Novel RT-PCR Using Sugar Chain-Immobilized Gold-Nanoparticles Correlates Patients’ Symptoms: The Follow-Up Study of COVID-19 Hospitalized Patients
Abstract
:1. Introduction
2. Methods
2.1. Diagnosis of COVID-19
2.2. Follow-Up of COVID-19 Patients
2.3. Quantitative RT-PCR
2.4. Culturing of Specimens
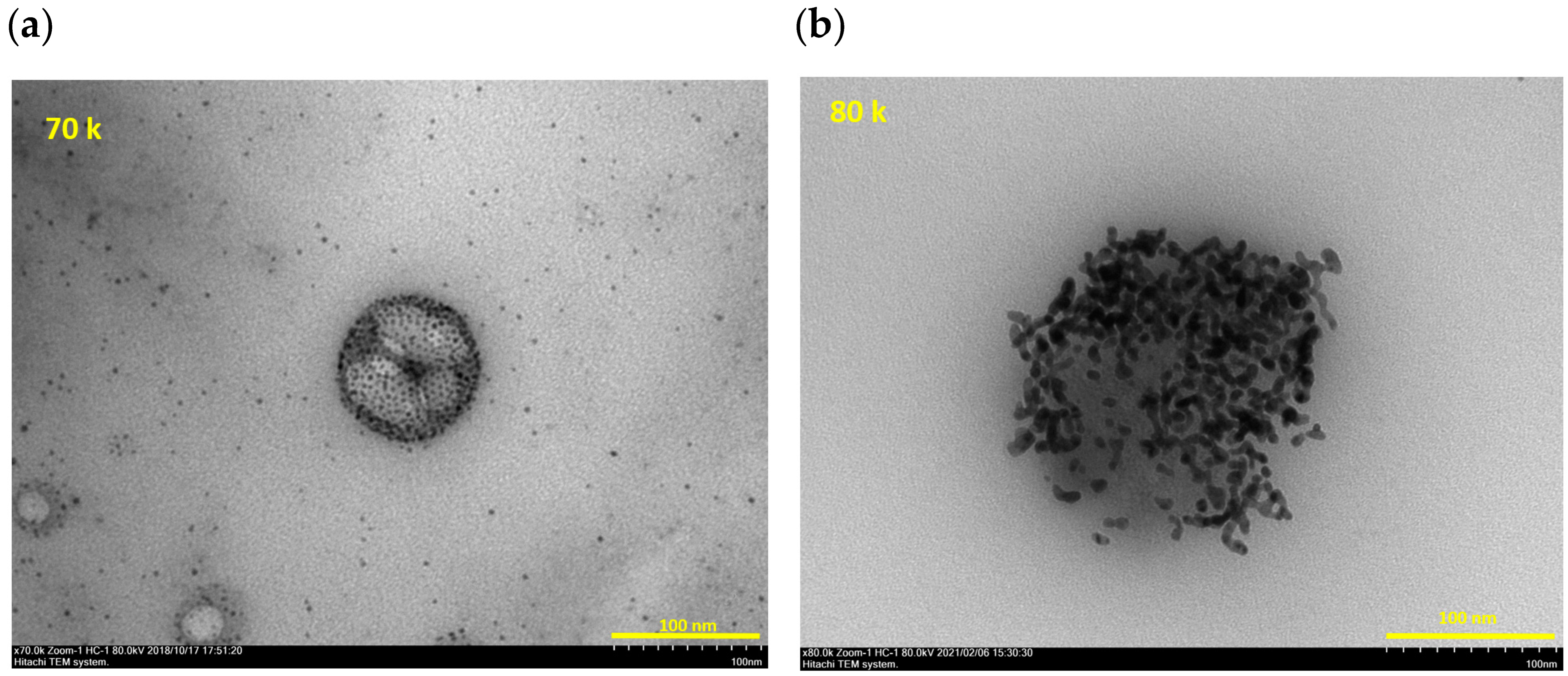
2.5. Transmittance Electro-Microscopy (TEM)
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Situation Report on COVID-19, Ministry of Health, Labour and Welfare, Japan. Available online: https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou_00006.html (accessed on 10 June 2022).
- COVID Data Tracker Weekly Review, Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html (accessed on 22 May 2022).
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet 2020, 20, 533–534. [Google Scholar] [CrossRef]
- COVID-19 Dashboard, Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://www.arcgis.com/apps/dashboards/bda7594740fd40299423467b48e9ecf6 (accessed on 22 May 2022).
- From Alpha to Omicron: Everything You Need to Know about SARS-CoV-2 Variants of Concern, Gavi, the Vaccine Alliance. Available online: https://www.gavi.org/vaccineswork/alpha-omicron-everything-you-need-know-about-coronavirus-variants-concern (accessed on 22 May 2022).
- Sheikh, A.; McMenamin, J.; Taylor, B.; Robertson, C. Robertson C, SARS-CoV-2 Delta VOC in Scotland: Demographics, risk of hospital admission, and vaccine effectiveness. Lancet 2021, 397, 2461–2462. [Google Scholar] [CrossRef]
- Omicron Variant: What You Need to Know, Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html (accessed on 26 May 2022).
- Iuliano, A.D.; Brunkard, J.M.; Boehmer, T.K.; Peterson, E.; Adjei, S.; Binder, A.M.; Cobb, S.; Graff, P.; Hidalgo, P.; Panaggio, M.J.; et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods—United States, December 2020–January 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Boom, R.C.J.A.; Sol, C.J.; Salimans, M.M.; Jansen, C.L.; Wertheim-van Dillen, P.M.; Van der Noordaa, J.P.M.E. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, Y.; Wakao, M.; Kodama, K. Method for Concentrating Viruses, Method for Concentrating Cells or Bacteria, and Magnetic Composit. U.S. Patent 9,464,281, 27 July 2010. [Google Scholar]
- Suda, Y.; Zhang, X.; Takahashi, Y.; Yokoyama, R.; Nagatomo, M.; Aoyama, K.; Okuno, T.; Saito, S.; Morikawa, S.; Hiroi, S.; et al. Discrimination of influenza virus strains and super high sensitive detection of viruses using sugar chip and sugar chain immobilized gold nanoparticles. In Tailored Polymer Architectures for Pharmaceutical and Biomedical Applications; Scholz, C., Jörg Kressler, J., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015; Volume 1135, pp. 331–350. [Google Scholar] [CrossRef]
- Saksono, B.; Dewi, B.E.; Nainggolan, L.; Suda, Y. A Highly Sensitive Diagnostic System for Detecting Dengue Viruses Using the Interaction between a Sulfated Sugar Chain and a Virion. PLoS ONE 2015, 10, e0123981. [Google Scholar] [CrossRef] [PubMed]
- Suda, Y.; Nagatomo, M.; Yokoyama, R.; Ohzono, M.; Aoyama, K.; Zhang, X.; Nakajima, K.; Murakami, N.; Shinoda, T.; Hirota, T.; et al. Highly sensitive detection of influenza virus in saliva by real-time PCR method using sugar chain-immobilized gold nanoparticles; application to clinical studies. Biotechnol. Rep. 2015, 7, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajima, Y.; Suda, Y.; Yano, K. A case report of SARS-CoV-2 confirmed in saliva specimens up to 37 days after onset: Proposal of saliva specimens for COVID-19 diagnosis and virus monitoring. J. Infect. Chemother. 2020, 26, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Manchester, M.; Steinmetz, N.F. (Eds.) Viruses and Noanotechnology. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2009; ISSN 0070-217x. [Google Scholar] [CrossRef]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramelecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tong, Z.; Jin, L.; Ye, B.; Zhou, J.; Sun, Z.; Yang, H.; Hong, L.; Huang, F.; Wang, W.; et al. An NIR discrete metallacycle constructed form perylene bisimide and tetraphenylethylene fluorophores for imaging-guided cancer radio-chemotherapy. Adv. Mater. 2022, 34, 2106388. [Google Scholar] [CrossRef]
- Gandhi, R.T.; Lynch, J.B.; Rio, C.D. Mild or Moderate COVID-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef]
- Lan, L.; Xu, D.; Ye, G.; Xia, C.; Wang, S.; Li, Y.; Xu, H. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA 2020, 323, 1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, Y.; Tajima, Y.; Nishi, J.; Kajiya, T. Diagnostic Method for COVID-19 Using Sugar Chain–Immobilized Nanoparticles and Saliva. In SARS-CoV-2 Methods and Protocols; Chu, J.J.H., Ahidjo, B.A., Mok, C.K., Eds.; Method in Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2022; Volume 2452, pp. 63–74. [Google Scholar] [CrossRef]
- Okamoto, M.; Toyama, M.; Baba, M. The chemokine receptor antagonist cenicriviroc inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020, 182, 104902. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin, P.; Morgeaua, S. Inactivation of DNA by β-propiolactone. Biologicals 1995, 23, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e15. [Google Scholar] [CrossRef] [PubMed]

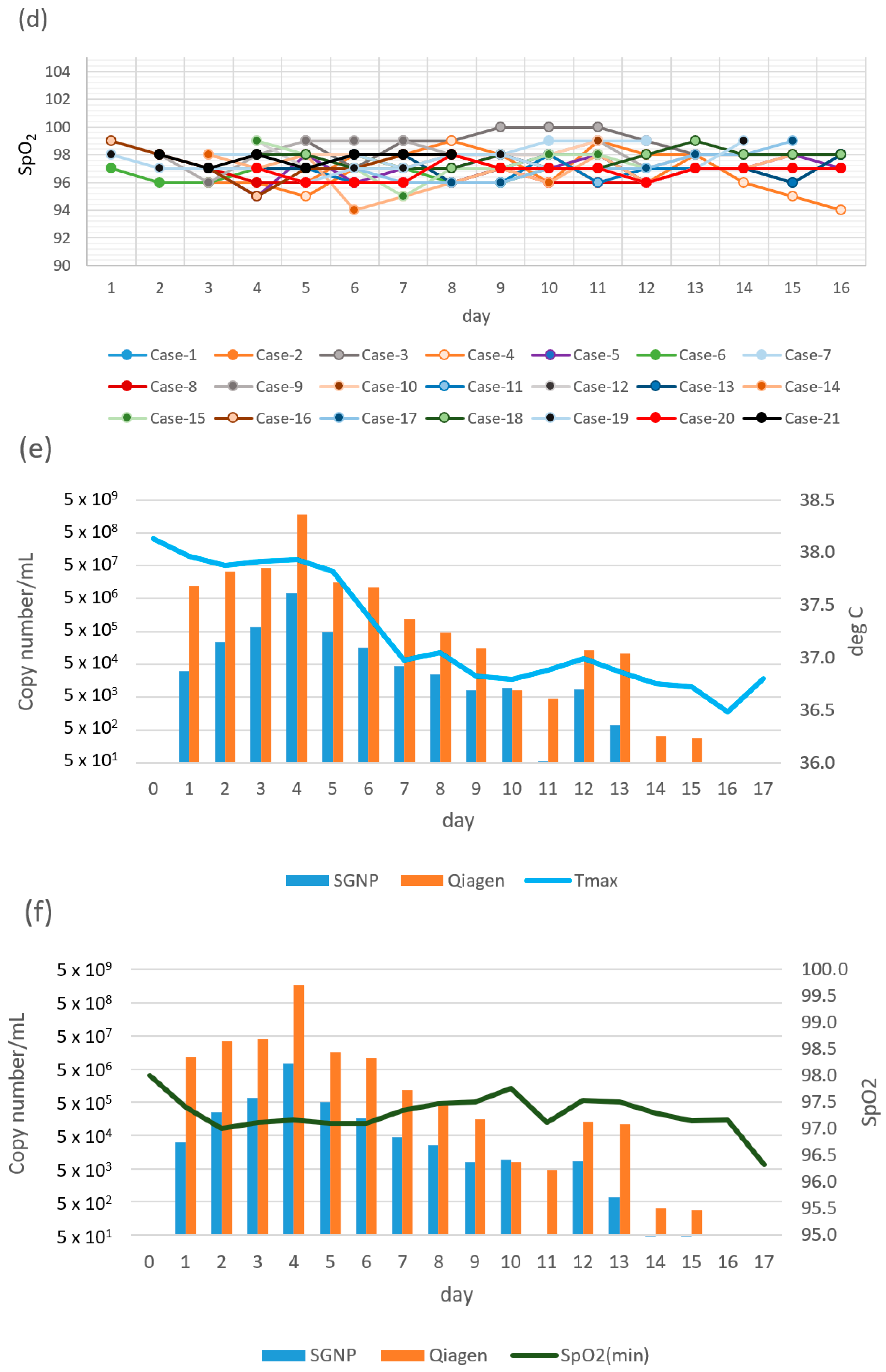
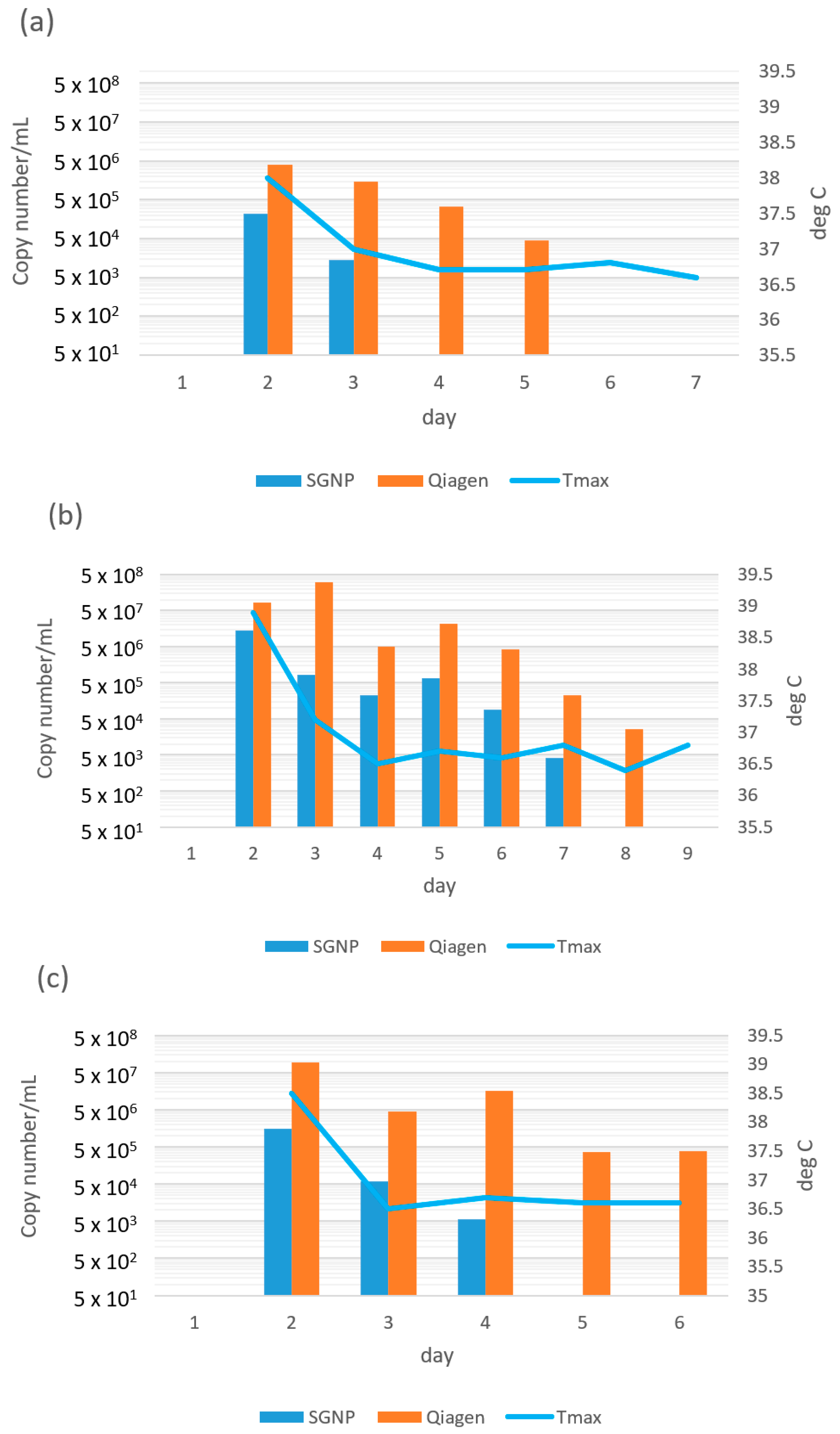
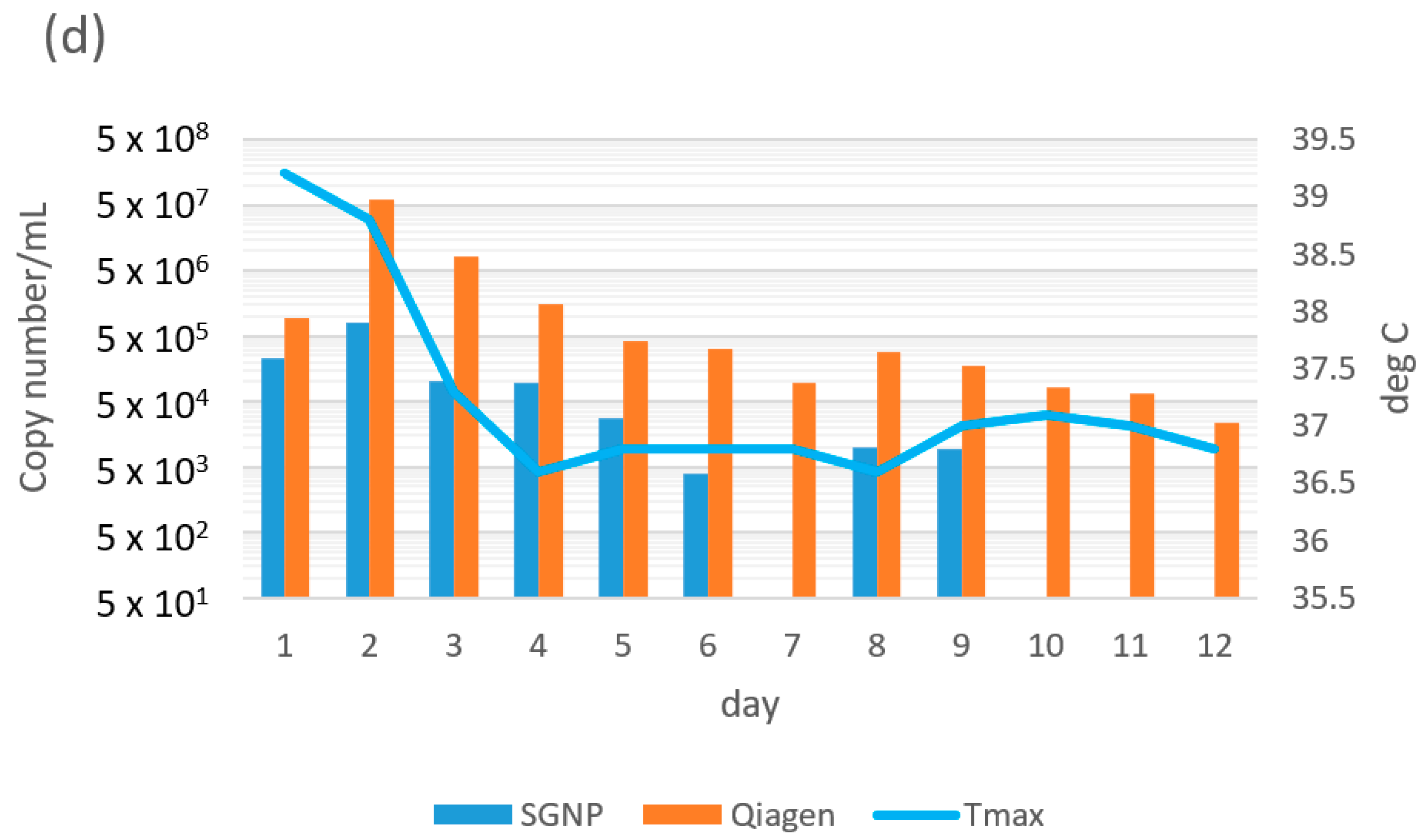
| Case | Days Since Onset to First Sampling | Variant | Diagnostic | Day 0 (Hospitalization) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 | Day 14 | Day 15 | Day 16 | Day 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | not available | SGNP | 3.4 × 103 | 1.1 × 102 | 4.0 × 10 | 3.1 × 102 | nd | nd | nd | nd | nd | nd | ||||||||
| Qiagen | 6.8 × 103 | 6.8 × 103 | 4.2 × 103 | 8.6 × 102 | 1.7 × 103 | nd | 1.3 × 103 | 3.8 × 103 | 7.5 × 103 | nd | |||||||||||
| Tmax (°C) | 37.3 | 36.8 | 36.8 | 36.9 | 36.9 | 37.2 | 37.0 | 36.9 | 37.3 | 37.3 | 37.3 | 36.8 | 37.0 | 37.1 | 37.0 | ||||||
| SpO2 (min) | 96 | 98 | 97 | 98 | 98 | 96 | 99 | 98 | 98 | 98 | 99 | 98 | 97 | 97 | 97 | ||||||
| 2 | 4 | not available | SGNP | 3.4 × 10 | 2.9 × 10 | 2.8 × 102 | nd | nd | 1.5 × 102 | ||||||||||||
| Qiagen | nd | 1.4 × 103 | 4.9 × 102 | nd | nd | 8.9 × 103 | |||||||||||||||
| Tmax (°C) | 37.7 | 37.7 | 38.4 | 37.6 | 37.7 | 37.3 | 36.9 | 36.3 | |||||||||||||
| SpO2 (min) | 96 | 95 | 96 | 96 | 96 | 90 | 99 | 97 | |||||||||||||
| 3 | 5 | not available | SGNP | 8.9 × 102 | 1.1 × 103 | 1.9 × 104 | 2.5 × 102 | 2.2 × 102 | 1.7 × 10 | nd | nd | nd | nd | ||||||||
| Qiagen | 1.8 × 102 | 5.5 × 10 | 2.4 × 103 | 7.9 × 103 | 8.2 × 102 | nd | nd | nd | nd | ||||||||||||
| Tmax (°C) | 37.3 | 36.7 | 36.8 | 36.9 | 36.8 | 36.8 | 36.5 | 36.8 | 36.8 | 36.8 | 36.6 | ||||||||||
| SpO2 (min) | 99 | 97 | 99 | 99 | 100 | 100 | 100 | 99 | 98 | 98 | 98 | ||||||||||
| 4 | 3 | not available | SGNP | 6.9 × 103 | 1.1 × 102 | 2.3 × 102 | 4.9 × 102 | nd | 5.0 | 2.0 × 10 | nd | nd | nd | nd | |||||||
| Qiagen | 9.4 × 103 | 6.3 × 102 | 1.9 × 103 | 5.1 × 102 | nd | nd | 1.5 × 102 | 1.0 × 102 | nd | 4.3 × 10 | nd | ||||||||||
| Tmax (°C) | 38.0 | 38.3 | 38.4 | 38.6 | 36.6 | 36.6 | 36.8 | 36.7 | 36.8 | 36.9 | 36.7 | 36.6 | 36.9 | 36.8 | 36.7 | 36.9 | 36.6 | 36.4 | |||
| SpO2 (min) | 96 | 96 | 95 | 97 | 98 | 99 | 98 | 98 | 96 | 96 | 98 | 96 | 95 | 94 | 96 | 95 | 98 | 98 | |||
| 5 | 3 | not available | SGNP | 6.0 × 103 | 6.2 × 103 | 4.4 × 103 | 1.8 × 104 | 1.7 × 103 | 5.8 × 10 | nd | nd | 1.3 × 102 | nd | nd | |||||||
| Qiagen | 2.0 × 106 | 6.0 × 105 | 7.7 × 105 | 2.8 × 106 | 3.1 × 105 | 8.0 × 103 | 5.3 × 102 | 2.9 × 102 | 5.5 × 103 | nd | nd | ||||||||||
| Tmax (°C) | 37.0 | 37.2 | 36.7 | 36.9 | 36.7 | 36.6 | 36.6 | 36.1 | 36.8 | 36.8 | 36.4 | 36.8 | 36.5 | 36.5 | 36.6 | 36.4 | 36.5 | ||||
| SpO2 (min) | 97 | 95 | 98 | 96 | 97 | 97 | 98 | 97 | 98 | 96 | 97 | 97 | 98 | 97 | 96 | 96 | 98 | ||||
| 6 | 1 | not available | SGNP | 1.4 × 104 | 3.2 × 102 | 2.2 × 102 | 9.1 × 103 | 7.1 × 102 | 1.6 × 102 | 5.4 × 10 | nd | nd | nd | ||||||||
| Qiagen | 3.0 × 107 | 3.8 × 105 | 2.0 × 104 | 1.2 × 106 | 1.2 × 105 | 2.5 × 105 | 1.2 × 104 | 1.0 × 104 | 1.3 × 103 | nd | |||||||||||
| Tmax (°C) | 36.5 | 36.5 | 36.5 | 37.0 | 36.1 | 36.2 | 36.3 | 36.3 | 36.4 | 36.2 | |||||||||||
| SpO2 (min) | 97 | 96 | 96 | 97 | 97 | 98 | 97 | 96 | 96 | 97 | |||||||||||
| 7 | 3 | not available | SGNP | 9.9 × 102 | 8.7 × 102 | 3.7 × 102 | 2.2 × 102 | nd | nd | nd | nd | nd | |||||||||
| Qiagen | 6.1 × 106 | 5.1 × 105 | 2.6 × 105 | 6.0 × 103 | nd | 2.6 × 102 | nd | nd | nd | ||||||||||||
| Tmax (°C) | 36.9 | 36.8 | 36.6 | 36.8 | 36.6 | 36.7 | 36.8 | 36.8 | 36.4 | 36.2 | |||||||||||
| SpO2 (min) | 98 | 98 | 97 | 98 | 97 | 98 | 98 | 99 | 99 | 99 | |||||||||||
| 8 | 3 | not available | SGNP | 2.7 × 105 | 1.8 × 105 | 2.8 × 104 | 1.6 × 102 | 2.2 × 102 | 3.3 × 102 | nd | 6.1 × 10 | 3.8 × 10 | 3.1 × 10 | 8.5 × 10 | |||||||
| Qiagen | 1.4 × 106 | 8.8 × 107 | 5.7 × 106 | 8.2 × 102 | 6.2 × 102 | 1.4 × 103 | nd | 6.1 × 102 | 4.7 × 104 | 1.5 × 104 | nd | ||||||||||
| Tmax (°C) | 36.8 | 37.4 | 38.0 | 37.6 | 37.5 | 37.5 | 38.2 | 37.5 | 36.8 | 36.7 | 36.4 | ||||||||||
| SpO2 (min) | 97 | 96 | 96 | 96 | 96 | 96 | 97 | 96 | 96 | 96 | 97 | ||||||||||
| 9 | 2 | δ | SGNP | 1.1 × 106 | 1.1 × 106 | 4.3 × 107 | 6.7 × 104 | 6.3 × 104 | 2.6 × 104 | 1.5 × 104 | nd | nd | nd | nd | |||||||
| Qiagen | 1.3 × 108 | 1.3 × 108 | 2.7 × 109 | 6.9 × 107 | 3.6 × 107 | 3.3 × 106 | 4.5 × 106 | 2.0 × 105 | nd | nd | nd | ||||||||||
| Tmax (°C) | 36.8 | 37.9 | 37.3 | 38.3 | 39.3 | 39.2 | 36.8 | 36.4 | 36.7 | 36.8 | 36.7 | 36.9 | 36.8 | 36.8 | 36.8 | 36.9 | |||||
| SpO2 (min) | 98 | 96 | 98 | 99 | 99 | 99 | 98 | 98 | 98 | 99 | 97 | 98 | 98 | 98 | 98 | 98 | |||||
| 10 | 3 | δ | SGNP | 6.6 × 105 | 5.5 × 105 | 5.0 × 104 | 7.8 × 103 | 7.4 × 103 | nd | nd | nd | ||||||||||
| Qiagen | 6.6 × 107 | 4.3 × 107 | 2.7 × 106 | 3.8 × 105 | 7.5 × 105 | 3.7 × 105 | 1.0 × 105 | nd | |||||||||||||
| Tmax (°C) | 38.8 | 38.3 | 38.6 | 37.5 | 36.7 | 36.8 | 36.5 | 36.5 | 36.8 | ||||||||||||
| SpO2 (min) | 98 | 97 | 98 | 98 | 98 | 98 | 98 | 98 | 99 | ||||||||||||
| 11 | 4 | δ | SGNP | 2.0 × 107 | 1.7 × 106 | 3.4 × 105 | 6.6 × 103 | 2.8 × 104 | 6.5 × 103 | nd | nd | 6.0 × 104 | 4.5 × 103 | nd | nd | ||||||
| Qiagen | 2.6 × 109 | 1.7 × 108 | 9.2 × 107 | 1.1 × 106 | 4.2 × 106 | 2.4 × 106 | 9.7 × 104 | 1.1 × 103 | 9.1 × 105 | 8.3 × 105 | nd | nd | |||||||||
| Tmax (°C) | 38.5 | 38.8 | 38.3 | 38 | 36.9 | 36.7 | 36.4 | 36.7 | 37.2 | 39.5 | 38.1 | 36.6 | 36.7 | 36.5 | 36.8 | ||||||
| SpO2 (min) | 97 | 97 | 96 | 96 | 96 | 96 | 98 | 96 | 97 | 97 | 97 | 96 | 98 | 97 | 98 | ||||||
| 12 | 4 | δ | SGNP | 3.2 × 105 | 1.9 × 104 | 4.8 × 105 | |||||||||||||||
| Qiagen | 4.4 × 106 | 3.5 × 105 | 5.4 × 107 | ||||||||||||||||||
| Tmax (°C) | 36.4 | 38.4 | 36.6 | 36.9 | 36.6 | 36.8 | 36.8 | 36.5 | 36.6 | ||||||||||||
| SpO2 (min) | 99 | 97 | 98 | 98 | 98 | 98 | 98 | 98 | 100 | ||||||||||||
| 13 | 3 | δ | SGNP | 2.0 × 106 | 1.5 × 104 | 6.4 × 104 | 4.3 × 104 | 6.7 × 103 | nd | nd | |||||||||||
| Qiagen | 2.4 × 107 | 2.3 × 107 | 5.3 × 107 | 7.5 × 106 | 6.9 × 105 | nd | nd | ||||||||||||||
| Tmax (°C) | 39.1 | 39 | 38.9 | 39 | 38.4 | 37.5 | 37 | 36.9 | 37.4 | 38 | 36.6 | 36.6 | 36.8 | 36.6 | 35.8 | ||||||
| SpO2 (min) | 97 | 97 | 97 | 98 | 98 | 96 | 97 | 98 | 98 | 96 | 97 | 97 | 96 | 98 | 98 | ||||||
| 14 | 3 | δ | SGNP | 4.0 × 106 | 5.5 × 107 | 5.9 × 106 | 7.5 × 105 | 6.2 × 105 | 3.4 × 105 | 1.1 × 105 | 1.4 × 105 | ||||||||||
| Qiagen | 2.3 × 108 | 2.6 × 1010 | 3.3 × 107 | 2.7 × 107 | 1.5 × 107 | nd | 4.0 × 104 | nd | |||||||||||||
| Tmax (°C) | 38.9 | 39.4 | 39.9 | 39.6 | 38.9 | 38.2 | 37.1 | 37.3 | 37.4 | 37 | 37.4 | 37 | 36.9 | ||||||||
| SpO2 (min) | 98 | 97 | 98 | 94 | 95 | 96 | 97 | 96 | 98 | 96 | 97 | 97 | 98 | ||||||||
| 15 | 4 | δ | SGNP | 1.8 × 106 | 2.9 × 105 | 1.3 × 106 | 6.6 × 104 | 4.5 × 104 | 2.3 × 103 | 3.6 × 103 | nd | ||||||||||
| Qiagen | 6.4 × 107 | 8.5 × 106 | 2.3 × 107 | 3.7 × 105 | 4.3 × 104 | nd | 3.3 × 104 | 2.3 × 103 | |||||||||||||
| Tmax (°C) | 38 | 37.9 | 38.3 | 37 | 36.8 | 36.7 | 36.8 | 36.5 | 36.7 | ||||||||||||
| SpO2 (min) | 99 | 98 | 97 | 95 | 97 | 97 | 98 | 98 | 97 | ||||||||||||
| 16 | 1 | α | SGNP | 7.4 × 104 | 5.2 × 103 | nd | nd | nd | nd | nd | nd | ||||||||||
| Qiagen | 6.4 × 106 | 8.9 × 105 | 6.3 × 104 | nd | nd | nd | nd | nd | |||||||||||||
| Tmax (°C) | 38 | 37.9 | 38.3 | 37 | 36.8 | 36.7 | 36.8 | 36.5 | 36.7 | ||||||||||||
| SpO2 (min) | 99 | 98 | 97 | 95 | 97 | 97 | 98 | 98 | 97 | ||||||||||||
| 17 | 6 | α | SGNP | 2.6 × 104 | nd | nd | nd | nd | nd | nd | nd | ||||||||||
| Qiagen | 2.2 × 105 | 1.1 × 104 | nd | nd | nd | nd | nd | nd | |||||||||||||
| Tmax (°C) | 38.8 | 37.2 | 36.7 | 36.9 | 36.6 | 36.6 | 36.6 | 36.7 | 36.5 | 36.6 | |||||||||||
| SpO2 (min) | 97 | 96 | 96 | 96 | 97 | 97 | 97 | 98 | 98 | 99 | |||||||||||
| 18 | 4 | δ | SGNP | 2.4 × 106 | nd | 4.0 × 104 | nd | nd | nd | nd | |||||||||||
| Qiagen | 4.2 × 107 | 9.7 × 105 | 3.1 × 106 | 3.0 × 106 | 3.5 × 105 | nd | nd | ||||||||||||||
| Tmax (°C) | 38 | 37.7 | 36.7 | 38.2 | 37 | 38.6 | 36.9 | 36 | 36.8 | 36.5 | 36.6 | 36.5 | 36.9 | 35.9 | |||||||
| SpO2 (min) | 98 | 98 | 97 | 97 | 97 | 98 | 97 | 97 | 98 | 99 | 98 | 98 | 98 | 98 | |||||||
| 19 | 1 | δ | SGNP | 8.3 × 103 | 5.7 × 104 | 1.5 × 104 | nd | nd | nd | ||||||||||||
| Qiagen | 5.2 × 105 | nd | nd | nd | nd | nd | |||||||||||||||
| Tmax (°C) | 39.9 | 40.4 | 38.6 | 39.4 | 38.2 | 39.5 | 39 | 38.4 | 36.9 | 36.7 | 36.9 | 37 | 36.9 | 36.8 | |||||||
| SpO2 (min) | 98 | 97 | 97 | 97 | 96 | 97 | 97 | 98 | 98 | 97 | 97 | 96 | 97 | 99 | |||||||
| 20 | 4 | δ | SGNP | 2.7 × 105 | 1.9 × 106 | 2.9 × 105 | 7.6 × 104 | 1.2 × 102 | |||||||||||||
| Qiagen | 7.2 × 105 | 3.1 × 106 | 1.7 × 105 | nd | nd | ||||||||||||||||
| Tmax (°C) | 39 | 39.6 | 39.2 | 38 | 37.3 | 38.4 | 37.9 | 37.6 | 37.2 | 36.8 | 36.5 | 36.8 | 36.7 | ||||||||
| SpO2 (min) | 97 | 96 | 96 | 96 | 98 | 97 | 97 | 97 | 96 | 97 | 97 | 97 | 97 | ||||||||
| 21 | 2 | δ | SGNP | 6.2 × 104 | 7.0 × 104 | 3.9 × 104 | 7.6 × 102 | 9.4 × 102 | nd | ||||||||||||
| Qiagen | 4.5 × 105 | 2.1 × 105 | |||||||||||||||||||
| Tmax (°C) | 38.2 | 37.7 | 37.5 | 37.2 | 37.3 | 36.4 | 36.5 | ||||||||||||||
| SpO2 (min) | 98 | 97 | 98 | 97 | 98 | 98 | 98 | ||||||||||||||
| 22 | 4 | δ | SGNP | 3.7 × 105 | 4.1 × 106 | ||||||||||||||||
| Qiagen | 3.6 × 107 | ||||||||||||||||||||
| Tmax (°C) | 38.2 | 37.5 | 37.4 | 37 | |||||||||||||||||
| 23 | 7 | δ | SGNP | 1.7 × 103 | 1.6 × 103 | 1.1 × 103 | |||||||||||||||
| Qiagen | 4.6 × 104 | 3.0 × 104 | 6.1 × 103 | ||||||||||||||||||
| Tmax (°C) | 38.5 | 36.8 | 36.8 | 36.2 | 36.6 | ||||||||||||||||
| 24 | 1 | δ | SGNP | 1.3 × 103 | nd | ||||||||||||||||
| Qiagen | 2.3 × 105 | nd | |||||||||||||||||||
| Tmax (°C) | 37 | 37.2 | 36.9 | 36.9 | 37 | ||||||||||||||||
| 25 | 2 | O | SGNP | 2.2 × 105 | 1.4 × 104 | nd | nd | nd | nd | ||||||||||||
| Qiagen | 4.0 × 106 | 1.4 × 106 | 3.4 × 105 | 4.5 × 104 | nd | nd | |||||||||||||||
| Tmax (°C) | 38 | 37 | 36.7 | 36.7 | 36.8 | 36.6 | |||||||||||||||
| 26 | 2 | δ | SGNP | 1.4. × 107 | 8.2 × 105 | 2.3 × 105 | 6.9 × 105 | 9.1 × 104 | 4.2 × 103 | nd | nd | ||||||||||
| Qiagen | 8.3 × 107 | 3.0 × 108 | 5.1 × 106 | 2.2 × 107 | 4.2 × 106 | 2.3 × 105 | 2.6 × 104 | nd | |||||||||||||
| Tmax (°C) | 38.9 | 37.2 | 36.5 | 36.7 | 36.6 | 36.8 | 36.4 | 36.8 | |||||||||||||
| 27 | 2 | O | SGNP | 1.5 × 106 | 6.0 × 104 | 5.7 × 103 | nd | nd | |||||||||||||
| Qiagen | 9.3 × 107 | 4.5 × 106 | 1.6 × 107 | 3.6 × 105 | 3.8 × 105 | ||||||||||||||||
| Tmax (°C) | 38.5 | 36.5 | 36.7 | 36.6 | 36.6 | ||||||||||||||||
| 28 | 6 | O | SGNP | 8.1 × 104 | 5.8 × 103 | nd | nd | ||||||||||||||
| Qiagen | 6.9 × 105 | 1.4 × 105 | 2.3 × 103 | nd | |||||||||||||||||
| Tmax (°C) | 36.4 | 36.4 | 36.1 | 36.6 | |||||||||||||||||
| 29 | 1 | δ | SGNP | 2.3 × 105 | 7.9 × 105 | 1.0 × 105 | 9.8 × 104 | 2.8 × 104 | 4.0 × 103 | nd | 1.0 × 104 | 9.4 × 103 | nd | nd | nd | ||||||
| Qiagen | 9.4 × 105 | 5.9 × 107 | 8.0 × 106 | 1.5 × 106 | 4.3 × 105 | 3.2 × 105 | 9.9 × 104 | 2.8 × 105 | 1.8 × 105 | 8.1 × 104 | 6.7 × 104 | 2.4 × 104 | |||||||||
| Tmax (°C) | 39.2 | 38.8 | 37.3 | 36.6 | 36.8 | 36.8 | 36.8 | 36.6 | 37 | 37.1 | 37 | 36.8 | |||||||||
| 30 | 5 | O | SGNP | 3.1 × 106 | 1.2 × 104 | nd | |||||||||||||||
| Qiagen | 1.2 × 109 | 1.6 × 106 | nd | ||||||||||||||||||
| Tmax (°C) | 38.9 | 38.1 | 38.1 | 36.9 |
| Case | Days Since Onset to First Sampling | Variant | Saliva | Day 0 (Hospitalization) | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 | 1 | not available | SGNP | 1.4 × 104 | 3.2 × 102 | 2.2 × 102 | 9.1 × 103 | 7.1 × 102 | 1.6 × 102 | 5.4 × 10 | nd | nd | nd | ||
| Qiagen | 3.0 × 107 | 3.8 × 105 | 2.0 × 104 | 1.2 × 106 | 1.2 × 105 | 2.5 × 105 | 1.2 × 104 | 1.0 × 104 | 1.3 × 103 | nd | |||||
| Tmax (°C) | 36.5 | 36.5 | 36.5 | 37.0 | 36.1 | 36.2 | 36.3 | 36.3 | 36.4 | 36.2 | |||||
| SpO2 (min) | 97.0 | 96.0 | 96.0 | 97.0 | 97.0 | 98.0 | 97.0 | 96.0 | 96.0 | 97.0 | |||||
| Culturing | CPE+ | CPE− | CPE− | CPE− | |||||||||||
| 8 | 3 | not available | SGNP | 2.7 × 105 | 1.8 × 105 | 2.8 × 104 | 1.6 × 102 | 2.2 × 102 | 3.3 × 102 | nd | 6.1 × 10 | 3.8 × 10 | 3.1 × 10 | 8.5 × 10 | |
| Qiagen | 1.4 × 106 | 8.8 × 107 | 5.7 × 106 | 8.2 × 102 | 6.2 × 102 | 1.4 × 103 | nd | 6.1 × 102 | 4.7 × 104 | 1.5 × 104 | nd | ||||
| Tmax (°C) | 36.8 | 37.4 | 38.0 | 37.6 | 37.5 | 37.5 | 38.2 | 37.5 | 36.8 | 36.7 | 36.4 | ||||
| SpO2 (min) | 97.0 | 96.0 | 96.0 | 96.0 | 96.0 | 96.0 | 97.0 | 96.0 | 96.0 | 96.0 | 97.0 | ||||
| Culturing | CPE− | CPE− | CPE− | ||||||||||||
| 22 | 4 | δ | SGNP | 3.7 × 105 | 4.1 × 106 | ||||||||||
| Qiagen | 3.6 × 107 | ||||||||||||||
| Tmax (°C) | 38.2 | 37.5 | 37.4 | 37 | |||||||||||
| Culturing | CPE+ | ||||||||||||||
| 23 | 7 | δ | SGNP | 1.7 × 103 | 1.6 × 103 | 1.1 × 103 | |||||||||
| Qiagen | 4.6 × 104 | 3.0 × 104 | 6.1 × 103 | ||||||||||||
| Tmax (°C) | 38.5 | 36.8 | 36.8 | 36.2 | 36.6 | ||||||||||
| Culturing | CPE− | CPE− | CPE− | ||||||||||||
| 24 | 1 | δ | SGNP | 1.3 × 103 | nd | ||||||||||
| Qiagen | 2.3 × 105 | nd | |||||||||||||
| Tmax (°C) | 37 | 37.2 | 36.9 | 36.9 | 37 | ||||||||||
| Culturing | CPE+ | CPE− |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajiya, T.; Sawayama, H.; Arima, E.; Okamoto, M.; Baba, M.; Toyama, M.; Okuya, K.; Ozawa, M.; Atsuchi, N.; Nishi, J.; et al. Novel RT-PCR Using Sugar Chain-Immobilized Gold-Nanoparticles Correlates Patients’ Symptoms: The Follow-Up Study of COVID-19 Hospitalized Patients. Viruses 2022, 14, 2577. https://doi.org/10.3390/v14112577
Kajiya T, Sawayama H, Arima E, Okamoto M, Baba M, Toyama M, Okuya K, Ozawa M, Atsuchi N, Nishi J, et al. Novel RT-PCR Using Sugar Chain-Immobilized Gold-Nanoparticles Correlates Patients’ Symptoms: The Follow-Up Study of COVID-19 Hospitalized Patients. Viruses. 2022; 14(11):2577. https://doi.org/10.3390/v14112577
Chicago/Turabian StyleKajiya, Takashi, Hayate Sawayama, Eriko Arima, Mika Okamoto, Masanori Baba, Masaaki Toyama, Kosuke Okuya, Makoto Ozawa, Nobuhiko Atsuchi, Junichiro Nishi, and et al. 2022. "Novel RT-PCR Using Sugar Chain-Immobilized Gold-Nanoparticles Correlates Patients’ Symptoms: The Follow-Up Study of COVID-19 Hospitalized Patients" Viruses 14, no. 11: 2577. https://doi.org/10.3390/v14112577





