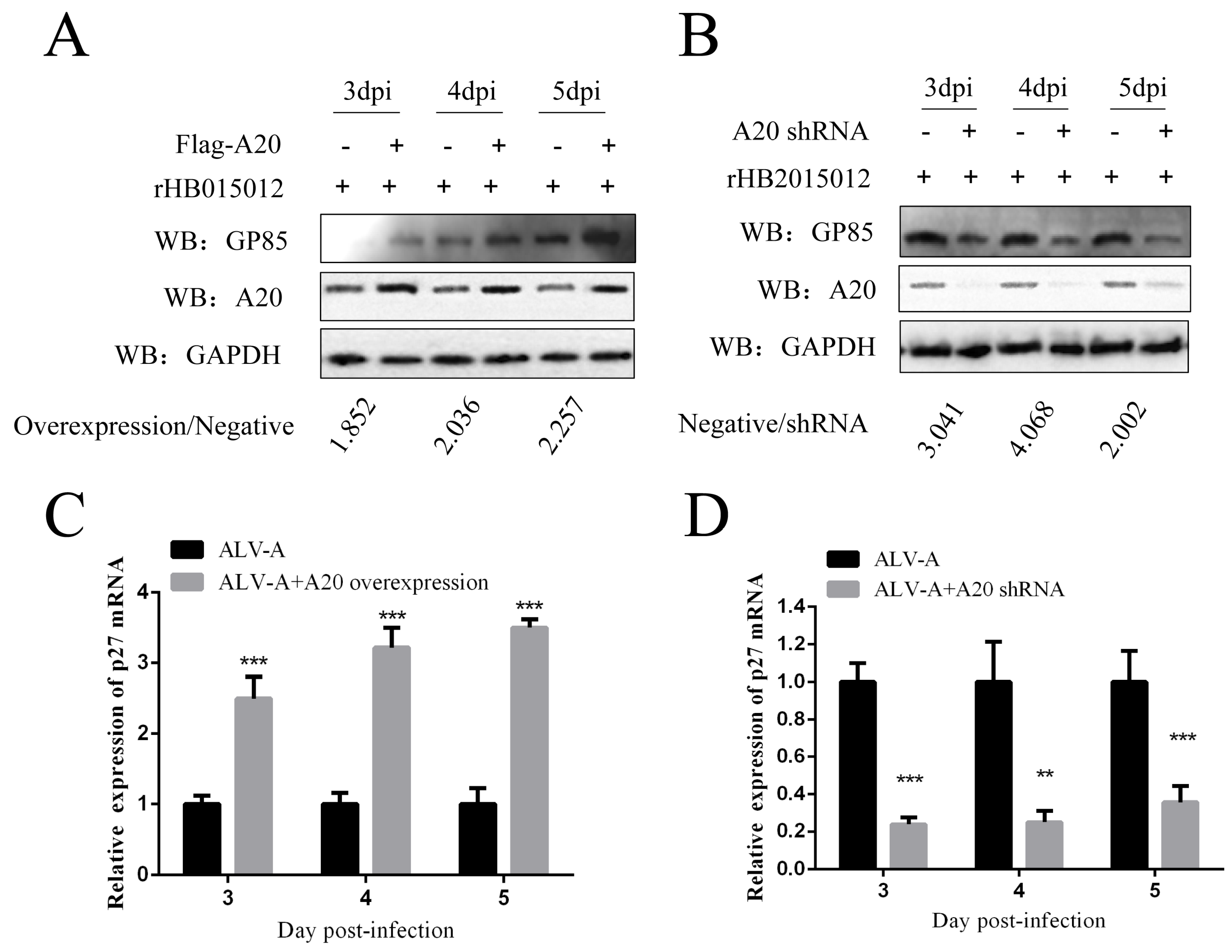

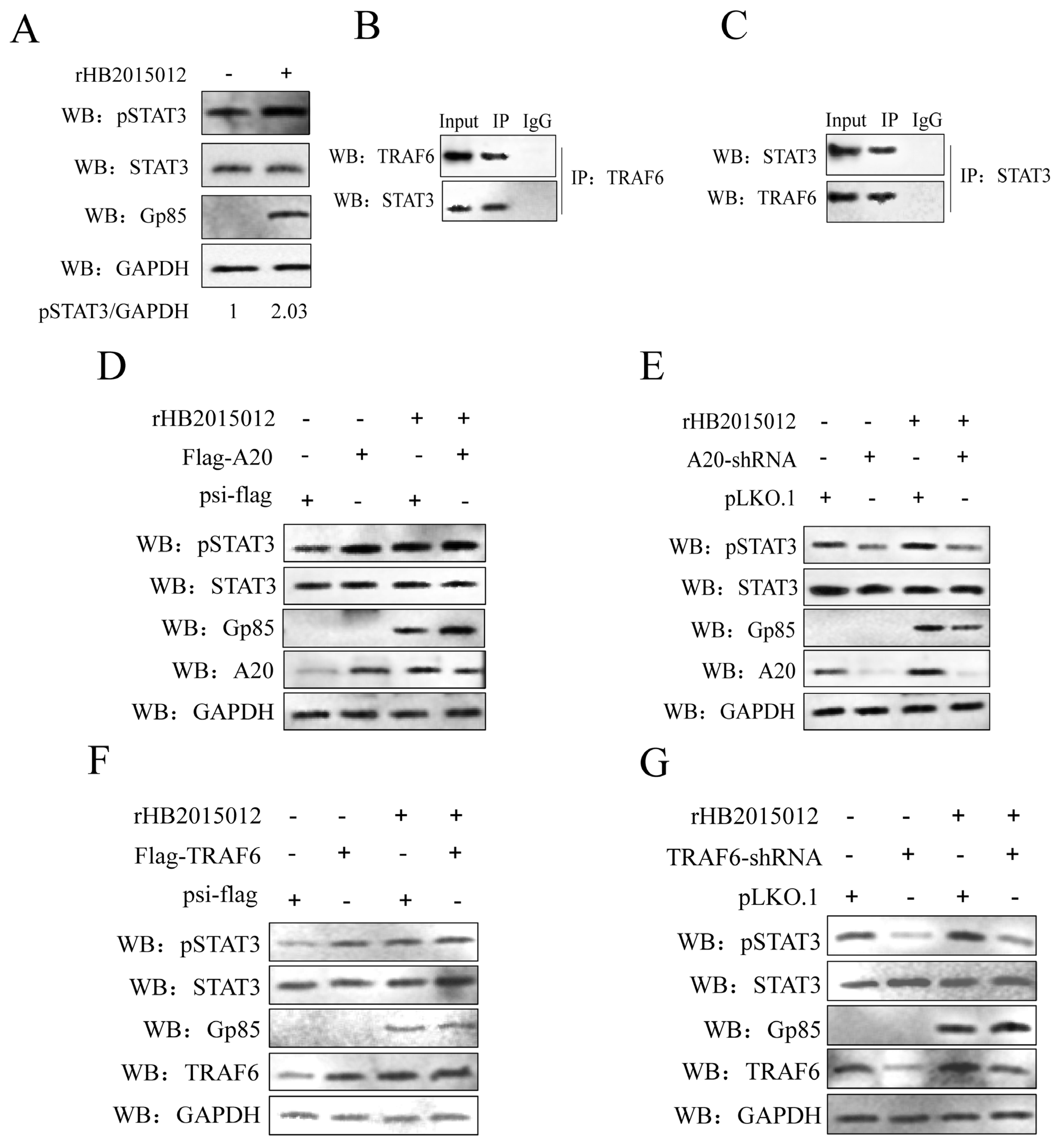
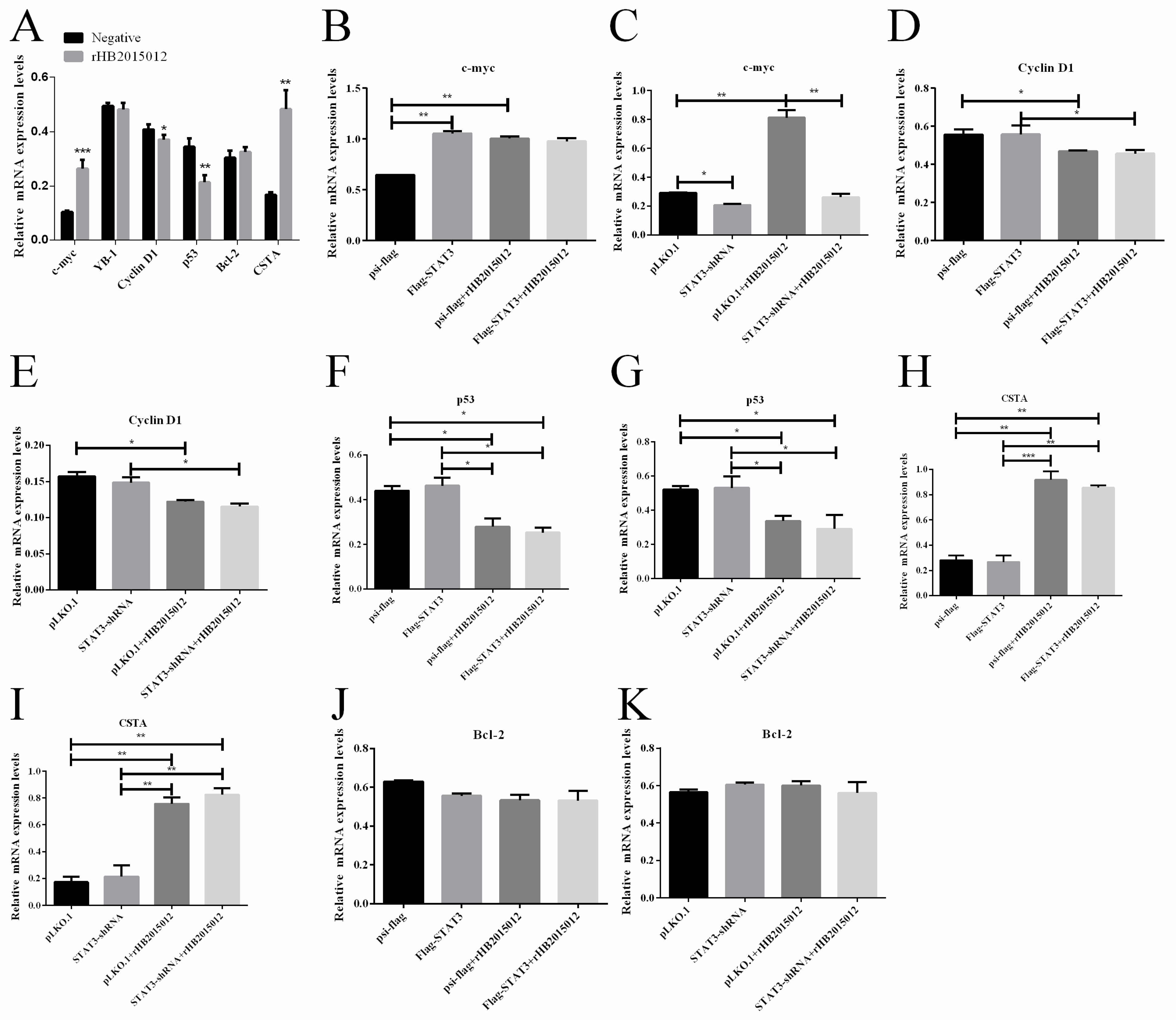
| Plasmids | Primers | Sequences (5′-3′) |
|---|---|---|
| psi-flag-A20 | F | CGCGGATCCATGGCTGGCCAACACATCCTTCCTC |
| R | CCGCTCGAGGCCGTAGATCTGTTTGAACTGGTAGCATTCA | |
| pCMV-HA-A20 | F | GCCATGGAGGCCCGAATTCGGTCGACCATGGCTGGCCAACACATCCT |
| R | ATCCCCGCGGCCGCGGTACCTCGAGGCCGTAGATCTGTTTGAACTGGT | |
| psi-flag-TRAF6 | F | CGGGATCCATGAGCTTGCTACACAGTGATAG |
| R | CCGCTCGAGTTACGCAGCTCCATCAGTACTG | |
| pCMV-HA-TRAF6 | F | CCATGGAGGCCCGAATTCGGTCGACCATGAGCTTGCTACACAGTGATAGC |
| R | ATCCCCGCGGCCGCGGTACCTCGAGTTACGCAGCTCCATCAGTACTG | |
| psi-flag-STAT3 | F | CGGGATCCATGGCGCAGTGGAACCAACT |
| R | CCGCTCGAGTCACATTGGTGAGGAAGCACACT | |
| pCMV-HA-STAT3 | F | CCATGGAGGCCCGAATTCGGTCGACCATGGCGCAGTGGAACCAACT |
| R | ATCCCCGCGGCCGCGGTACCTCGAGTCACATTGGTGAGGAAGCACACT | |
| pLKO.1-GFP-A20 | F | GATCCGCTTTGTATCAGAGCAATATGTTCAAGAGACATATTGCTCTGATACAAAGCTTTTTTG |
| R | AATTCAAAAAAGCTTTGTATCAGAGCAATATGTCTCTTGAACATATTGCTCTGATACAAAGCG | |
| pLKO.1-GFP-TRAF6 | F | GATCCGCAGCAGATGCCTAACCATTATTCAAGAGATAATGGTTAGGCATCTGCTGCTTTTTTG |
| R | AATTCAAAAAAGCAGCAGATGCCTAACCATTATCTCTTGAATAATGGTTAGGCATCTGCTGCG | |
| pLKO.1-GFP-STAT3 | F | GATCCGCTGTCAGCCATGGAGTATGTTTCAAGAGAACATACTCCATGGCTGACAGCTTTTTTG |
| R | AATTCAAAAAAGCTGTCAGCCATGGAGTATGTTCTCTTGAAACATACTCCATGGCTGACAGCG | |
| psi-flag-myc-chUb | F | GGGCGGATCCGCGATACCGGAATTCGAGCAGAAGCTGATCTCAGAGGAGGACCTGATGCAGATCTTCGTGAAGACCCTG |
| R | TGCGCCTGAGGGGAGGCTAACTCGAGCAATTCGTCGAGGGACCTAA |
| Gene | Primers | Sequences (5′-3′) |
|---|---|---|
| YB-1 | F | ACCGTGGAGTTTGATGTGGTT |
| R | CTTCCGGGATGTTCTCTGCTC | |
| Cyclin D1 | F | TCAAGTGCGTGCAGAAGGAA |
| R | CTGCGGTCAGAGGAATCGTT | |
| c-myc | F | TTCTTTGCCCTGCGTGACC |
| R | GCCTCAACTGCTCTTTCTCTGC | |
| β-actin | F | CAACACACTGCTGTCTGGTGGTA |
| R | ATCGTACTCCTGCTTGCTGATCC | |
| A20 | F | TGGGGCTCGAAACAGACTTC |
| R | TTGTCGTAGCCGAGCACAAT | |
| P27 | F | CCGGGGGAATTGGTTGCTAT |
| R | ATCTGGCTGTGACTTCTGCC | |
| Bcl2 | F | CGCTACCAGAGGGACTTCG |
| R | TTGACCCCATCACGGAAGAG | |
| CSTA | F | ACCGGACTCAAGTGGTTGC |
| R | CCGGTAAGGCTGGGATGTT | |
| P53 | F | ATGGCGGAGGAGATGGAACC |
| R | CAATGGCAGAGGTGGTGGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, X.; Yang, Y.; Fang, C.; Liu, J.; Liang, X.; Yang, Y. A20 Enhances the Expression of the Proto-Oncogene C-Myc by Downregulating TRAF6 Ubiquitination after ALV-A Infection. Viruses 2022, 14, 2210. https://doi.org/10.3390/v14102210
Chen X, Wang X, Yang Y, Fang C, Liu J, Liang X, Yang Y. A20 Enhances the Expression of the Proto-Oncogene C-Myc by Downregulating TRAF6 Ubiquitination after ALV-A Infection. Viruses. 2022; 14(10):2210. https://doi.org/10.3390/v14102210
Chicago/Turabian StyleChen, Xueyang, Xingming Wang, Yuxin Yang, Chun Fang, Jing Liu, Xiongyan Liang, and Yuying Yang. 2022. "A20 Enhances the Expression of the Proto-Oncogene C-Myc by Downregulating TRAF6 Ubiquitination after ALV-A Infection" Viruses 14, no. 10: 2210. https://doi.org/10.3390/v14102210




