Aerosol Delivery of Palivizumab in a Neonatal Lamb Model of Respiratory Syncytial Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. In-Life Measures and Sample Collection for Assessment of RSV Infection
2.3. RSV RT-qPCR
2.4. Lung Histopathology
2.5. Light Microscopy Analysis
2.6. Statistical Analysis
3. Results
3.1. Aerosol Administration
3.2. Clinical Observations
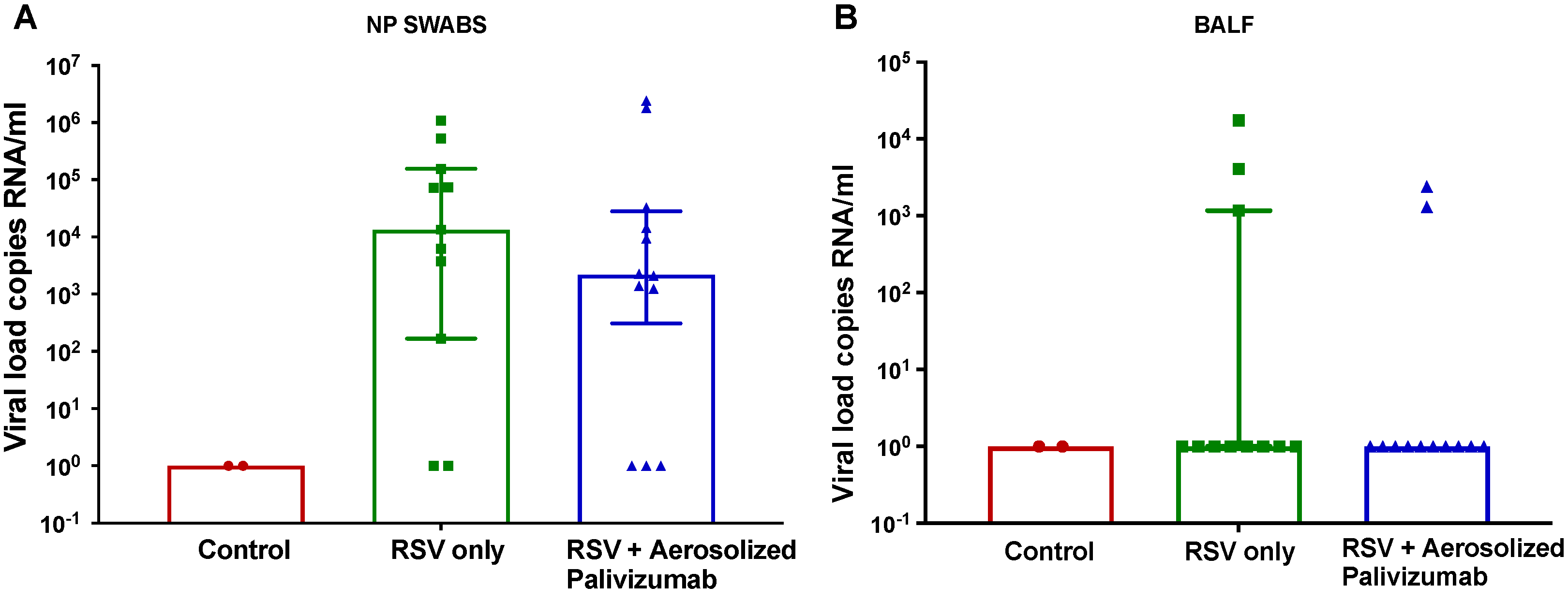
3.3. Viral Load
3.4. Lung Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mejias, A.; Rodríguez-Fernández, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The journey to a respiratory syncytial virus vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Taleb, S.A.; Al Thani, A.A.; Al Ansari, K.; Yassine, H.M. Human respiratory syncytial virus: Pathogenesis, immune responses, and current vaccine approaches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Do, L.A.H.; Wurzel, D.; Quan Toh, Z.; Mulholland, K.; Pellicci, D.G.; Licciardi, P.V. Severe respiratory syncytial virus disease in preterm infants: A case of innate immaturity. Thorax 2021, 76, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, R.; Pecenka, C.; Baral, R. Cost of childhood RSV management and cost-effectiveness of RSV interventions: A systematic review from a low- and middle-income country perspective. BMC Med. 2023, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Boytchev, H. Maternal RSV vaccine: Further analysis is urged on preterm births. BMJ 2023, 381, p1021. [Google Scholar] [CrossRef]
- Domachowske, J.; Madhi, S.A.; Simões, E.A.F.; Atanasova, V.; Cabañas, F.; Furuno, K.; Garcia-Garcia, M.L.; Grantina, I.; Nguyen, K.A.; Brooks, D.; et al. Safety of Nirsevimab for RSV in Infants with Heart or Lung Disease or Prematurity. N. Engl. J. Med. 2022, 386, 892–894. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef]
- Pfizer. Pfizer Announces Positive Top-Line Data of Phase 3 Global Maternal Immunization Trial for its Bivalent Respiratory Syncytial Virus (RSV) Vaccine Candidate. 2022. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-phase-3-global (accessed on 8 September 2023).
- Resch, B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum. Vaccines Immunother. 2017, 13, 2138–2149. [Google Scholar] [CrossRef]
- Rajapaksa, A.E.; Do, L.A.H.; Suryawijaya Ong, D.; Sourial, M.; Veysey, D.; Beare, R.; Hughes, W.; Yang, W.; Bischof, R.J.; McDonnell, A.; et al. Pulmonary Deposition of Radionucleotide-Labeled Palivizumab: Proof-of-Concept Study. Front. Pharmacol. 2020, 11, 1291. [Google Scholar] [CrossRef]
- Sitthicharoenchai, P.; Alnajjar, S.; Ackermann, M.R. A model of respiratory syncytial virus (RSV) infection of infants in newborn lambs. Cell Tissue Res. 2020, 380, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Olivier, A.; Gallup, J.; de Macedo, M.M.; Varga, S.M.; Ackermann, M. Human respiratory syncytial virus A2 strain replicates and induces innate immune responses by respiratory epithelia of neonatal lambs. Int. J. Exp. Pathol. 2009, 90, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Derscheid, R.J.; Ackermann, M.R. Perinatal lamb model of respiratory syncytial virus (RSV) infection. Viruses 2012, 4, 2359–2378. [Google Scholar] [CrossRef] [PubMed]
- Larios Mora, A.; Detalle, L.; Van Geelen, A.; Davis, M.S.; Stohr, T.; Gallup, J.M.; Ackermann, M.R. Kinetics of Respiratory Syncytial Virus (RSV) Memphis Strain 37 (M37) Infection in the Respiratory Tract of Newborn Lambs as an RSV Infection Model for Human Infants. PLoS ONE 2015, 10, e0143580. [Google Scholar] [CrossRef]
- Ackermann, M.R. Lamb model of respiratory syncytial virus-associated lung disease: Insights to pathogenesis and novel treatments. ILAR J. 2014, 55, 4–15. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLOS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- Do, L.A.H.; Tse, R.; Nathanielsz, J.; Anderson, J.; Ong, D.S.; Chappell, K.; Mulholland, K.; Licciardi, P.V. An Improved and High Throughput Respiratory Syncytial Virus (RSV) Micro-neutralization Assay. J. Vis. Exp. 2019, 143, e59025. [Google Scholar] [CrossRef]
- Do, L.A.; van Doorn, H.R.; Bryant, J.E.; Nghiem, M.N.; Nguyen Van, V.C.; Vo, C.K.; Nguyen, M.D.; Tran, T.H.; Farrar, J.; de Jong, M.D. A sensitive real-time PCR for detection and subgrouping of human respiratory syncytial virus. J. Virol. Methods 2012, 179, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Larios Mora, A.; Detalle, L.; Gallup, J.M.; Van Geelen, A.; Stohr, T.; Duprez, L.; Ackermann, M.R. Delivery of ALX-0171 by inhalation greatly reduces respiratory syncytial virus disease in newborn lambs. mAbs 2018, 10, 778–795. [Google Scholar] [CrossRef]
- Martinez, M.E.; Harder, O.E.; Rosas, L.E.; Joseph, L.; Davis, I.C.; Niewiesk, S. Pulmonary function analysis in cotton rats after respiratory syncytial virus infection. PLoS ONE 2020, 15, e0237404. [Google Scholar] [CrossRef] [PubMed]
- Pickles, R.J.; DeVincenzo, J.P. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J. Pathol. 2015, 235, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Knisely, J.M.; Buyon, L.E.; Mandt, R.; Farkas, R.; Balasingam, S.; Bok, K.; Buchholz, U.J.; D’Souza, M.P.; Gordon, J.L.; King, D.F.L.; et al. Mucosal vaccines for SARS-CoV-2: Scientific gaps and opportunities-workshop report. npj Vaccines 2023, 8, 53. [Google Scholar] [CrossRef] [PubMed]




| Group No. | Group Name | RSV Inoculation | Aerosolised Palivizumab | Study Duration (Days) | No. of Lambs |
|---|---|---|---|---|---|
| 0 | Control | - | - | 6 | 2 |
| 1a | RSV/untreated–D6 | + | - | 6 | 6 |
| 1b | RSV/untreated–D10 | + | - | 10 | 6 |
| 2a | RSV/palivizumab–D6 | + | + | 6 | 6 |
| 2b | RSV/palivizumab–D10 | + | + | 10 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edirisinghe, H.S.; Rajapaksa, A.E.; Royce, S.G.; Sourial, M.; Bischof, R.J.; Anderson, J.; Sarila, G.; Nguyen, C.D.; Mulholland, K.; Do, L.A.H.; et al. Aerosol Delivery of Palivizumab in a Neonatal Lamb Model of Respiratory Syncytial Virus Infection. Viruses 2023, 15, 2276. https://doi.org/10.3390/v15112276
Edirisinghe HS, Rajapaksa AE, Royce SG, Sourial M, Bischof RJ, Anderson J, Sarila G, Nguyen CD, Mulholland K, Do LAH, et al. Aerosol Delivery of Palivizumab in a Neonatal Lamb Model of Respiratory Syncytial Virus Infection. Viruses. 2023; 15(11):2276. https://doi.org/10.3390/v15112276
Chicago/Turabian StyleEdirisinghe, Hasindu S., Anushi E. Rajapaksa, Simon G. Royce, Magdy Sourial, Robert J. Bischof, Jeremy Anderson, Gulcan Sarila, Cattram D. Nguyen, Kim Mulholland, Lien Anh Ha Do, and et al. 2023. "Aerosol Delivery of Palivizumab in a Neonatal Lamb Model of Respiratory Syncytial Virus Infection" Viruses 15, no. 11: 2276. https://doi.org/10.3390/v15112276






