Predictable Chikungunya Infection Dynamics in Brazil
Abstract
:1. Introduction
2. Materials and Methods
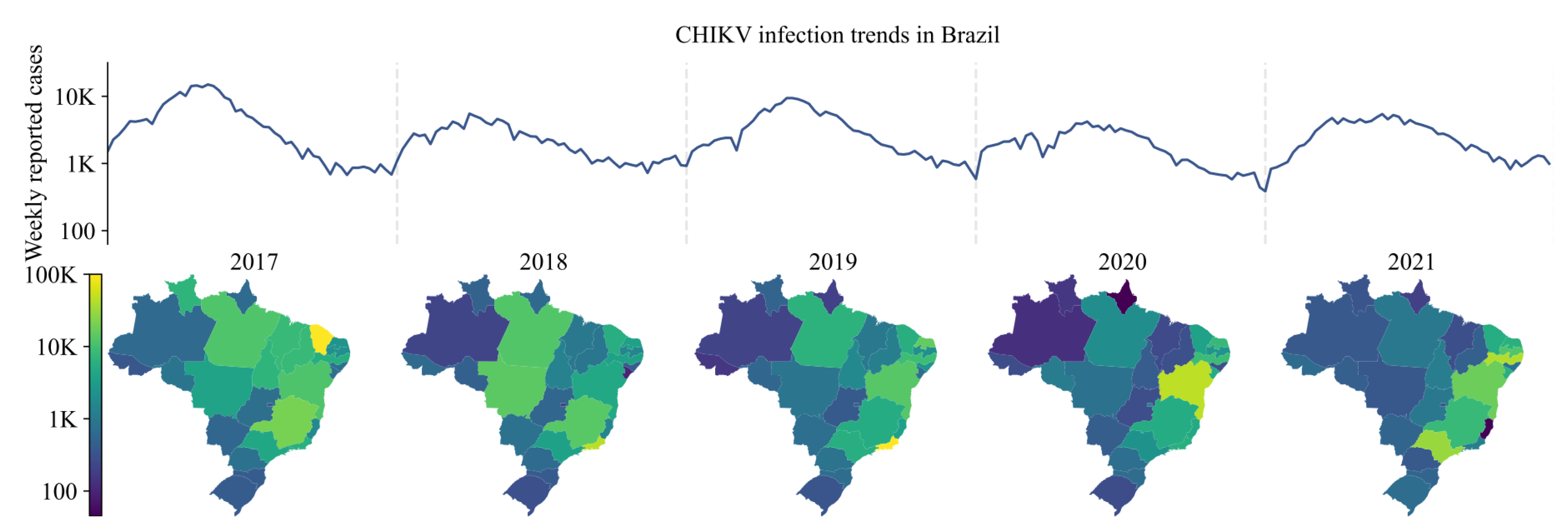
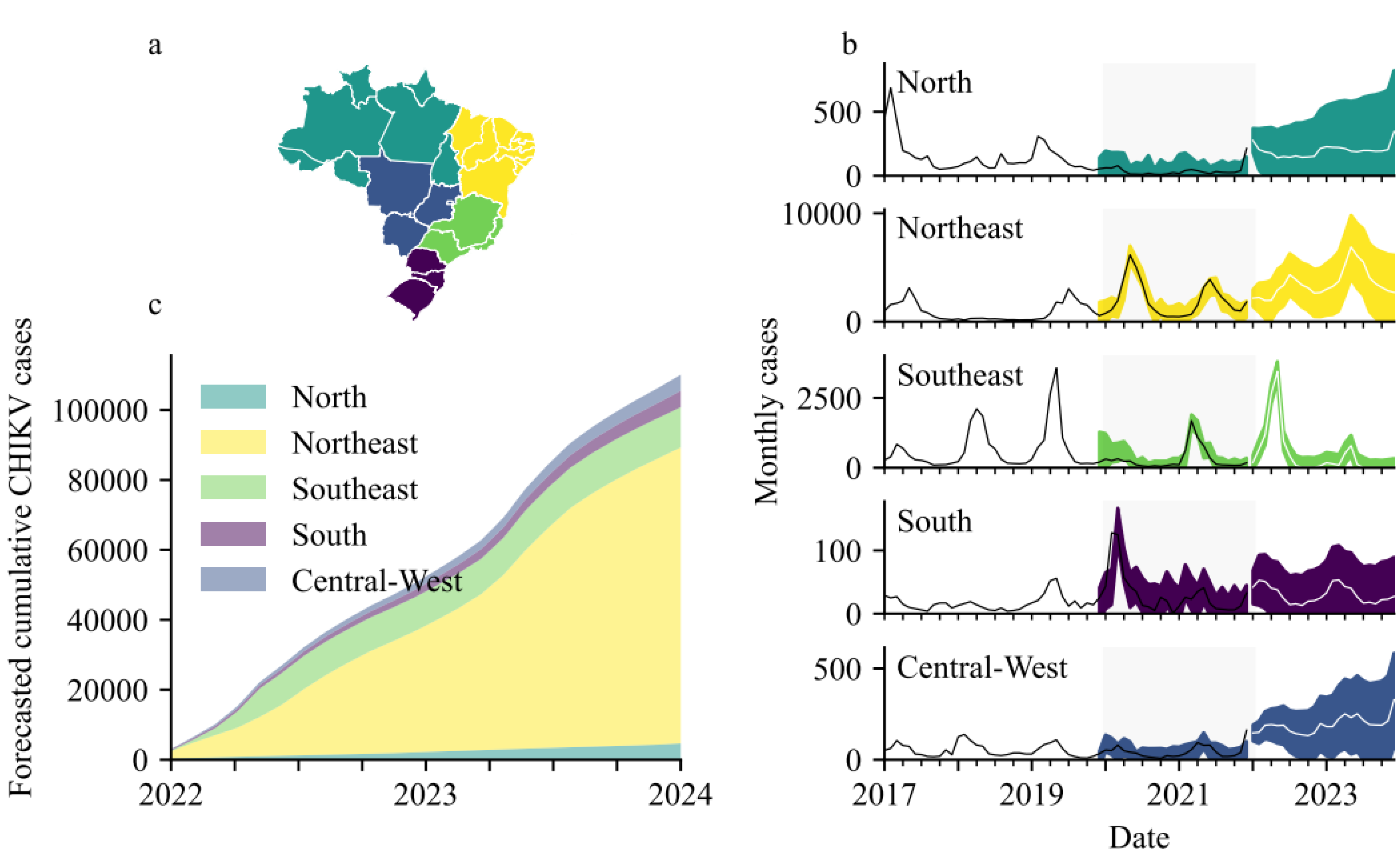
3. Results
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, R.; Mukhopadhyay, S.; Merits, A.; Bolling, B.; Nasar, F.; Coffey, L.L.; Powers, A.; Weaver, S.C.; Ictv Report Consortium. ICTV Virus Taxonomy Profile: Togaviridae. J. Gen. Virol. 2018, 99, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- de Lima, S.T.S.; de Souza, W.M.; Cavalcante, J.W.; da Silva Candido, D.; Fumagalli, M.J.; Carrera, J.P.; Simões Mello, L.M.; De Carvalho Araújo, F.M.; Cavalcante Ramalho, I.L.; de Almeida Barreto, F.K.; et al. Fatal Outcome of Chikungunya Virus Infection in Brazil. Clin. Infect. Dis. 2021, 73, e2436–e2443. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.M.; Brault, A.C.; Tesh, R.B.; Weaver, S.C. Re-emergence of chikungunya and o’nyong-nyong viruses: Evidence for distinct geographical lineages and distant evolutionary relationships. J. Gen. Virol. 2000, 81, 471–479. [Google Scholar] [CrossRef]
- Weaver, S.C.; Osorio, J.E.; Livengood, J.A.; Chen, R.; Stinchcomb, D.T. Chikungunya virus and prospects for a vaccine. Expert Rev. Vaccines 2012, 11, 1087–1101. [Google Scholar] [CrossRef]
- Leparc-Goffart, I.; Nougairede, A.; Cassadou, S.; Prat, C.; de Lamballerie, X. Chikungunya in the Americas. Lancet 2014, 383, 514. [Google Scholar] [CrossRef]
- de Oliveira, E.C.; Fonseca, V.; Xavier, J.; Adelino, T.; Morales Claro, I.; Fabri, A.; Marques Macario, E.; Viniski, A.E.; Campos Souza, C.L.; Gomes da Costa, E.S.; et al. Short report: Introduction of chikungunya virus ECSA genotype into the Brazilian Midwest and its dispersion through the Americas. PLOS Negl. Trop. Dis. 2021, 15, e0009290. [Google Scholar] [CrossRef]
- Del Valle, S.Y.; McMahon, B.H.; Asher, J.; Hatchett, R.; Lega, J.C.; Brown, H.E.; Leany, M.E.; Pantazis, Y.; Roberts, D.J.; Moore, S.; et al. Summary results of the 2014–2015 DARPA Chikungunya challenge. BMC Infect. Dis. 2018, 18, 245. [Google Scholar] [CrossRef]
- Portal da Secretaria de Vigilância em Saúde do Ministério da Saúde. Informações sobre o Sinan (Sistema Informação Agravos Notif. e RESP (Registro Eventos Saúde Pública) [Internet]. Available online: http://tabnet.datasus.gov.br/ (accessed on 8 June 2022).
- Dzul-Manzanilla, F.; Correa-Morales, F.; Che-Mendoza, A.; Palacio-Vargas, J.; Sánchez-Tejeda, G.; González-Roldan, J.F.; López-Gatell, H.; Flores-Suárez, A.E.; Gómez-Dantes, H.; Coelho, G.E.; et al. Identifying urban hotspots of dengue, chikungunya, and Zika transmission in Mexico to support risk stratification efforts: A spatial analysis. Lancet Planet. Health 2021, 5, e277–e285. [Google Scholar] [CrossRef]
- Hyndman, R.J.; Athanasopoulos, G. Forecasting: Principles and Practice, 2nd ed.; OTexts: Melbourne, VIC, Australia, 2018. [Google Scholar]
- Powell, M.J. An efficient method for finding the minimum of a function of several variables without calculating derivatives. Comput. J. 1964, 7, 155–162. [Google Scholar] [CrossRef]
- Available online: https://www.ibge.gov.br/en/home-eng.html (accessed on 8 June 2022).
- Moran, P.A.P. Notes on ontinuous Stochastic Phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Cliff, A.D.; Ord, J.K. Spatial Processes: Models and Applications; Pion Ltd.: London, UK, 1981. [Google Scholar]
- ECDC. Situation Update. 2 June 2022. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Communicable-disease-threats-report-3-June-2022_2.pdf (accessed on 8 June 2022).
- Scott, D.W. Multivariate Density Estimation: Theory, Practice, and Visualization; John Wiley & Sons: Chicester, NY, USA, 1992. [Google Scholar]
- Nsoesie, E.O.; Kraemer, M.U.; Golding, N.; Pigott, D.M.; Brady, O.J.; Moyes, C.L.; Johansson, M.A.; Gething, P.W.; Velayudhan, R.; Khan, K.; et al. Global distribution and environmental suitability for chikungunya virus, 1952 to 2015. Euro Surveill. 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Bloch, D. The Cost and Burden of Chikungunya in the Americas. Ph.D. Thesis, Yale School of Public Health, New Haven, CT, USA, 2016. [Google Scholar]
- Plisa Health Information Platform for the Americas–Chikungunya Cases [Internet]. 2021. Available online: https://www3.paho.org/data/index.php/en/mnu-topics/chikv-en/550-chikv-weekly-en.html (accessed on 8 June 2022).
- Salje, H.; Cauchemez, S.; Alera, M.T.; Rodriguez-Barraquer, I.; Thaisomboonsuk, B.; Srikiatkhachorn, A.; Lago, C.B.; Villa, D.; Klungthong, C.; Tac-An, I.A.; et al. Reconstruction of 60 Years of Chikungunya Epidemiology in the Philippines Demonstrates Episodic and Focal Transmission. J. Infect. Dis. 2015, 213, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Bettis, A.A.; L’Azou Jackson, M.; Yoon, I.K.; Breugelmans, J.G.; Goios, A.; Gubler, D.J.; Powers, A.M. The global epidemiology of chikungunya from 1999 to 2020: A systematic literature review to inform the development and introduction of vaccines. PLOS Negl. Trop. Dis. 2022, 16, e0010069. [Google Scholar] [CrossRef]
- Johansson, M.A.; Apfeldorf, K.M.; Dobson, S.; Devita, J.; Buczak, A.L.; Baugher, B.; Moniz, L.J.; Bagley, T.; Babin, S.M.; Guven, E.; et al. An open challenge to advance probabilistic forecasting for dengue epidemics. Proc. Natl. Acad. Sci. USA 2019, 116, 24268–24274. [Google Scholar] [CrossRef]
- Yakob, L. Zika Virus after the Public Health Emergency of International Concern Period, Brazil. Emerg. Infect. Dis. 2022, 28, 837–840. [Google Scholar] [CrossRef]
- George, D.B.; Taylor, W.; Shaman, J.; Rivers, C.; Paul, B.; O’Toole, T.; Johansson, M.A.; Hirschman, L.; Biggerstaff, M.; Asher, J.; et al. Technology to advance infectious disease forecasting for outbreak management. Nat. Commun. 2019, 10, 3932. [Google Scholar] [CrossRef]
- de Lima Cavalcanti, T.Y.; Pereira, M.R.; de Paula, S.O.; Franca, R.F. A Review on Chikungunya Virus Epidemiology, Pathogenesis and Current Vaccine Development. Viruses 2022, 14, 969. [Google Scholar] [CrossRef]
- Rezza, G.; Weaver, S.C. Chikungunya as a paradigm for emerging viral diseases: Evaluating disease impact and hurdles to vaccine development. PLoS Negl. Trop. Dis. 2019, 13, e0006919. [Google Scholar] [CrossRef]
- Ministério da Saúde do Brasil. Boletins Epidemiológicos. 2020. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/edicoes/2020 (accessed on 8 June 2022).
- Furuya-Kanamori, L.; Liang, S.; Milinovich, G.; Soares Magalhaes, R.J.; Clements, A.C.; Hu, W.; Brasil, P.; Frentiu, F.D.; Dunning, R.; Yakob, L. Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect. Dis. 2016, 16, 84. [Google Scholar] [CrossRef] [Green Version]
- Bustos Carrillo, F.; Collado, D.; Sanchez, N.; Ojeda, S.; Lopez Mercado, B.; Burger-Calderon, R.; Gresh, L.; Gordon, A.; Balmaseda, A.; Kuan, G.; et al. Epidemiological Evidence for Lineage-Specific Differences in the Risk of Inapparent Chikungunya Virus Infection. J. Virol. 2019, 93, e01622-18. [Google Scholar] [CrossRef] [PubMed]
- Puntasecca, C.J.; King, C.H.; LaBeaud, A.D. Measuring the global burden of chikungunya and Zika viruses: A systematic review. PLOS Negl. Trop. Dis. 2021, 15, e0009055. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakob, L. Predictable Chikungunya Infection Dynamics in Brazil. Viruses 2022, 14, 1889. https://doi.org/10.3390/v14091889
Yakob L. Predictable Chikungunya Infection Dynamics in Brazil. Viruses. 2022; 14(9):1889. https://doi.org/10.3390/v14091889
Chicago/Turabian StyleYakob, Laith. 2022. "Predictable Chikungunya Infection Dynamics in Brazil" Viruses 14, no. 9: 1889. https://doi.org/10.3390/v14091889





