Drivers of Spatial Expansions of Vampire Bat Rabies in Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. National Surveillance VBR Data
2.2. Colombian Biogeographical Regions and Municipality Data
2.3. Identifying Changes in the Area Affected by VBR
2.4. Seasonality Analysis
2.5. Identifying Drivers of Potential Spatial Expansions
3. Results
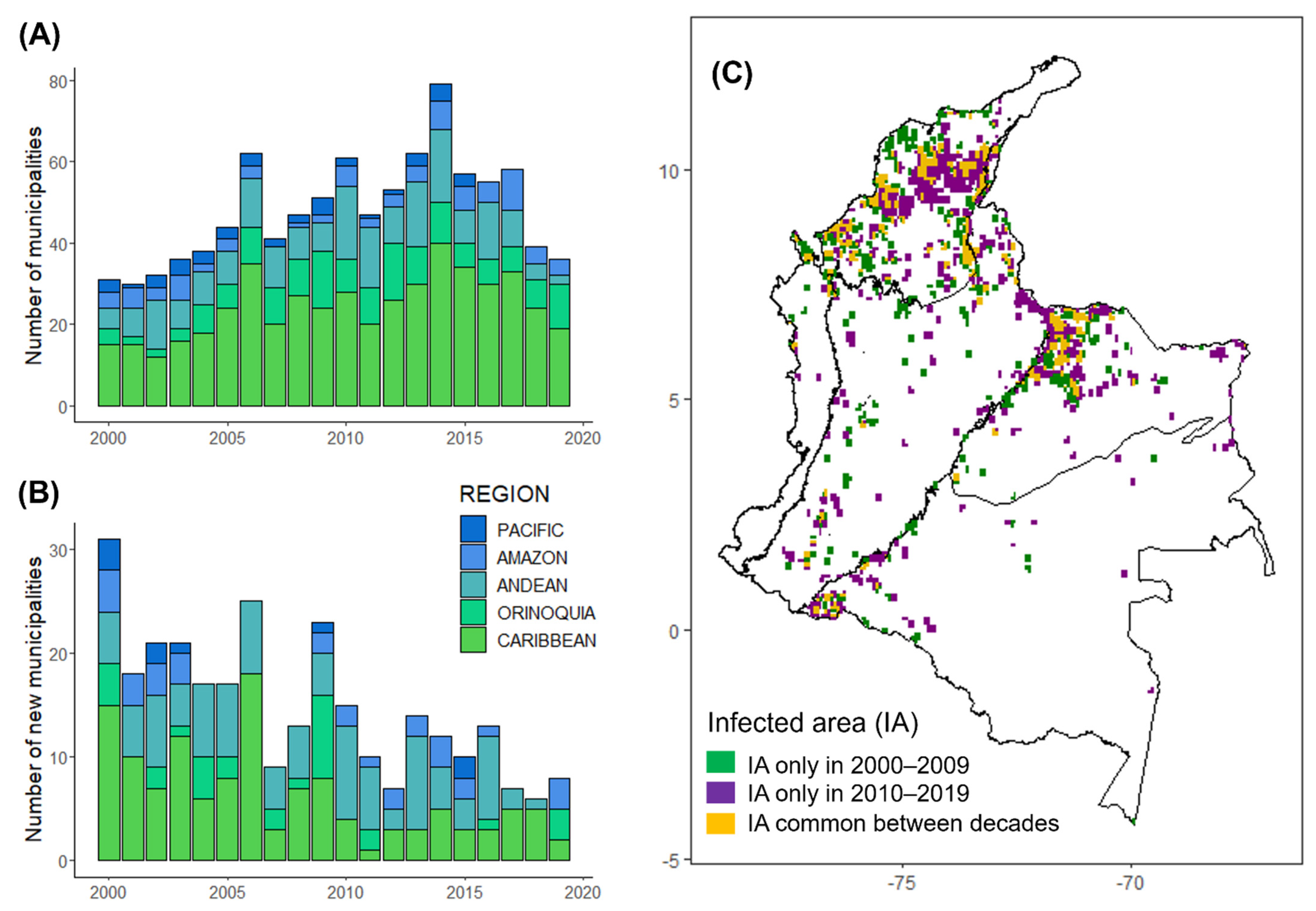
3.1. Spatio-Temporal Distribution of VBR
3.2. Changes in the VBR-Infected Area
3.3. Potential Drivers of Spatial Expansions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, N.; Aréchiga-Ceballos, N.; Aguilar-Setien, A. Vampire Bat Rabies: Ecology, Epidemiology and Control. Viruses 2014, 6, 1911–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benavides, J.A.; Valderrama, W.; Streicker, D.G. Spatial Expansions and Travelling Waves of Rabies in Vampire Bats. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160328. [Google Scholar] [CrossRef] [Green Version]
- Benavides, J.A.; Rojas Paniagua, E.; Hampson, K.; Valderrama, W.; Streicker, D.G. Quantifying the Burden of Vampire Bat Rabies in Peruvian Livestock. PLoS Negl. Trop. Dis. 2017, 11, e0006105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito-Hoyos, D.M.; Sierra, E.B.; Álvarez, R.V. Distribución Geográfica Del Riesgo de Rabia de Origen Silvestre y Evaluación de Los Factores Asociados Con Su Incidencia En Colombia, 1982–2010. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2013, 33, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Bárcenas-Reyes, I.; Loza-Rubio, E.; Zendejas-Martínez, H.; Luna-Soria, H.; Cantó-Alarcón, G.J.; Milián-Suazo, F. Comportamiento Epidemiológico de La Rabia Paralítica Bovina En La Región Central de México, 2001-2013. Rev. Panam. Salud Pública 2015, 38, 396–402. [Google Scholar] [PubMed]
- Cárdenas-Contreras, Z. Análisis Espacio Temporal de La Rabia Bovina de Origen Silvestre En Colombia (2005–2014). Master’s Thesis, Universidad Autónoma de Barcelona, Barcelona, Spain, 2017. [Google Scholar]
- Botto Nuñez, G.; Becker, D.J.; Plowright, R.K. The Emergence of Vampire Bat Rabies in Uruguay within a Historical Context. Epidemiol. Infect. 2019, 147, e180. [Google Scholar] [CrossRef] [Green Version]
- Blackwood, J.C.; Streicker, D.G.; Altizer, S.; Rohani, P. Resolving the Roles of Immunity, Pathogenesis, and Immigration for Rabies Persistence in Vampire Bats. Proc. Natl. Acad. Sci. USA 2013, 110, 20837–20842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escobar, L.E.; Peterson, T.; Favi, M.; Yung, V.; Medina-Vogel, G. Bat-Borne Rabies in Latin America. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 63–72. [Google Scholar] [CrossRef]
- McClure, K.M.; Gilbert, A.T.; Chipman, R.B.; Rees, E.E.; Pepin, K.M. Variation in Host Home Range Size Decreases Rabies Vaccination Effectiveness by Increasing the Spatial Spread of Rabies Virus. J. Anim. Ecol. 2020, 89, 1375–1386. [Google Scholar] [CrossRef] [Green Version]
- Mungrue, K.; Mahabir, R. The Rabies Epidemic in Trinidad of 1923 to 1937: An Evaluation with a Geographic Information System. Wilderness Environ. Med. 2011, 22, 28–36. [Google Scholar] [CrossRef]
- Torres, C.; Lema, C.; Gury Dohmen, F.; Beltran, F.; Novaro, L.; Russo, S.; Freire, M.C.; Velasco-Villa, A.; Mbayed, V.A.; Cisterna, D.M. Phylodynamics of Vampire Bat-Transmitted Rabies in Argentina. Mol. Ecol. 2014, 23, 2340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streicker, D.G.; Winternitzc, J.C.; Satterfield, D.A.; Condori-Condori, R.E.; Broos, A.; Tello, C.; Recuenco, S.; Velasco-Villa, A.; Altizer, S.; Valderrama, W. Host-Pathogen Evolutionary Signatures Reveal Dynamics and Future Invasions of Vampire Bat Rabies. Proc. Natl. Acad. Sci. USA 2016, 113, 10926–10931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, B.L.; Bruhn, F.R.P.; Coelho, A.C.B.; Estima-Silva, P.; Echenique, J.V.; Sallis, E.S.V.; Schild, A.L. Epidemiological Study of Rabies in Cattle in Southern Brazil: Spatial and Temporal Distribution from 2008 to 20171. Pesqui. Vet. Bras. 2019, 39, 460–468. [Google Scholar] [CrossRef]
- Delpietro, H.A.; Marchevsky, N.; Simonetti, E. Relative Population Densities and Predation of the Common Vampire Bat (Desmodus Rotundus) in Natural and Cattle-Raising Areas in North-East Argentina. Prev. Vet. Med. 1992, 14, 13–20. [Google Scholar] [CrossRef]
- Lee, D.N.; Papeş, M.; Van Den Bussche, R.A. Present and Potential Future Distribution of Common Vampire Bats in the Americas and the Associated Risk to Cattle. PLoS ONE 2012, 7, e42466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Streicker, D.G.; Lemey, P.; Velasco-Villa, A.; Rupprecht, C.E. Rates of Viral Evolution Are Linked to Host Geography in Bat Rabies. PLoS Pathog. 2012, 8, e1002720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, R.D. Seasonal Reproduction of Vampire Bats and Its Relation to Seasonality of Bovine Rabies. J. Wildl. Dis. 1992, 28, 292–294. [Google Scholar] [CrossRef] [PubMed]
- ICA. Sanidad Animal 2016; Dirección Tecnica de Vigilancia Epidemiológica. Subgerencia de Protección Animal. Instituto Colombiano Agropecuario; Produmedios: Bogotá, Colombia, 2019. [Google Scholar]
- Etter, A.; McAlpine, C.; Wilson, K.; Phinn, S.; Possingham, H. Regional Patterns of Agricultural Land Use and Deforestation in Colombia. Agric. Ecosyst. Environ. 2006, 114, 369–386. [Google Scholar] [CrossRef]
- Gonzáles, M.; García, H.; Corzo, G.; Madriñan, S. Ecosistemas Terrestres de Colombia y El Mundo. In Biodiversidad, Conservación y Desarrollo; Sánchez, J.A., Mandriñán, S., Eds.; Universidad de los Andes, Ediciones Uniandes: Bogotá, Colombia, 2012; Volume I, pp. 69–113. [Google Scholar]
- Urrea, V.; Ochoa, A.; Mesa, O. Seasonality of Rainfall in Colombia. Water Resour. Res. 2019, 55, 4149–4162. [Google Scholar] [CrossRef]
- Mantilla-Meluk, H.; Mantilla-Meluk, H.; Jimenez-Ortega, A.M.; Baker, R.J. Phyllostomid Bats of Colombia: Annotated Checklist, Distribution, and Biogeography; Museum of Texas Tech University: Lubbock TX, USA, 2009; Volume 56. [Google Scholar]
- Escobar, L.E.; Peterson, A.T. Spatial Epidemiology of Bat-Borne Rabies in Colombia. Rev. Panam. Salud Pública 2013, 34, 135–136. [Google Scholar]
- Streicker, D.G.; Fallas González, S.L.; Luconi, G.; Barrientos, R.G.; Leon, B. Phylodynamics Reveals Extinction–Recolonization Dynamics Underpin Apparently Endemic Vampire Bat Rabies in Costa Rica. Proc. R. Soc. B Biol. Sci. 2019, 286. [Google Scholar] [CrossRef] [PubMed]
- OIE Rabies (Infection with Rabies Virus and Other Lyssaviruses). Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.17_RABIES.pdf (accessed on 5 August 2020).
- QGIS.org. QGIS Geographic Information System. Available online: http://www.qgis.org (accessed on 29 June 2020).
- IGAC Grandes Biomas y Biomas Continentales de Colombia. Insituto Geográfico Agustin Codazzi. Available online: https://geoportal.igac.gov.co/sites/geoportal.igac.gov.co/files/geoportal/grandes_biomas.pdf (accessed on 29 June 2020).
- Wand, M. KernSmooth: Functions for Kernel Smoothing Supporting Wand & Jones, 1995. R Package Version 2.23-20. Available online: https://cran.r-project.org/package=KernSmooth (accessed on 1 September 2021).
- Ávila-Flores, R.; Bolaina-Badal, A.L.; Gallegos-Ruiz, A.; Sánchez-Gómez, W.S.; Ávila-Flores, R.; Bolaina-Badal, A.L.; Gallegos-Ruiz, A.; Sánchez-Gómez, W.S. Use of Linear Features by the Common Vampire Bat (Desmodus Rotundus) in a Tropical Cattle-Ranching Landscape. Therya 2019, 10, 229–234. [Google Scholar] [CrossRef]
- Ulloa-Stanojlovic, F.M.; Dias, R.A. Spatio-temporal Description of Bovine Rabies Cases in Peru, 2003–2017. Transbound. Emerg. Dis. 2020, 67, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- George, D.B.; Webb, C.T.; Farnsworth, M.L.; O’Shea, T.J.; Bowen, R.A.; Smith, D.L.; Stanley, T.R.; Ellison, L.E.; Rupprecht, C.E. Host and Viral Ecology Determine Bat Rabies Seasonality and Maintenance. Proc. Natl. Acad. Sci. USA 2011, 108, 10208–10213. [Google Scholar] [CrossRef] [Green Version]
- Streicker, D.G.; Recuenco, S.; Valderrama, W.; Benavides, J.G.; Vargas, I.; Pacheco, V.; Condori Condori, R.E.; Montgomery, J.; Rupprecht, C.E.; Rohani, P.; et al. Ecological and Anthropogenic Drivers of Rabies Exposure in Vampire Bats: Implications for Transmission and Control. Proc. R. Soc. B Biol. Sci. 2012, 279, 3384–3392. [Google Scholar] [CrossRef] [Green Version]
- Benavides, J.A.; Valderrama, W.; Recuenco, S.; Uieda, W.; Suzán, G.; Avila-Flores, R.; Velasco-Villa, A.; Almeida, M.; de Andrade, F.A.G.; Molina-Flores, B.; et al. Defining New Pathways to Manage the Ongoing Emergence of Bat Rabies in Latin America. Viruses 2020, 12, 1002. [Google Scholar] [CrossRef]
- Griffiths, M.E.; Bergner, L.M.; Broos, A.; Meza, D.K.; Filipe, A. da S.; Davison, A.; Tello, C.; Becker, D.J.; Streicker, D.G. Epidemiology and Biology of a Herpesvirus in Rabies Endemic Vampire Bat Populations. Nat. Commun. 2020, 11, 5951. [Google Scholar] [CrossRef]
- Anderson, A.; Shwiff, S.; Gebhardt, K.; Ramírez, A.J.; Shwiff, S.; Kohler, D.; Lecuona, L. Economic Evaluation of Vampire Bat (Desmodus Rotundus) Rabies Prevention in Mexico. Transbound. Emerg. Dis. 2014, 61, 140–146. [Google Scholar] [CrossRef] [Green Version]
- ICA. Sanidad Animal 2005; Boletines Epidemiológicos; Dirección Tecnica de Vigilancia Epidemiológica. Subgerencia de Protección Animal. Instituto Colombiano Agropecuario; Imprenta Nacional de Colombia: Bogotá, Colombia, 2007. [Google Scholar]
- Seetahal, J.F.R.; Vokaty, A.; Carrington, C.V.F.; Adesiyun, A.A.; Mahabir, R.; Hinds, A.Q.J.; Rupprecht, C.E. The History of Rabies in Trinidad: Epidemiology and Control Measures. Trop. Med. Infect. Dis. 2017, 2, 27. [Google Scholar] [CrossRef]
- ICA. No Resolución 00383 de 2015; Ministerio de Agricultura y Desarrollo Rural: Bogotá, Colombia, 2015; p. 20. [Google Scholar]
- ICA. Resolución 23132 de 2018; Ministerio de Agricultura y Desarrollo Rural: Bogotá, Colombia, 2018; p. 37. [Google Scholar]


| Variable | Value | Std. Error | DF | t-Value | p-Value |
|---|---|---|---|---|---|
| (Intercept) | 1.88 | 0.15 | 239 | 12.39 | 0.00 |
| Altitude | 1.59 × 10−5 | 8.02 × 10−5 | 239 | 0.20 | 0.84 |
| Precipitation | 5.16 × 10−5 | 5.66 × 10−5 | 239 | 0.91 | 0.36 |
| Cattle population | −2.00 × 10−6 | 9.50 × 10−7 | 239 | −2.12 | 0.04 * |
| Distance to the reporting office | 2.34 × 10−3 | 9.43 × 10−4 | 239 | 2.48 | 0.01 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Sereno, Z.E.; Streicker, D.G.; Medina-Rodríguez, A.T.; Benavides, J.A. Drivers of Spatial Expansions of Vampire Bat Rabies in Colombia. Viruses 2022, 14, 2318. https://doi.org/10.3390/v14112318
Rojas-Sereno ZE, Streicker DG, Medina-Rodríguez AT, Benavides JA. Drivers of Spatial Expansions of Vampire Bat Rabies in Colombia. Viruses. 2022; 14(11):2318. https://doi.org/10.3390/v14112318
Chicago/Turabian StyleRojas-Sereno, Zulma E., Daniel G. Streicker, Andrea Tatiana Medina-Rodríguez, and Julio A. Benavides. 2022. "Drivers of Spatial Expansions of Vampire Bat Rabies in Colombia" Viruses 14, no. 11: 2318. https://doi.org/10.3390/v14112318







