Pseudorabies Virus ICP0 Abolishes Tumor Necrosis Factor Alpha-Induced NF-κB Activation by Degrading P65
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Antibodies and Reagents
2.3. Plasmids
2.4. Western Blotting
2.5. Co-Immunoprecipitation Assay
2.6. RNA Extraction and RT-qPCR
2.7. Transfection
2.8. Nuclear and Cytoplasmic Extraction
2.9. Virus Titer
2.10. ICP0 mRNA Detection
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. P65 Polyubiquitination Assay
2.13. Statistical Analysis
3. Results
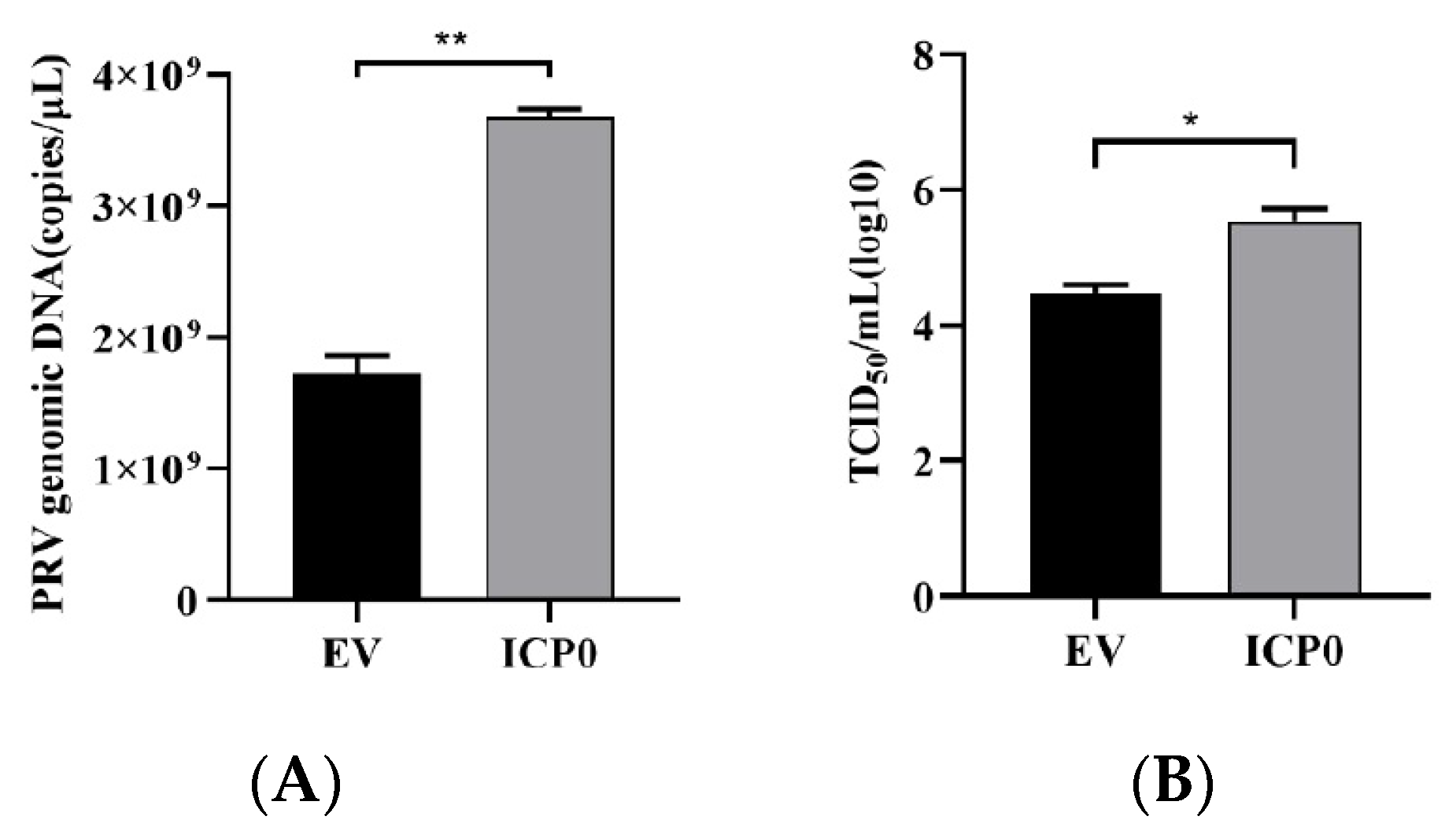
3.1. ICP0 Promotes PRV Replication in PK15 Cells
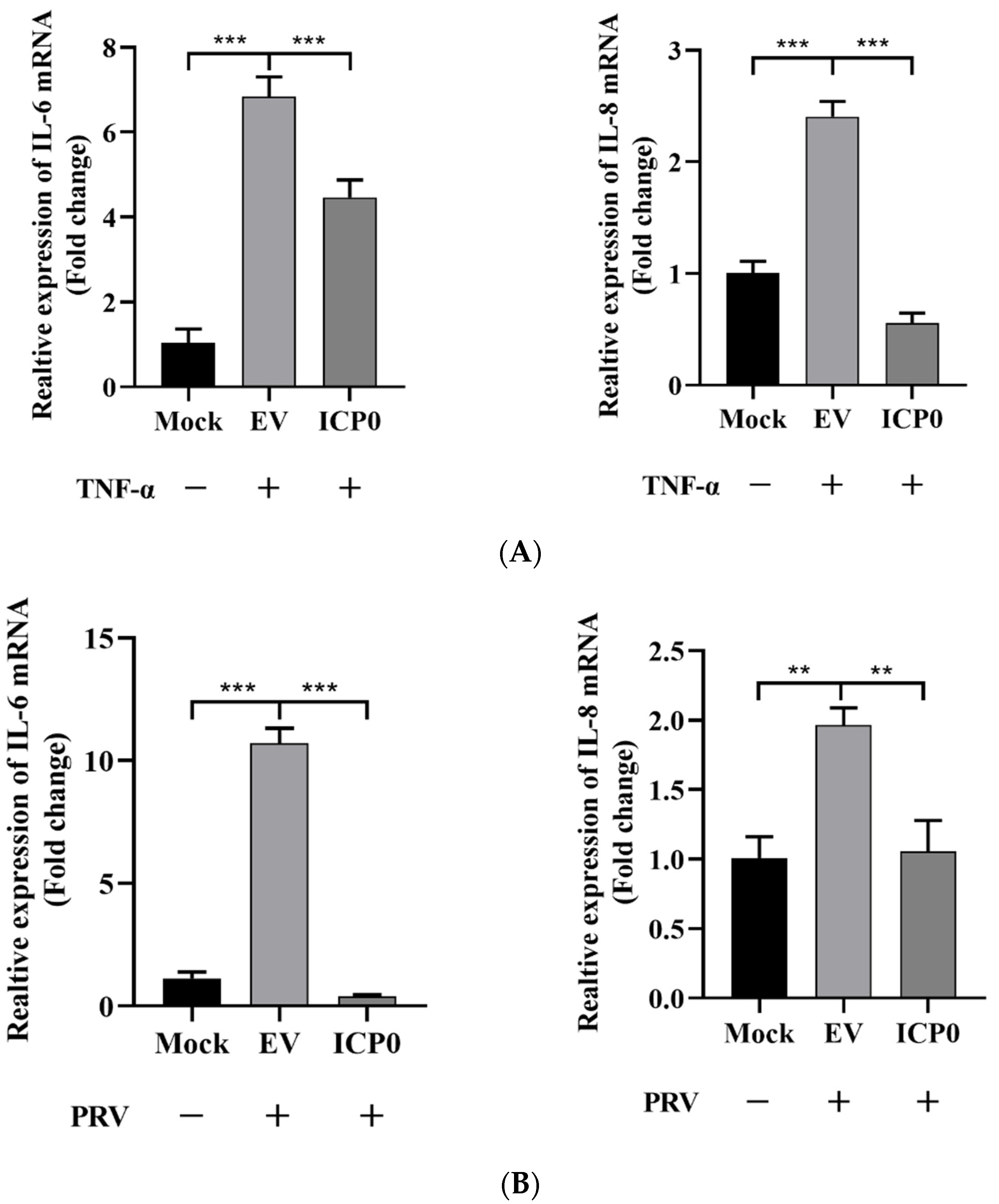
3.2. ICP0 Inhibits the Transcription of Inflammatory Factors
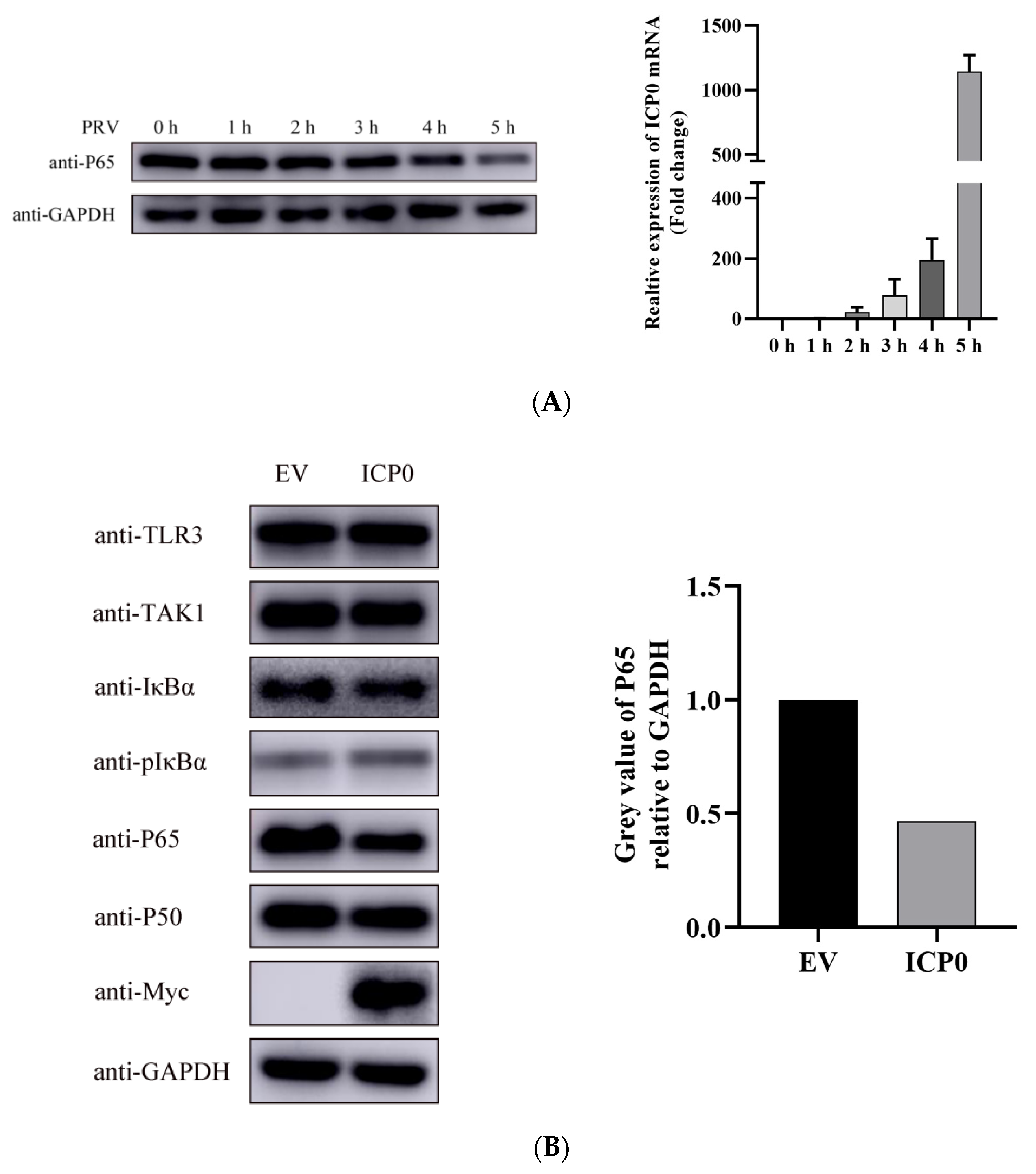
3.3. p65 May Be a Target of Pseudorabies Virus ICP0 Protein
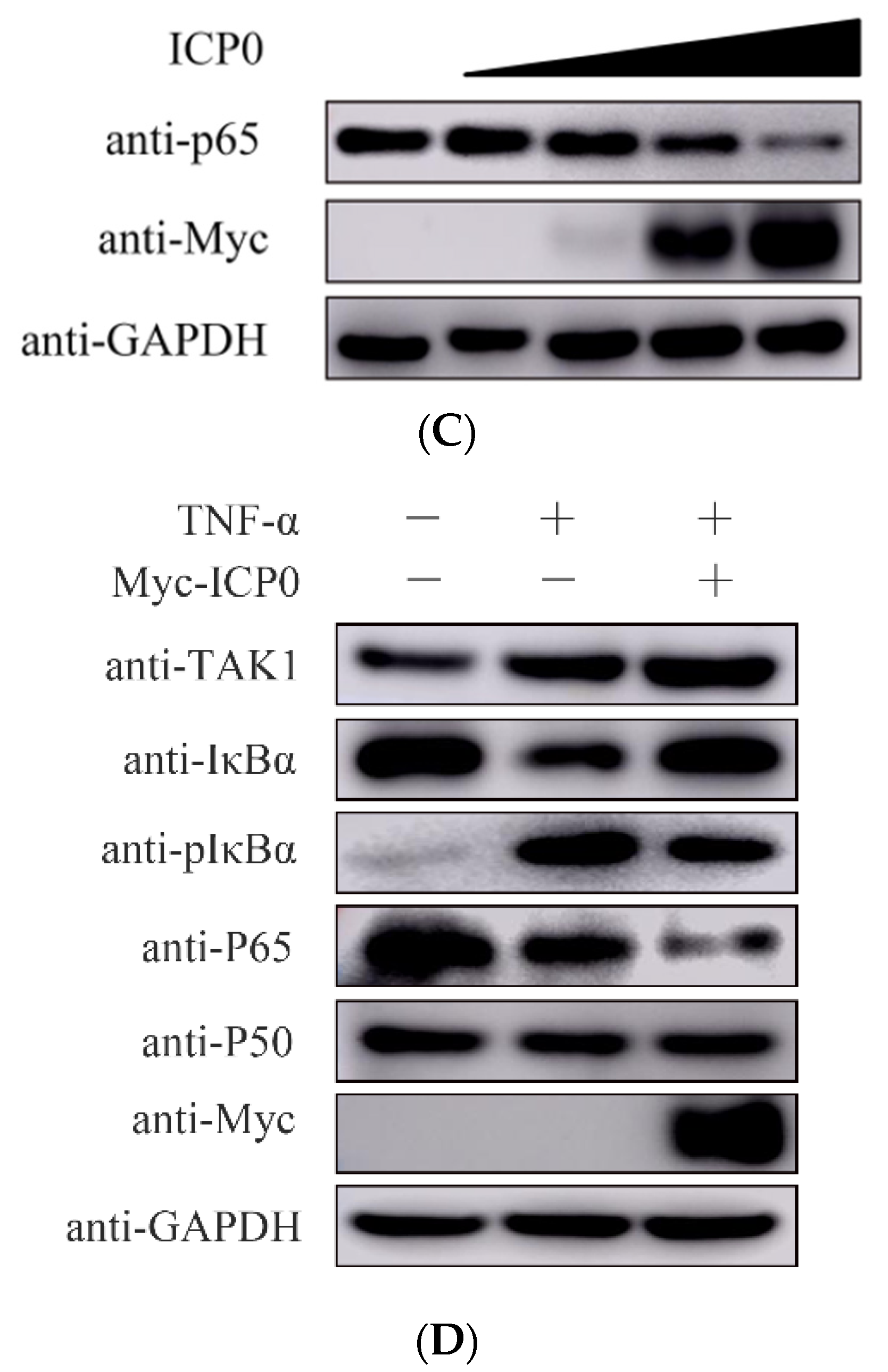
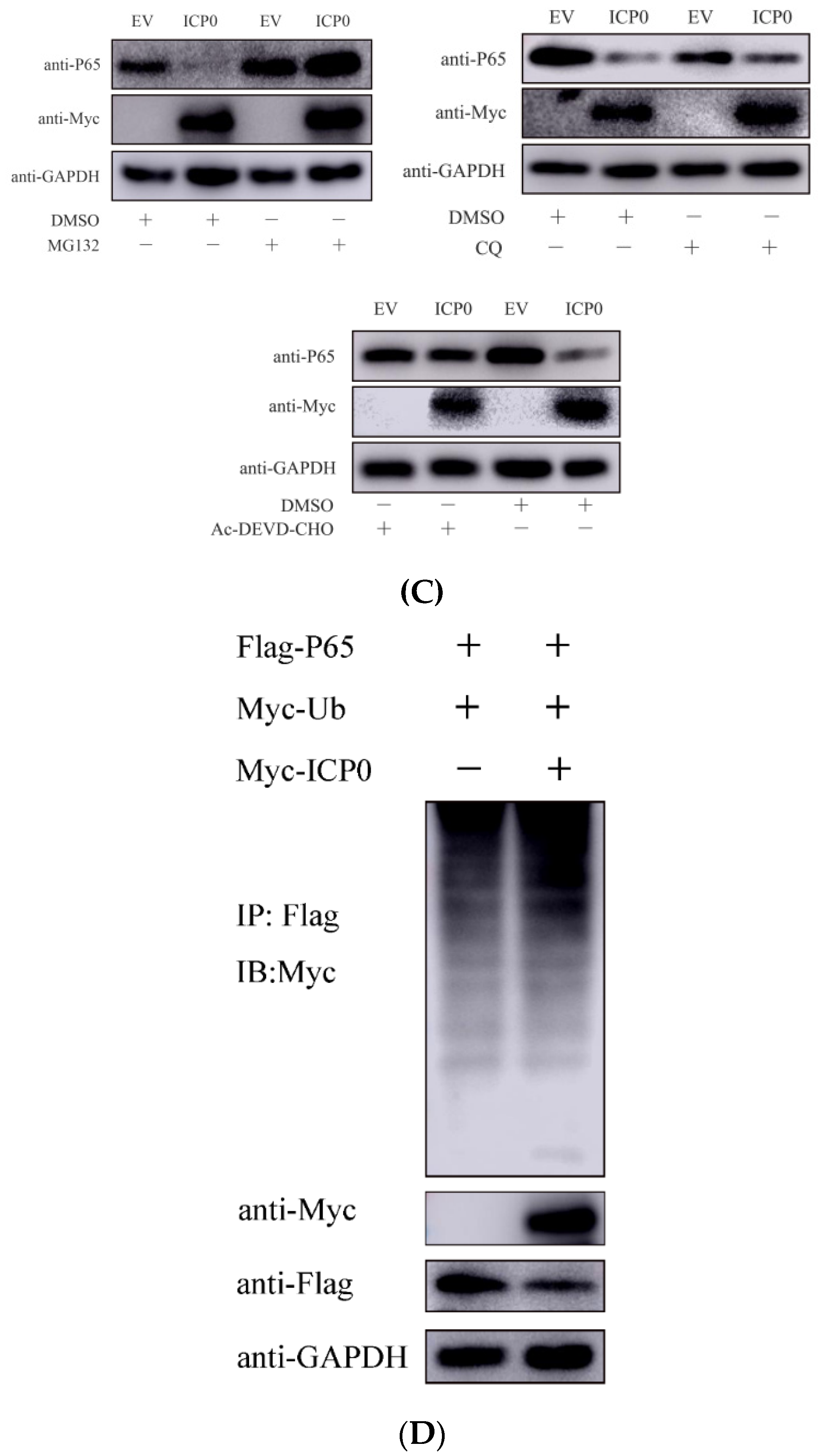
3.4. ICP0 Interacts with p65 and Degrades p65 through the Proteasome Pathway
3.5. ICP0 Protein Suppresses p65 Phosphorylation
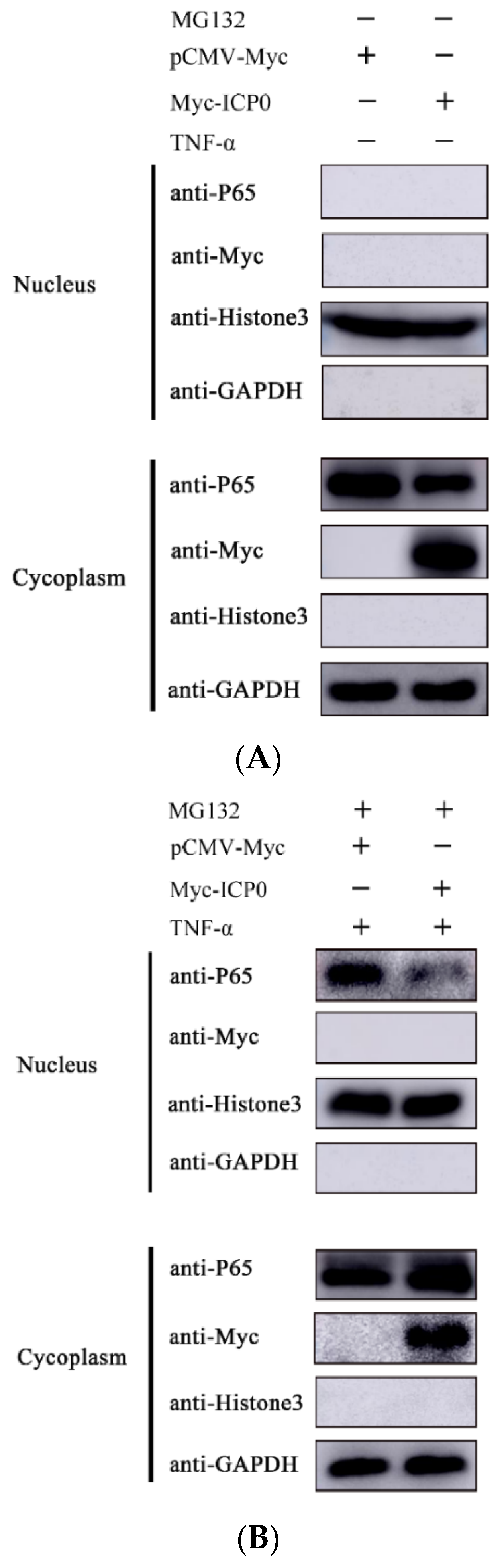
3.6. ICP0 Protein Blocks p65 Nuclear Translocation
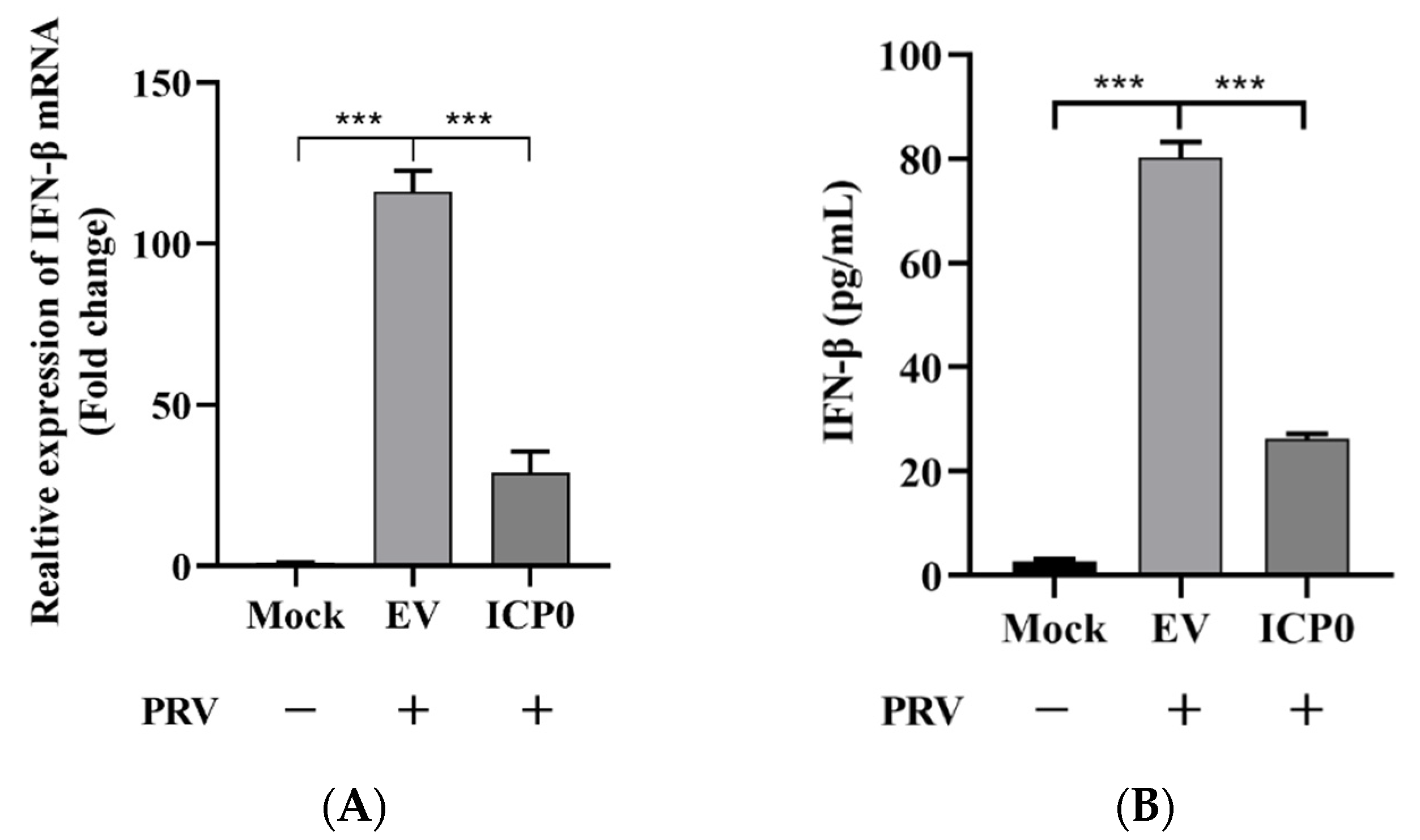
3.7. ICP0 Protein Promotes PRV Proliferation via Decreasing IFN-β Production
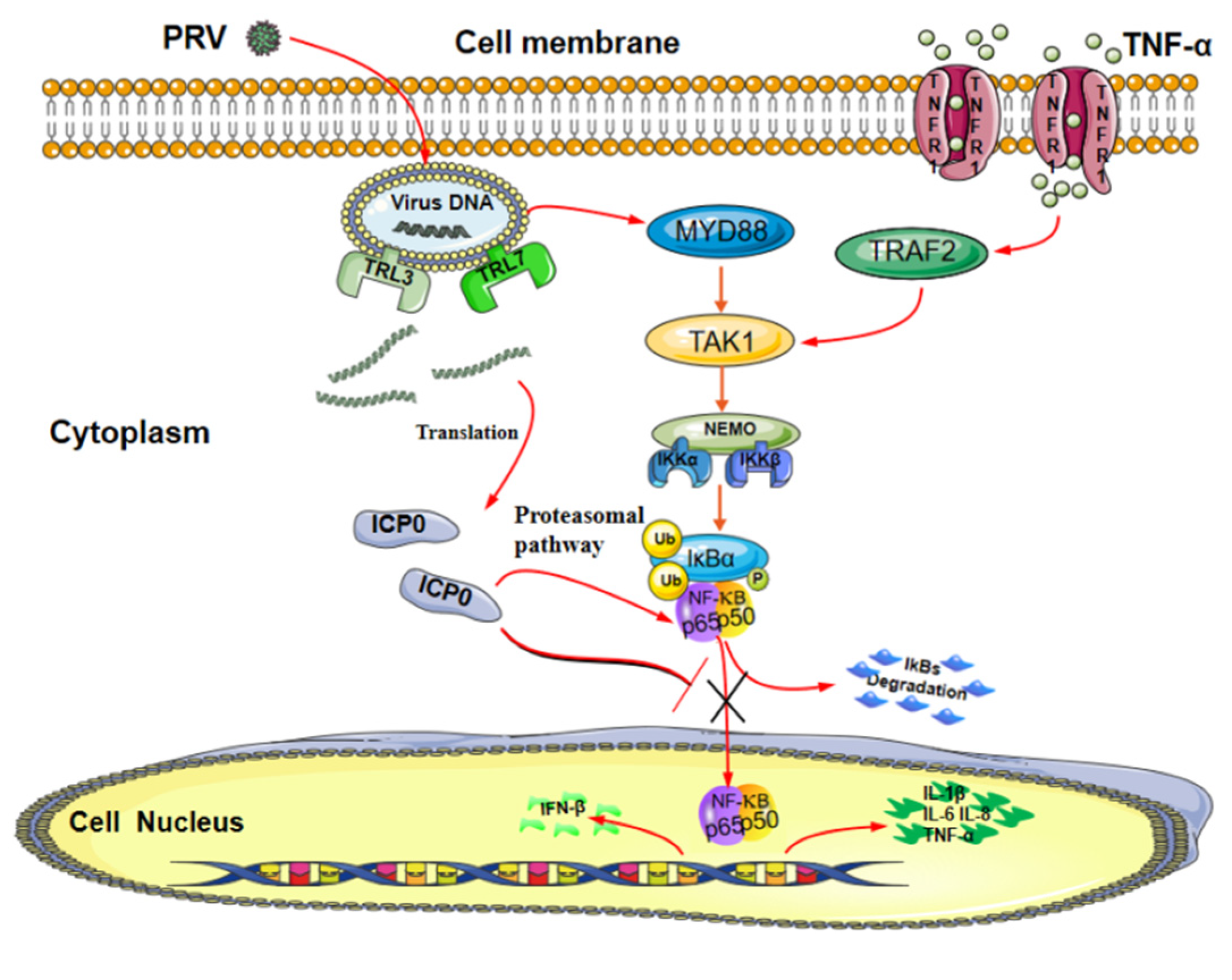
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-ΚB and the Link between Inflammation and Cancer: NF-ΚB Links Inflammation and Cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-ΚB in Immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-ΚB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-Receptors: From Mediators of Cell Death and Inflammation to Therapeutic Giants–Past, Present and Future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, L.M.; Gilmore, T.D. Looking Down on NF-ΚB. Mol. Cell Biol. 2020, 40, e00104-20. [Google Scholar] [CrossRef]
- Zhao, X.; Cui, Q.; Fu, Q.; Song, X.; Jia, R.; Yang, Y.; Zou, Y.; Li, L.; He, C.; Liang, X.; et al. Antiviral Properties of Resveratrol against Pseudorabies Virus Are Associated with the Inhibition of IκB Kinase Activation. Sci. Rep. 2017, 7, 8782. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-ΚB Signaling Pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Everett, R.D.; Boutell, C.; McNair, C.; Grant, L.; Orr, A. Comparison of the Biological and Biochemical Activities of Several Members of the Alphaherpesvirus ICP0 Family of Proteins. J. Virol. 2010, 84, 3476–3487. [Google Scholar] [CrossRef] [Green Version]
- Ly, C.Y.; Yu, C.; McDonald, P.R.; Roy, A.; Johnson, D.K.; Davido, D.J. Simple and Rapid High-Throughput Assay to Identify HSV-1 ICP0 Transactivation Inhibitors. Antivir. Res. 2021, 194, 105160. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Dybas, J.M.; Hughes, J.; Weitzman, M.D.; Boutell, C. The HSV-1 Ubiquitin Ligase ICP0: Modifying the Cellular Proteome to Promote Infection. Virus Res. 2020, 285, 198015. [Google Scholar] [CrossRef]
- Song, K.; Li, S. The Role of Ubiquitination in NF-ΚB Signaling during Virus Infection. Viruses 2021, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Boutell, C.; Everett, R.D. Regulation of Alphaherpesvirus Infections by the ICP0 Family of Proteins. J. Gen. Virol. 2013, 94, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Shahnazaryan, D.; Khalil, R.; Wynne, C.; Jefferies, C.A.; Ní Gabhann-Dromgoole, J.; Murphy, C.C. Herpes Simplex Virus 1 Targets IRF7 via ICP0 to Limit Type I IFN Induction. Sci. Rep. 2020, 10, 22216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, K.; Wang, S.; Zheng, C. Herpes Simplex Virus 1 E3 Ubiquitin Ligase ICP0 Protein Inhibits Tumor Necrosis Factor Alpha-Induced NF-ΚB Activation by Interacting with P65/RelA and P50/NF-ΚB1. J. Virol. 2013, 87, 12935–12948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bo, Z.; Miao, Y.; Xi, R.; Zhong, Q.; Bao, C.; Chen, H.; Sun, L.; Qian, Y.; Jung, Y.-S.; Dai, J. PRV UL13 Inhibits CGAS–STING-Mediated IFN-β Production by Phosphorylating IRF3. Vet. Res. 2020, 51, 118. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Bai, J.; Gao, Y.; Jin, L.; Wang, X.; Cao, M.; Liu, X.; Jiang, P. Peroxiredoxin 1 Interacts with TBK1/IKKε and Negatively Regulates Pseudorabies Virus Propagation by Promoting Innate Immunity. J. Virol. 2021, 95, e00923-21. [Google Scholar] [CrossRef]
- Lv, L.; Cao, M.; Bai, J.; Jin, L.; Wang, X.; Gao, Y.; Liu, X.; Jiang, P. PRV-Encoded UL13 Protein Kinase Acts as an Antagonist of Innate Immunity by Targeting IRF3-Signaling Pathways. Vet. Microbiol. 2020, 250, 108860. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, M.; Ye, C.; Ruan, K.; Xu, A.; Gao, F.; Tong, G.; Zheng, H. Inhibition of the DNA-Sensing Pathway by Pseudorabies Virus UL24 Protein via Degradation of Interferon Regulatory Factor 7. Vet. Microbiol. 2021, 255, 109023. [Google Scholar] [CrossRef]
- Lu, M.; Qiu, S.; Zhang, L.; Sun, Y.; Bao, E.; Lv, Y. Pseudorabies Virus Glycoprotein GE Suppresses Interferon-β Production via CREB-Binding Protein Degradation. Virus Res. 2021, 291, 198220. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Chen, L.; Bi, Y.; Idris, A.; Xu, S.; Li, X.; Zhang, Y.; Feng, R. Pseudorabies Virus US3 Protein Inhibits IFN-β Production by Interacting with IRF3 to Block Its Activation. Front. Microbiol. 2021, 12, 761282. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Yang, Y.-L.; Feng, C.; Sun, M.-X.; Peng, J.-M.; Tian, Z.-J.; Tang, Y.-D.; Cai, X.-H. Pseudorabies Virus UL24 Abrogates Tumor Necrosis Factor Alpha-Induced NF-ΚB Activation by Degrading P65. Viruses 2020, 12, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Bi, Y.; Xu, S.; Han, Y.; Idris, A.; Zhang, H.; Li, X.; Bai, J.; Zhang, Y.; Feng, R. Host Antiviral Protein IFITM2 Restricts Pseudorabies Virus Replication. Virus Res. 2020, 287, 198105. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-ΚB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [Green Version]
- Santoro, M.G.; Rossi, A.; Amici, C. NF-KB and Virus Infection: Who Controls Whom. EMBO J. 2003, 22, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Barrado-Gil, L.; del Puerto, A.; Galindo, I.; Cuesta-Geijo, M.Á.; García-Dorival, I.; de Motes, C.M.; Alonso, C. African Swine Fever Virus Ubiquitin-Conjugating Enzyme Is an Immunomodulator Targeting NF-ΚB Activation. Viruses 2021, 13, 1160. [Google Scholar] [CrossRef] [PubMed]
- Neidel, S.; Ren, H.; Torres, A.A.; Smith, G.L. NF-ΚB Activation Is a Turn on for Vaccinia Virus Phosphoprotein A49 to Turn off NF-ΚB Activation. Proc. Natl. Acad. Sci. USA 2019, 116, 5699–5704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, W.H.; Joosten, J.; Overheul, G.J.; Jansen, P.W.; Vermeulen, M.; Obbard, D.J.; Van Rij, R.P. Induction and Suppression of NF-ΚB Signalling by a DNA Virus of Drosophila. J. Virol. 2019, 93, e01443-18. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Luo, Y.; Wang, C.-H.; Yuan, J.; Li, N.; Song, K.; Qiu, H.-J. Control of Swine Pseudorabies in China: Opportunities and Limitations. Vet. Microbiol. 2016, 183, 119–124. [Google Scholar] [CrossRef]
- Sumner, R.P.; Maluquer de Motes, C.; Veyer, D.L.; Smith, G.L. Vaccinia Virus Inhibits NF-ΚB-Dependent Gene Expression Downstream of P65 Translocation. J. Virol. 2014, 88, 3092–3102. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xie, J.; Gao, M.; Yan, Z.; Chen, L.; Wei, S.; Feng, R. Pseudorabies Virus ICP0 Abolishes Tumor Necrosis Factor Alpha-Induced NF-κB Activation by Degrading P65. Viruses 2022, 14, 954. https://doi.org/10.3390/v14050954
Zhang X, Xie J, Gao M, Yan Z, Chen L, Wei S, Feng R. Pseudorabies Virus ICP0 Abolishes Tumor Necrosis Factor Alpha-Induced NF-κB Activation by Degrading P65. Viruses. 2022; 14(5):954. https://doi.org/10.3390/v14050954
Chicago/Turabian StyleZhang, Xiangbo, Jingying Xie, Ming Gao, Zhenfang Yan, Lei Chen, Suocheng Wei, and Ruofei Feng. 2022. "Pseudorabies Virus ICP0 Abolishes Tumor Necrosis Factor Alpha-Induced NF-κB Activation by Degrading P65" Viruses 14, no. 5: 954. https://doi.org/10.3390/v14050954





