Infectivity and Morphology of Bovine Coronavirus Inactivated In Vitro by Cationic Photosensitizers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus Propagation and Titration
2.2. Photodynamic Inactivation
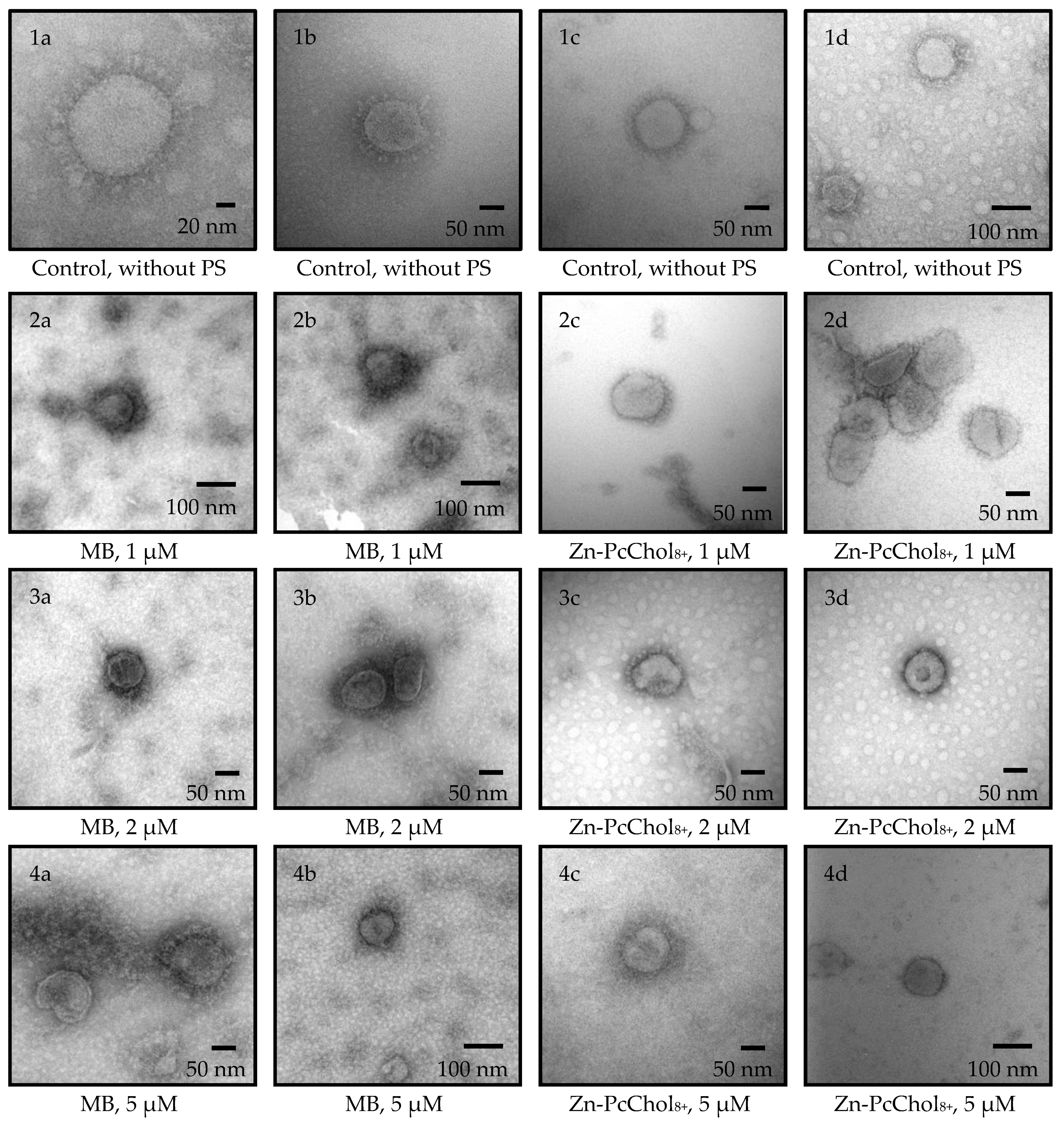
2.3. Transmission Electron Microscopy with Negative Contrast
3. Results
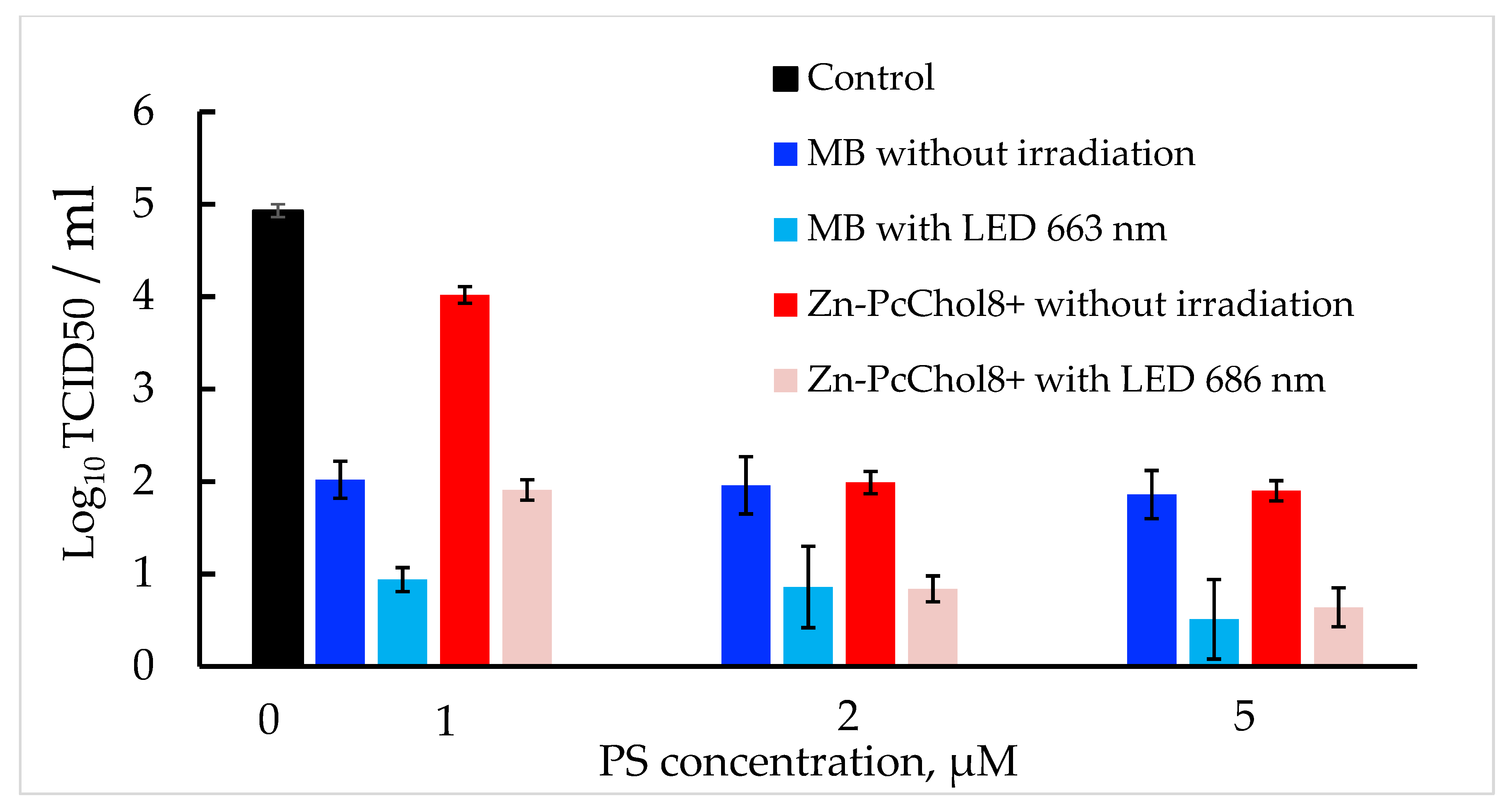
3.1. Antiviral Activity of MB and Zn-PcChol8+ without Irradiation
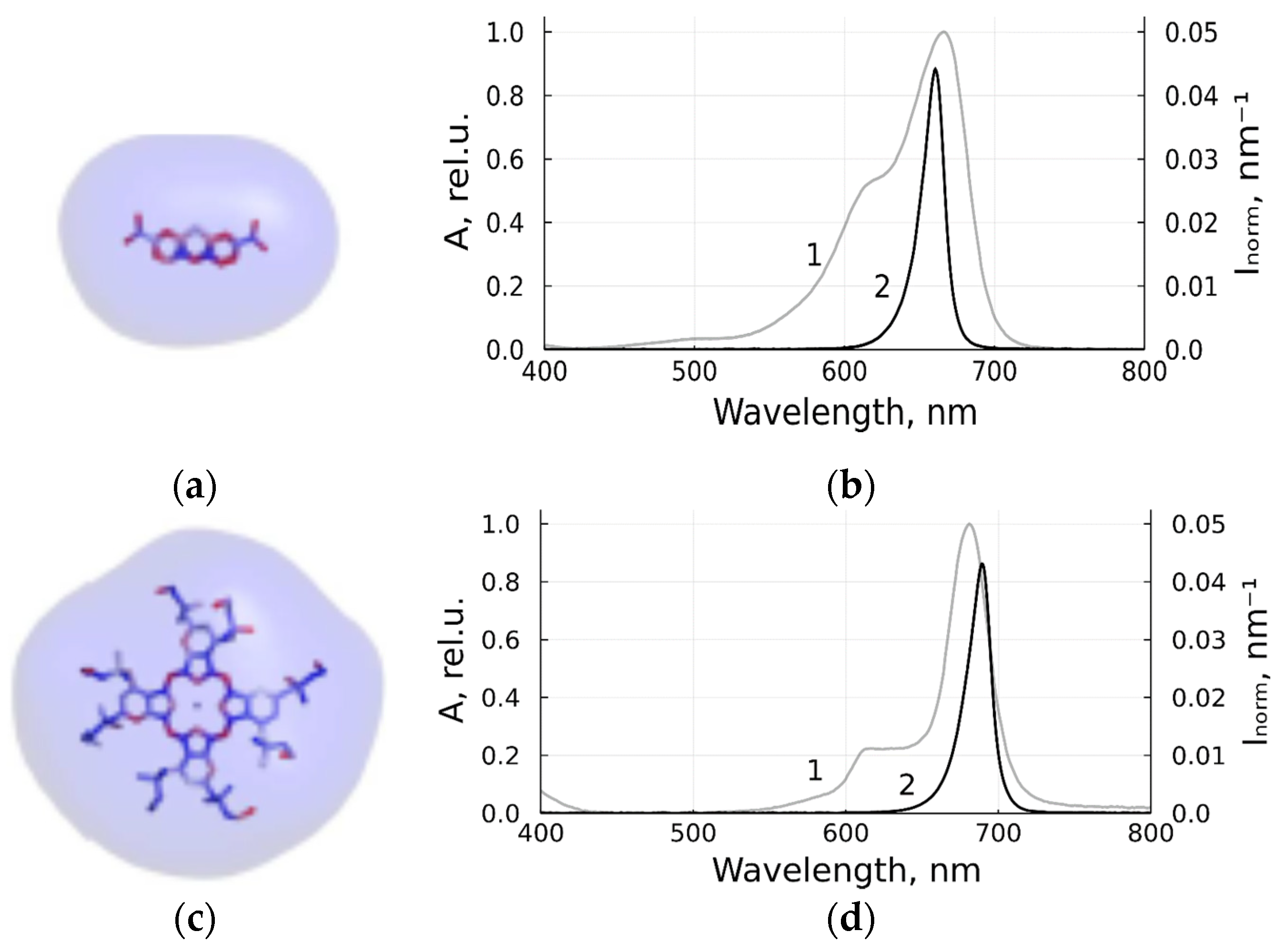
3.2. Photodynamic Inactivation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Islam, A.; Ferdous, J.; Islam, S.; Sayeed, M.A.; Dutta Choudhury, S.; Saha, O.; Hassan, M.M.; Shirin, T. Evolutionary Dynamics and Epidemiology of Endemic and Emerging Coronaviruses in Humans, Domestic Animals, and Wildlife. Viruses 2021, 13, 1908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.R.; Li, X.; Zhao, X.; Lin, H. Cell Entry of Animal Coronaviruses. Viruses 2021, 13, 1977. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Drigo, M.; Legnardi, M.; Grassi, L.; Pasotto, D.; Menandro, M.L.; Cecchinato, M.; Tucciarone, C.M. Bovine Coronavirus: Variability, Evolution, and Dispersal Patterns of a No Longer Neglected Betacoronavirus. Viruses 2020, 12, 1285. [Google Scholar] [CrossRef] [PubMed]
- Abi, K.M.; Zhang, Q.; Zhang, B.; Zhou, L.; Yue, H.; Tang, C. An emerging novel bovine coronavirus with a 4-amino-acid insertion in the receptor-binding domain of the hemagglutinin-esterase gene. Arch. Virol. 2020, 165, 3011–3015. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Storm, N.; Ilan, W.; Sol, A. Characterization of Winter Dysentery Bovine Coronavirus Isolated from Cattle in Israel. Viruses 2021, 13, 1070. [Google Scholar] [CrossRef]
- King, A. Down on the farm: Farm coronaviruses are a large reservoir for new spillover events into humans. EMBO Rep. 2021, 22, e53701. [Google Scholar] [CrossRef]
- Vijgen, L.; Keyaerts, E.; Moës, E.; Thoelen, I.; Wollants, E.; Lemey, P.; Vandamme, A.M.; Van Ranst, M. Complete genomic sequence of human coronavirus OC43: Molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005, 79, 1595–1604. [Google Scholar] [CrossRef] [Green Version]
- Saif, L.J. Bovine respiratory coronavirus. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 349–364. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 2020, 9, e57309. [Google Scholar] [CrossRef]
- Neuman, B.W.; Kiss, G.; Kunding, A.H.; Bhella, D.; Baksh, M.F.; Connelly, S.; Droese, B.; Klaus, J.P.; Makino, S.; Sawicki, S.G.; et al. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011, 174, 11–22. [Google Scholar] [CrossRef]
- Simon, M.; Veit, M.; Osterrieder, K.; Gradzielski, M. Surfactants—Compounds for inactivation of SARS-CoV-2 and other enveloped viruses. Curr. Opin. Colloid Interface Sci. 2021, 55, 101479. [Google Scholar] [CrossRef] [PubMed]
- Keha, A.; Xue, L.; Yan, S.; Yue, H.; Tang, C. Prevalence of a novel bovine coronavirus strain with a recombinant hemagglutinin/esterase gene in dairy calves in China. Transbound. Emerg. Dis. 2019, 66, 1971–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kienzle, T.E.; Abraham, S.; Hogue, B.G.; Brian, D.A. Structure and orientation of expressed bovine coronavirus hemagglutinin-esterase protein. J. Virol. 1990, 64, 1834–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wainwright, M. Photodynamic Antimicrobial Chemotherapy (PACT). J. Antimicrob. Chemother. 1998, 42, 13. [Google Scholar] [CrossRef]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Laser Surg. Med. 2006, 38, 468. [Google Scholar] [CrossRef]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565. [Google Scholar] [CrossRef]
- Fekrazad, R.; Asefi, S.; Pourhajibagher, M.; Vahdatinia, F.; Fekrazad, S.; Bahador, A.; Abrahamse, H.; Hamblin, M.R. Photobiomodulation and Antiviral Photodynamic Therapy in COVID-19 Management. Adv. Exp. Med. Biol. 2021, 1318, 517. [Google Scholar] [CrossRef]
- Costa, L.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacterio-phages. Viruses 2012, 4, 1034. [Google Scholar] [CrossRef] [Green Version]
- Conrado, P.C.V.; Sakita, K.M.; Arita, G.S.; Galinari, C.B.; Gonçalves, R.S.; Lopes, L.D.G.; Lonardoni, M.V.C.; Teixeira, J.J.V.; Bonfim-Mendonça, P.S.; Kioshima, E.S. A systematic review of photodynamic therapy as an antiviral treatment: Potential guidance for dealing with SARS-CoV-2. Photodiagn. Photodyn. Ther. 2021, 34, 102221. [Google Scholar] [CrossRef]
- Sabino, C.P.; Ball, A.R.; Baptista, M.S.; Dai, T.; Hamblin, M.R.; Ribeiro, M.S.; Santos, A.L.; Sellera, F.P.; Tegos, G.P.; Wainwright, M. Light-based technologies for management of COVID-19 pandemic crisis. J. Photochem. Photobiol. B Biol. 2020, 212, 111999. [Google Scholar] [CrossRef]
- Strakhovskaya, M.G.; Meerovich, G.A.; Kuskov, A.N.; Gonchukov, S.A.; Loschenov, V.B. Photoinactivation of coronaviruses: Going along the optical spectrum. Laser Phys. Lett. 2020, 17, 093001. [Google Scholar] [CrossRef]
- Fedorov, V.; Kholina, E.; Khruschev, S.; Kovalenko, I.; Rubin, A.; Strakhovskaya, M. What Binds Cationic Photosensitizers Better: Brownian Dynamics Reveals Key Interaction Sites on Spike Proteins of SARS-CoV, MERS-CoV, and SARS-CoV-2. Viruses 2021, 13, 1615. [Google Scholar] [CrossRef] [PubMed]
- Sharshov, K.; Solomatina, M.; Kurskaya, O.; Kovalenko, I.; Kholina, E.; Fedorov, V.; Meerovich, G.; Rubin, A.; Strakhovskaya, M. The Photosensitizer Octakis(cholinyl)zinc Phthalocyanine with Ability to Bind to a Model Spike Protein Leads to a Loss of SARS-CoV-2 Infectivity In Vitro When Exposed to Far-Red LED. Viruses 2021, 13, 643. [Google Scholar] [CrossRef] [PubMed]
- Korneev, D.; Kurskaya, O.; Sharshov, K.; Eastwood, J.; Strakhovskaya, M. Ultrastructural Aspects of Photodynamic Inactivation of Highly Pathogenic Avian H5N8 Influenza Virus. Viruses 2019, 11, 955. [Google Scholar] [CrossRef] [Green Version]
- Svyatchenko, V.A.; Nikonov, S.D.; Mayorov, A.P.; Gelfond, M.L.; Loktev, V.B. Antiviral Photodynamic Therapy: Inactivation and Inhibition of SARS-CoV-2 In Vitro Using Methylene Blue and Radachlorin. Photodiagn. Photodyn. Ther. 2020, 33, 102112. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Meerovich, G.; Romanishkin, I.; Akhlyustina, E.; Strakhovskaya, M.; Kogan, E.; Angelov, I.; Loschenov, V.; Borisova, E. Photodynamic action in thin sensitized layers: Estimating the utilization of light energy. J. Biomed. Photonics Eng. 2021, 7, 040301. [Google Scholar] [CrossRef]
- Meerovich, G.A.; Linkov, K.G.; Nekhoroshev, A.V.; Borodkin, A.V.; Akhlyustina, E.V.; Angelov, I.P.; Glechik, D.A.; Loschenov, V.B. Devices for Photodynamic Studies Based on Light-Emitting Diodes. J. Biomed. Photonics Eng. 2021, 7, 040308. [Google Scholar] [CrossRef]
- Roseto, A.; Bobulesco, P.; Laporte, J.; Escaig, J.; Gaches, D.; Peries, J. Bovine enteric coronavirus structure as studied by a freeze-drying technique. J. Gen. Virol. 1982, 63, 241–245. [Google Scholar] [CrossRef]
- Clark, M.A. Bovine coronavirus. Br. Vet. J. 1993, 149, 51–70. [Google Scholar] [CrossRef]
- Lutkus, L.V.; Rickenbach, S.S.; McCormick, T.M. Singlet oxygen quantum yields determined by oxygen consumption. J. Photochem. Photobiol. A Chem. 2019, 378, 131. [Google Scholar] [CrossRef]
- Seghatchian, J.; Struff, W.G.; Reichenberg, S. Main Properties of the THERAFLEX MB-Plasma System for Pathogen Reduction. Transfus. Med. Hemotherapy 2011, 38, 55. [Google Scholar] [CrossRef] [PubMed]
- Eickmann, M.; Gravemann, U.; Handke, W.; Tolksdorf, F.; Reichenberg, S.; Müller, N.H.; Seltsam, A. Inactivation of three emerging viruses—Severe acute respiratory syndrome coronavirus, Crimean-Congo haemorrhagic fever virus and Nipah virus—In platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox Sang. 2020, 115, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cagno, V.; Medaglia, C.; Cerny, A.; Cerny, T.; Zwygart, A.C.; Cerny, E.; Tapparel, C. Methylene Blue has a potent antiviral activity against SARS-CoV-2 and H1N1 influenza virus in the absence of UV-activation in vitro. Sci. Rep. 2021, 11, 14295. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lang, Y.; Sakamuru, S.; Samrat, S.; Trudeau, N.; Kuo, L.; Rugenstein, N.; Tharappel, A.; D’Brant, L.; Koetzner, C.A.; et al. Methylene blue is a potent and broad-spectrum inhibitor against Zika virus in vitro and in vivo. Emerg. Microbes Infect. 2020, 9, 2404. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; da Fonseca Orcina, B.; Brito Reia, V.C.; Ribeiro, L.G.; Grotto, R.M.T.; Prudenciatti, A.; de Moraes, L.N.; Ragghianti Zangrando, M.; Vilhena, F.V.; da Silva Santos, P.S. Virucidal Activity of the Antiseptic Mouthwash and Dental Gel Containing Anionic Phthalocyanine Derivative: In vitro Study. Clin. Cosmet. Investig. Dent. 2021, 13, 269. [Google Scholar] [CrossRef]
- Smetana, Z.; Mendelson, E.; Manor, J.; van Lier, J.E.; Ben-Hur, E.; Salzberg, S.; Malik, Z. Photodynamic inactivation of herpes viruses with phthalocyanine derivatives. J. Photochem. Photobiol. B Biol. 1994, 22, 37. [Google Scholar] [CrossRef]
- Zarubaev, V.V.; Belousova, I.M.; Kiselev, O.I.; Piotrovsky, L.B.; Anfimov, P.M.; Krisko, T.C.; Muraviova, T.D.; Rylkov, V.V.; Starodubzev, A.M.; Sirotkin, A.C. Photodynamic inactivation of influenza virus with fullerene C60 suspension in allantoic fluid. Photodiagn. Photodyn. Ther. 2007, 4, 31. [Google Scholar] [CrossRef]
- Duguay, B.A.; Herod, A.; Pringle, E.S.; Monro, S.; Hetu, M.; Cameron, C.G.; McFarland, S.A.; McCormick, C. Photodynamic Inactivation of Human Coronaviruses. Viruses 2022, 14, 110. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukhovitsky, V.; Shevlyagina, N.; Zubasheva, M.; Russu, L.; Gushchin, V.; Meerovich, G.; Strakhovskaya, M. Infectivity and Morphology of Bovine Coronavirus Inactivated In Vitro by Cationic Photosensitizers. Viruses 2022, 14, 1053. https://doi.org/10.3390/v14051053
Zhukhovitsky V, Shevlyagina N, Zubasheva M, Russu L, Gushchin V, Meerovich G, Strakhovskaya M. Infectivity and Morphology of Bovine Coronavirus Inactivated In Vitro by Cationic Photosensitizers. Viruses. 2022; 14(5):1053. https://doi.org/10.3390/v14051053
Chicago/Turabian StyleZhukhovitsky, Vladimir, Natalia Shevlyagina, Margarita Zubasheva, Leonid Russu, Vladimir Gushchin, Gennady Meerovich, and Marina Strakhovskaya. 2022. "Infectivity and Morphology of Bovine Coronavirus Inactivated In Vitro by Cationic Photosensitizers" Viruses 14, no. 5: 1053. https://doi.org/10.3390/v14051053






