Evaluation of Rapid Dot-Immunoassay for Detection Orthopoxviruses Using Laboratory-Grown Viruses and Animal’s Clinical Specimens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Viruses
2.2.1. Antibodies
2.2.2. Clinical Materials
2.2.3. Colloidal Gold Conjugate Au-a/POX-IgG
2.2.4. OPV Detection Kit
2.2.5. Dot-Immunoassay
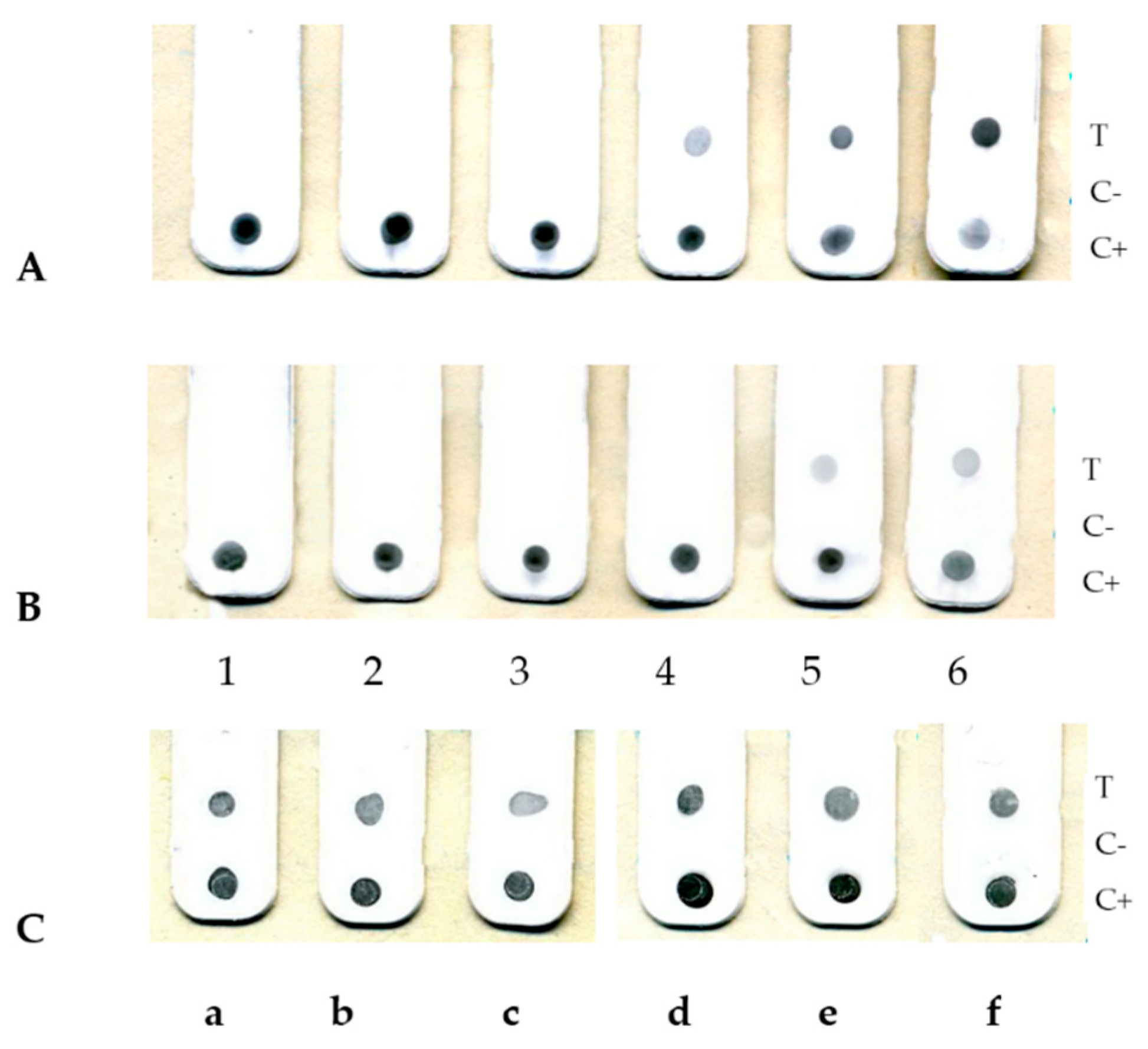
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitley, R.J. Smallpox: A Potential Agent of Bioterrorism. Antivir. Res. 2003, 57, 7–12. [Google Scholar] [CrossRef]
- Wallin, A.; Luksiene, Z.; Zagminas, K.; Surkiene, G. Public Health and Bioterrorism: Renewed Threat of Anthrax and Smallpox. Med. Kaunas Lith. 2007, 43, 278. [Google Scholar] [CrossRef] [Green Version]
- Vorou, R.M.; Papavassiliou, V.G.; Pierroutsakos, I.N. Cowpox Virus Infection: An Emerging Health Threat. Curr. Opin. Infect. Dis. 2008, 21, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Rimoin, A.W.; Graham, B.S. Whither Monkeypox Vaccination. Vaccine 2011, 29 (Suppl. 4), D60–D64. [Google Scholar] [CrossRef] [Green Version]
- Shchelkunov, S.N. An Increasing Danger of Zoonotic Orthopoxvirus Infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Lloyd Smith, J.O.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major Increase in Human Monkeypox Incidence 30 Years after Smallpox Vaccination Campaigns Cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [CrossRef] [Green Version]
- CDC Monkeypox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/index.html (accessed on 30 September 2022).
- Shchelkunov, S.N.; Marennikova, S.S.; Moyer, R.W. Orthopoxviruses Pathogenic for Humans; Springer Science & Business Media: New York, NY, USA, 2006; pp. 1–425. [Google Scholar] [CrossRef]
- Townsend, M.B.; MacNeil, A.; Reynolds, M.G.; Hughes, C.M.; Olson, V.A.; Damon, I.K.; Karem, K.L. Evaluation of the Tetracore Orthopox BioThreat® Antigen Detection Assay Using Laboratory Grown Orthopoxviruses and Rash Illness Clinical Specimens. J. Virol. Methods 2013, 187, 37–42. [Google Scholar] [CrossRef]
- Stern, D.; Olson, V.A.; Smith, S.K.; Pietraszczyk, M.; Miller, L.; Miethe, P.; Dorner, B.G.; Nitsche, A. Rapid and Sensitive Point-of-Care Detection of Orthopoxviruses by ABICAP Immunofiltration. Virol. J. 2016, 13, 207. [Google Scholar] [CrossRef] [Green Version]
- Stern, D.; Pauly, D.; Zydek, M.; Miller, L.; Piesker, J.; Laue, M.; Lisdat, F.; Dorner, M.B.; Dorner, B.G.; Nitsche, A. Development of a Genus-Specific Antigen Capture ELISA for Orthopoxviruses—Target Selection and Optimized Screening. PLoS ONE 2016, 11, e0150110. [Google Scholar] [CrossRef]
- Poltavchenko, A.G.; Ersh, A.V.; Filatov, P.V.; Yakubitskiy, S.N. Rapid Protocol of Dot-Immunnoassay for Orthopoxviruses Detection. J. Virol. Methods 2020, 279, 113859. [Google Scholar] [CrossRef]
- Mazurkov, O.Y.; Kabanov, A.S.; Shishkina, L.N.; Sergeev, A.A.; Skarnovich, M.O.; Bormotov, N.I.; Skarnovich, M.A.; Ovchinnikova, A.S.; Titova, K.A.; Galahova, D.O.; et al. New effective chemically synthesized anti-smallpox compound NIOCH-14. J. Gen. Virol. 2016, 97, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Yakubitskiy, S.N.; Kolosova, I.V.; Maksyutov, R.A.; Shchelkunov, S.N. Attenuation of vaccinia virus. Acta Nat. 2015, 7, 113–121. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Yakubitskiy, S.N.; Bauer, T.V.; Sergeev, A.A.; Kabanov, A.S.; Bulichev, L.E.; Yurganova, I.A.; Odnoshevskiy, D.A.; Kolosova, I.V.; Pyankov, S.A.; et al. The influence of an elevated production of extracellular enveloped virions of the vaccinia virus on its properties in infected mice. Acta Nat. 2020, 12, 120–132. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Safronov, P.F.; Totmenin, A.V.; Petrov, N.A.; Ryazankina, O.I.; Gutorov, V.V.; Kotwal, G.J. The genomic sequence analysis of the left and right species-specific terminal region of a cowpox virus strain reveals unique sequences and a cluster of intact ORFs for immunomodulatory and host range proteins. Virology 1998, 243, 432–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakubitskiy, S.N.; Kolosova, I.V.; Maksyutov, R.A.; Shchelkunov, S.N. Highly immunogenic variant of attenuated vaccinia virus. Dokl. Biochem. Biophys. 2016, 466, 35–38. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 7th ed.; The National Academies Press: Washington, DC, USA, 1996; p. 140. [Google Scholar] [CrossRef]
- Poltavchenko, A.G.; Zaitsev, B.N.; Ersh, A.V.; Korneev, D.V.; Taranov, O.S.; Filatov, P.V.; Nechitaylo, O.V. The Selection and Optimization of the Detection System for Self-Contained Multiplexed Dot-Immunoassay. J. Immunoass. Immunochem. 2016, 37, 540–554. [Google Scholar] [CrossRef]
- Meyer, H.; Perrichot, M.; Stemmler, M.; Emmerich, P.; Schmitz, H.; Varaine, F.; Shungu, R.; Tshioko, F.; Formenty, P. Outbreaks of Disease Suspected of Being Due to Human Monkeypox Virus Infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002, 40, 2919–2921. [Google Scholar] [CrossRef] [Green Version]
- Sklenovská, N. Monkeypox Virus. In Animal-Origin Viral Zoonoses; Malik, Y.S., Singh, R.K., Dhama, K., Eds.; Livestock Diseases and Management; Springer: Singapore, 2020; pp. 39–68. ISBN 9789811526510. [Google Scholar] [CrossRef]
- Sergeev, A.; Bulychev, L.; P’yankov, O.; Sergeev, A.; Bodnev, S.; Kabanov, A.; Tumanov, Y.; Yurganova, I.; Shishkina, L.; Agafonov, A.; et al. Sensitivity of Different Animal Species to Monkeypox Virus. Probl. Part. Danger. Infect. 2012, 111, 88–91. [Google Scholar] [CrossRef]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-Neutralizing and Protective Human Antibody Specificities to Poxvirus Infections. Cell 2016, 167, 684–694.e4. [Google Scholar] [CrossRef] [Green Version]
- Ryabinin, V.A.; Shundrin, L.A.; Kostina, E.B.; Laassri, M.; Chizhikov, V.; Shchelkunov, S.N.; Chumakov, K.; Sinyakov, A.N. Microarray Assay for Detection and Discrimination of Orthopoxvirus Species. J. Med. Virol. 2006, 78, 1325–1340. [Google Scholar] [CrossRef]
- Shchelkunov, S.N.; Shcherbakov, D.N.; Maksyutov, R.A.; Gavrilova, E.V. Species-Specific Identification of Variola, Monkeypox, Cowpox, and Vaccinia Viruses by Multiplex Real-Time PCR Assay. J. Virol. Methods 2011, 175, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Czerny, C.P.; Meyer, H.; Mahnel, H. Establishment of an ELISA for the Detection of Orthopox Viruses Based on Neutralizing Monoclonal and Polyclonal Antibodies. Zent. Vet. Reihe B J. Vet. Med. Ser. B 1989, 36, 537–546. [Google Scholar] [CrossRef] [PubMed]
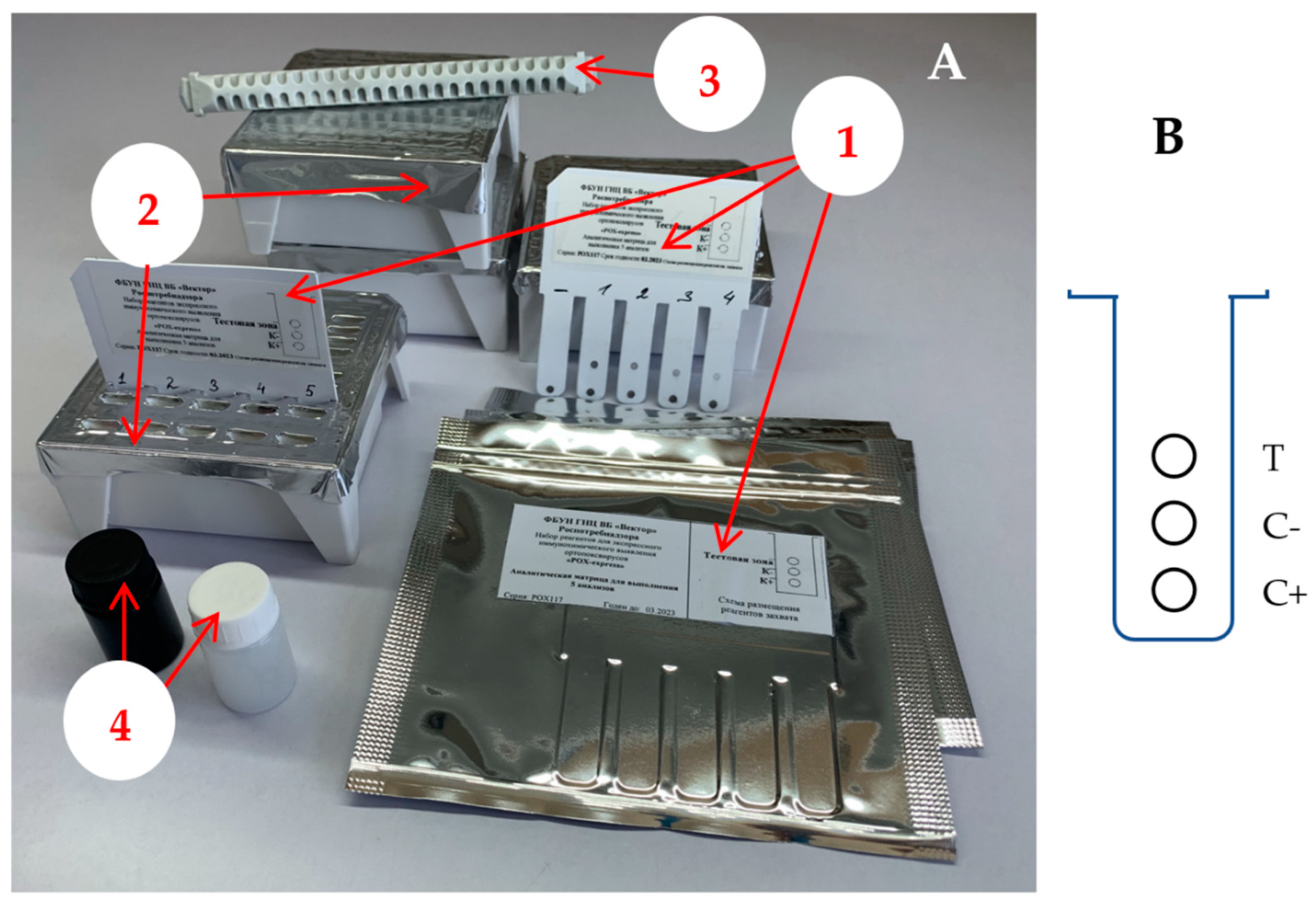
 —antibodies,
—antibodies, —antigens,
—antigens, —the multiplicity of washing.
—the multiplicity of washing.
 —antibodies,
—antibodies, —antigens,
—antigens, —the multiplicity of washing.
—the multiplicity of washing.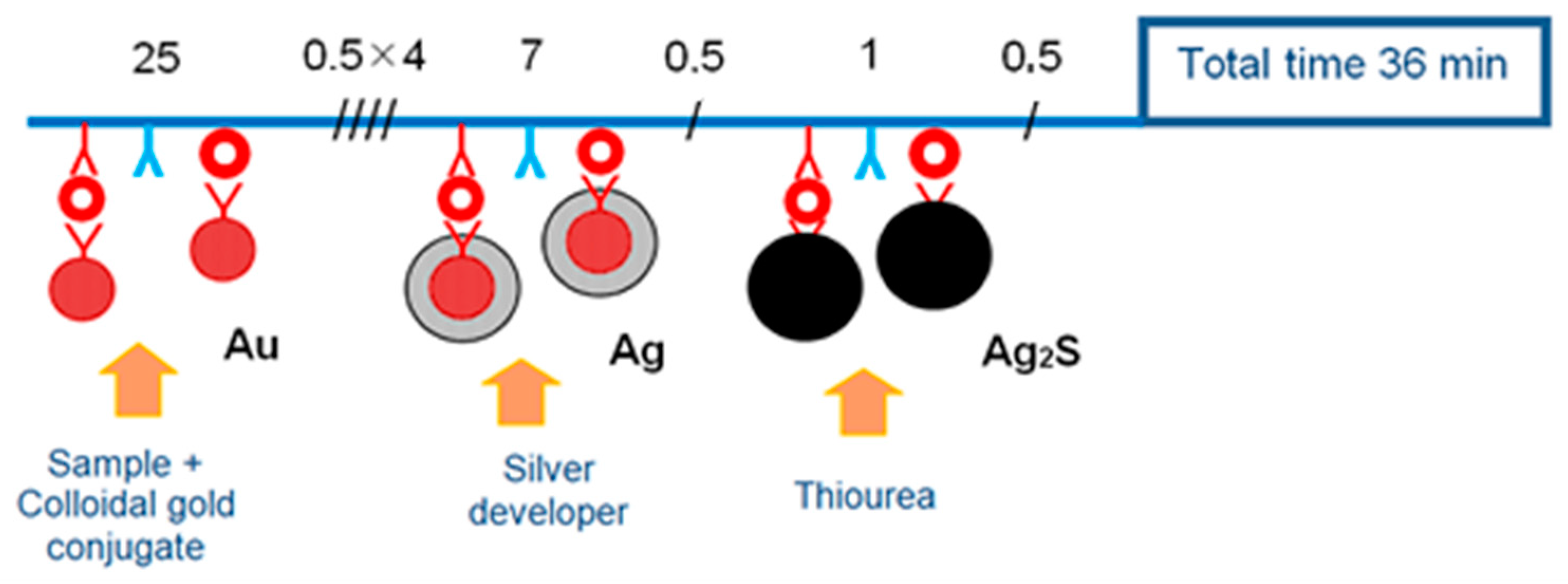
| Row | Composition of the Working Solution | Volume, µL |
|---|---|---|
| 1 | dilution of gold conjugate in PBS-T with 0.02 % PEG-20000, pH 7.4 | 200 |
| 2 | PBS-T | 300 |
| 3 | PBS-T | 350 |
| 4 | purified water | 400 |
| 5 | purified water | 400 |
| 6 | tablet of a dry mixture of citric acid and metol in a ratio of 5:3 | 4 mg |
| 7 | purified water | 400 |
| 8 | 1% thiourea and 1% sodium hydroxide in purified water | 300 |
| 9 | purified water | 400 |
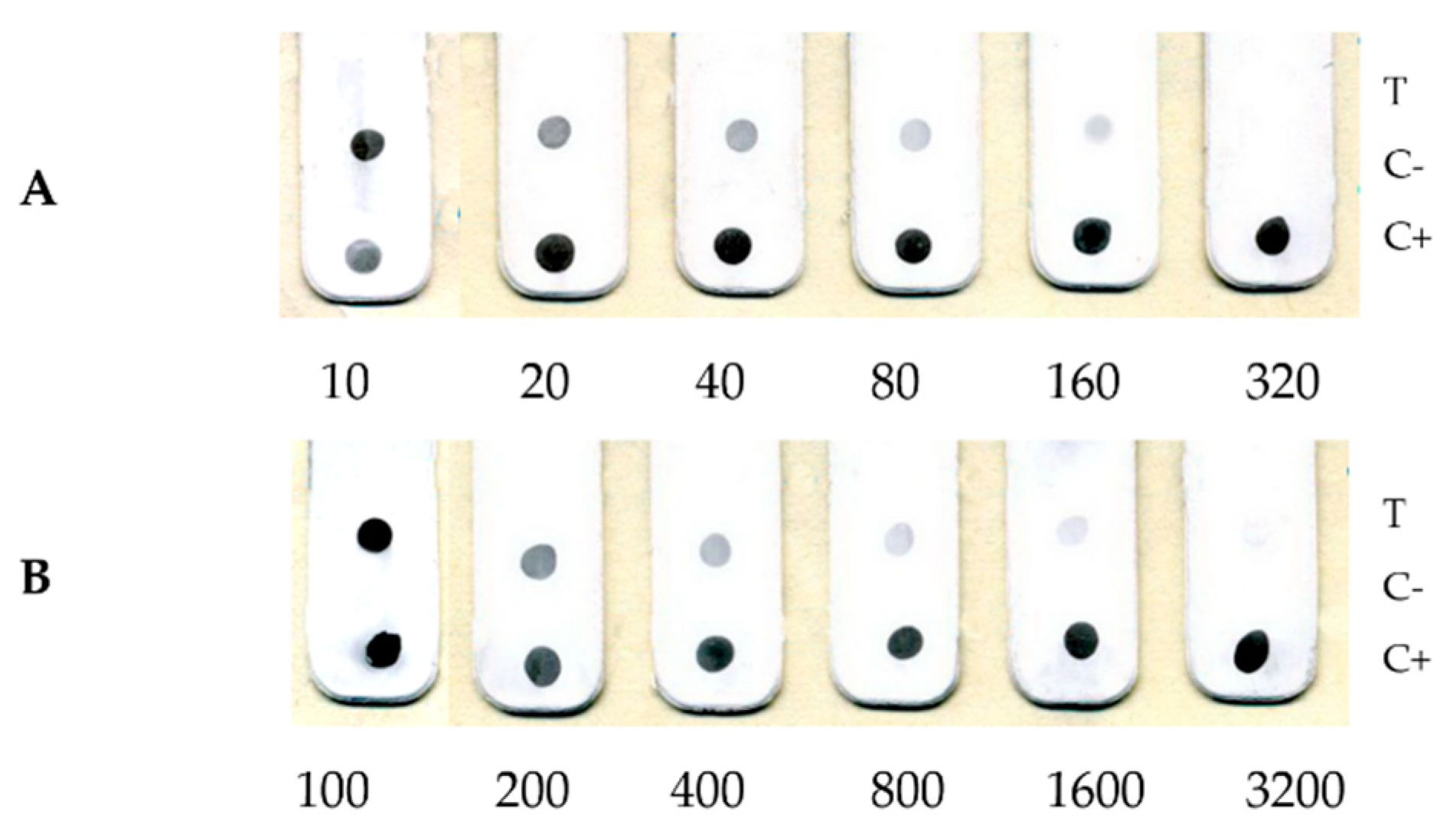
| Sample | Initial Titer, PFU/mL | Detection Limit of Dot-Immunoassay | ||
|---|---|---|---|---|
| Limiting Dilution | Titer, PFU/mL | |||
| Monkeypox virus, strain V79-1-005 | 4.0 × 106 | 1/1600 | 2.5 × 103 | |
| Vaccinia virus, strain LIVP | 8.5 × 106 | 1/12,800 | 6.6 × 102 | |
| Vaccinia virus, strain ABCNJ | 1.3 × 107 | 1/3200 | 4.1 × 103 | |
| Vaccinia virus, _A34R_ [D110N_K151E] | 1.1 × 106 | 1/800 | 1.4 × 103 | |
| Ectromelia virus, strain K-1 | 8.5 × 106 | 1/400 | 5.6 × 103 | |
| Cowpox virus, strain GRI-90 | 1.3 × 107 | 1/1600 | 8.0 × 103 | |
| Rabbitpox virus, strain Uetrecht | 1.0 × 106 | 1/1600 | 6.2 × 102 | |
| Cell culture control | 0 | 0 | 0 | |
| Heterogenous controls | Chickenpox virus | 0 | 0 | 0 |
| Measles virus | 0 | 0 | 0 | |
| Rubella virus | 0 | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ushkalenko, N.; Ersh, A.; Sergeev, A.; Filatov, P.; Poltavchenko, A. Evaluation of Rapid Dot-Immunoassay for Detection Orthopoxviruses Using Laboratory-Grown Viruses and Animal’s Clinical Specimens. Viruses 2022, 14, 2580. https://doi.org/10.3390/v14112580
Ushkalenko N, Ersh A, Sergeev A, Filatov P, Poltavchenko A. Evaluation of Rapid Dot-Immunoassay for Detection Orthopoxviruses Using Laboratory-Grown Viruses and Animal’s Clinical Specimens. Viruses. 2022; 14(11):2580. https://doi.org/10.3390/v14112580
Chicago/Turabian StyleUshkalenko, Nikita, Anna Ersh, Alexander Sergeev, Pavel Filatov, and Alexander Poltavchenko. 2022. "Evaluation of Rapid Dot-Immunoassay for Detection Orthopoxviruses Using Laboratory-Grown Viruses and Animal’s Clinical Specimens" Viruses 14, no. 11: 2580. https://doi.org/10.3390/v14112580





