Molecular and Pathological Characterization of Classical Swine Fever Virus Genotype 2 Strains Responsible for the 2013–2018 Outbreak in Colombia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Samples from Colombia
2.2. Nucleic Acid Extraction and RRT-PCR
2.3. Virus Isolation and Titration
2.4. Whole Genome Sequencing
2.5. Phylogenetic Analysis
2.6. Animal Experiment
2.7. Hematological and Biochemical Analysis
2.8. Antibody Detection
2.9. Histopathology and Immunohistochemistry (IHC)
3. Results
3.1. Laboratory Analysis of the Clinical Samples from Colombia
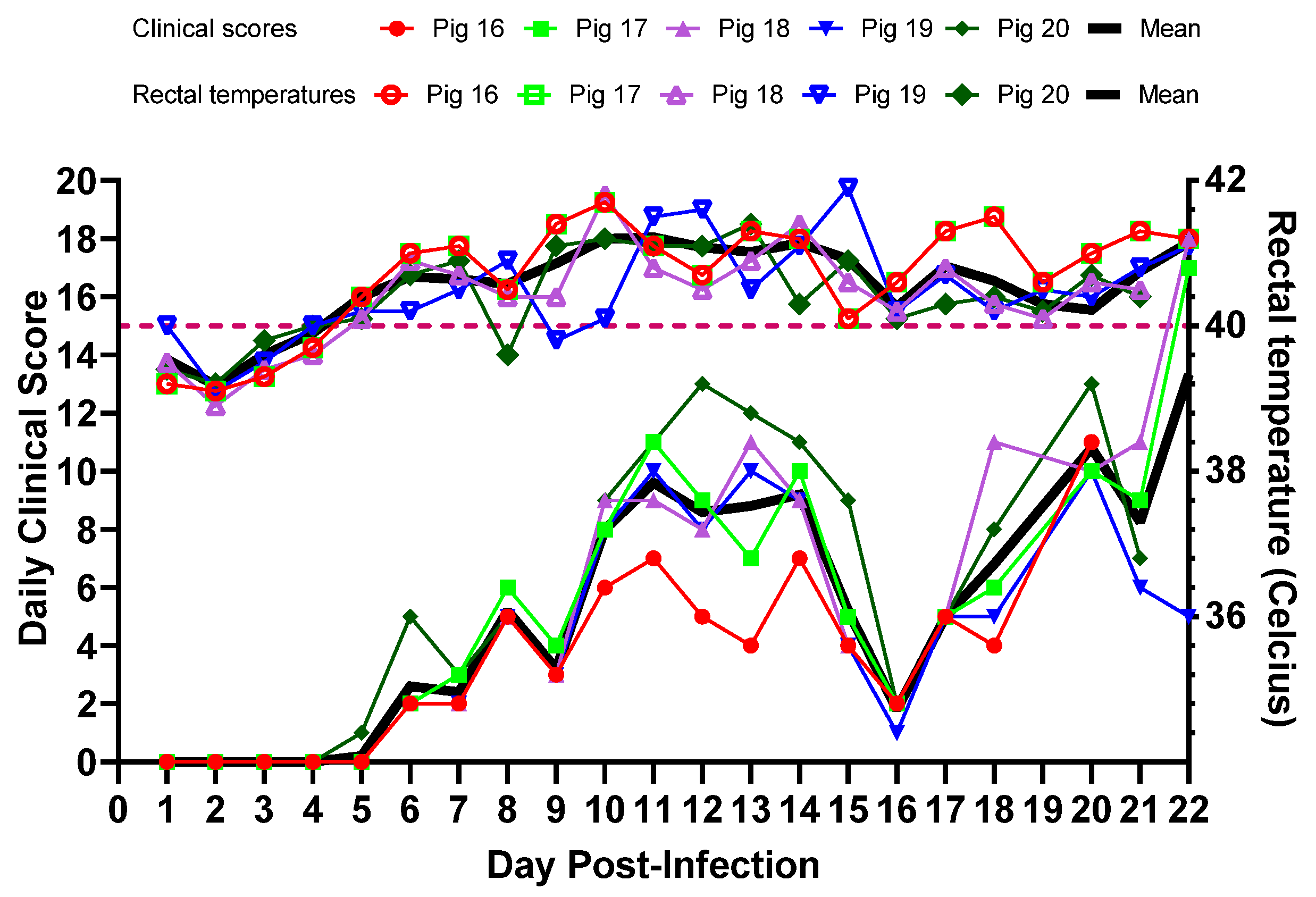
3.2. Experimental Inoculation—Clinical Signs and Clinical Scores
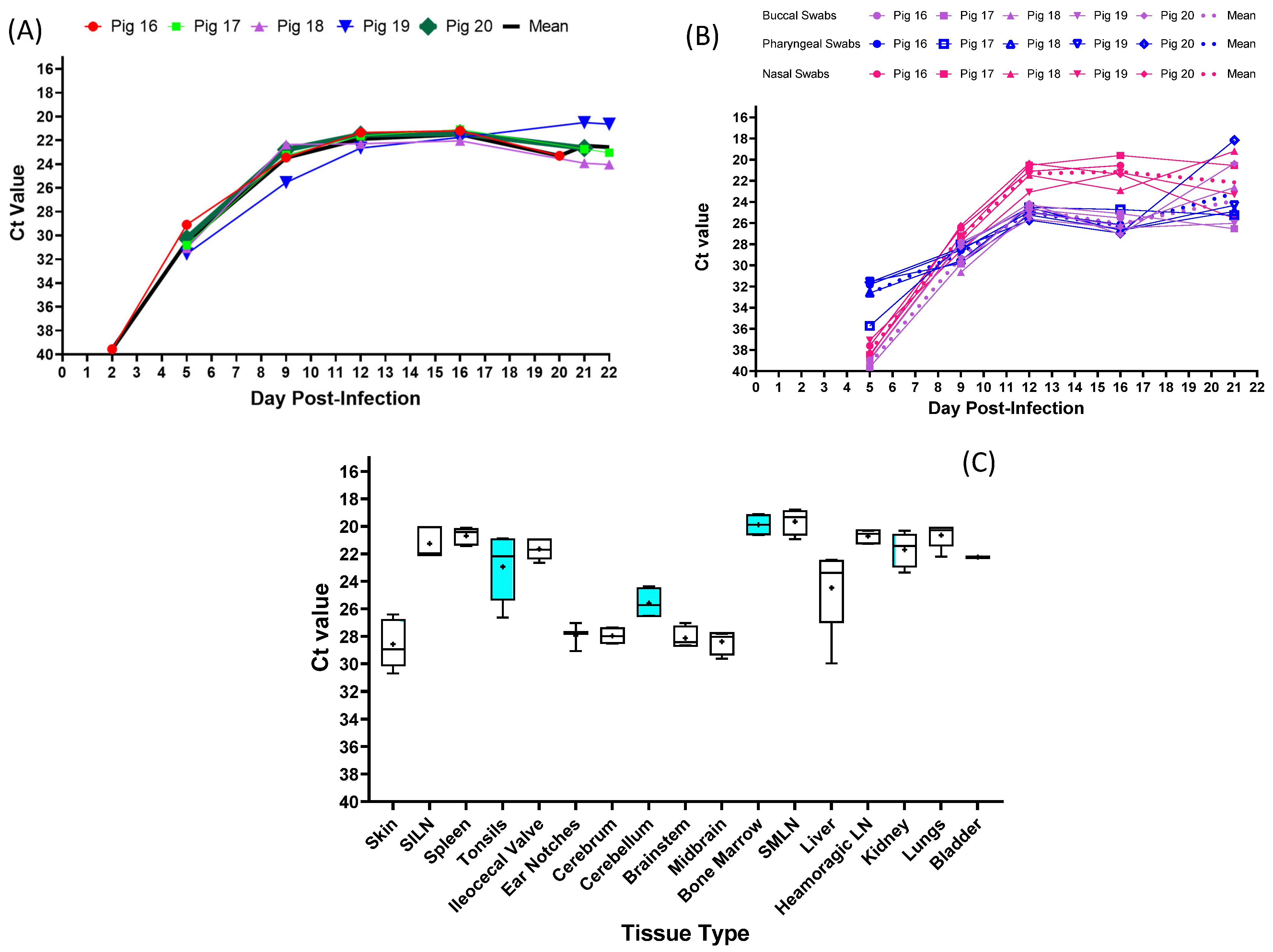
3.3. Experimental Inoculation—CSFV Genome Detection
3.4. Experimental Inoculation—Serology
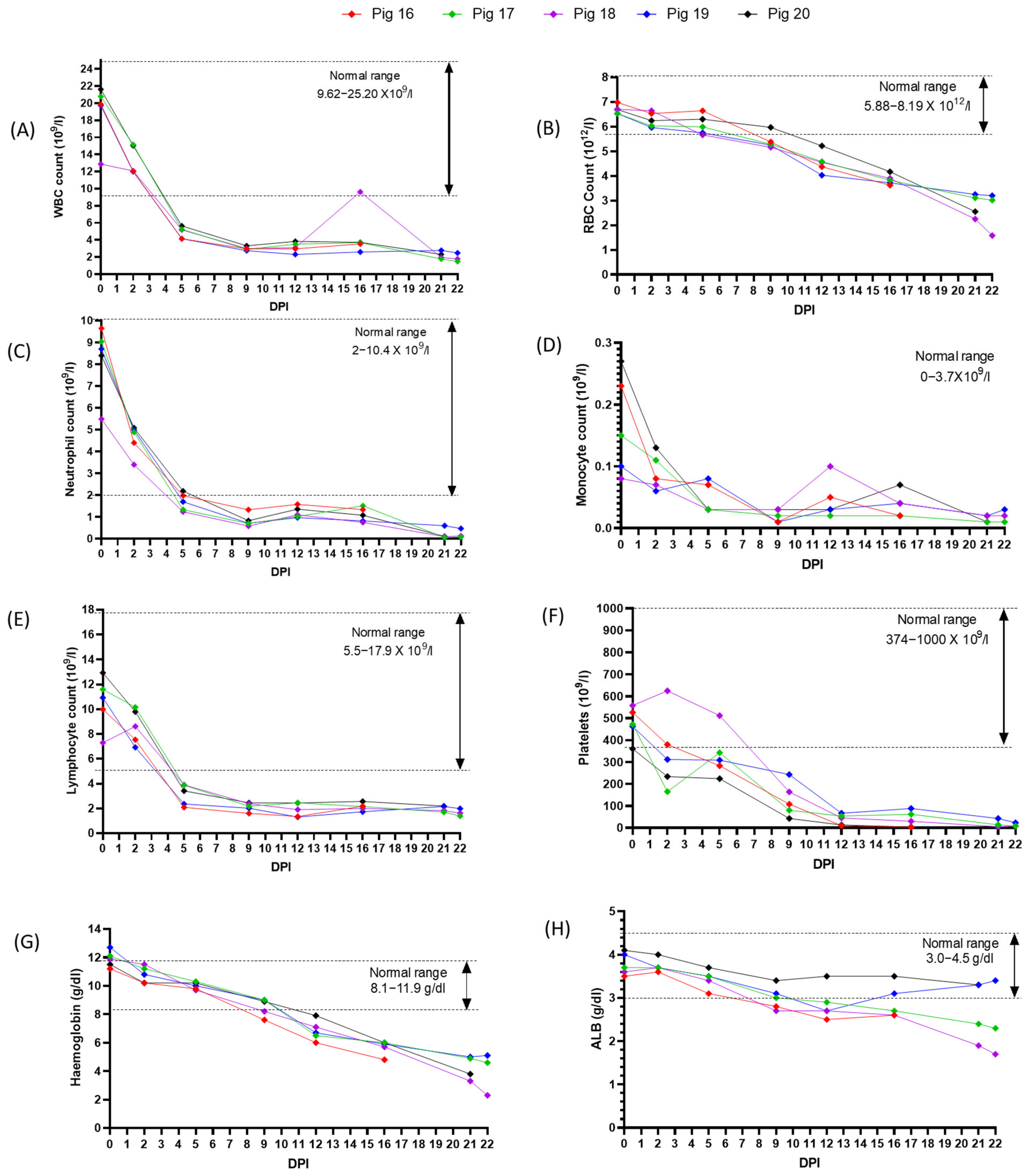
3.5. Experimental Inoculation—Hematology
3.6. Experimental Inoculation—Gross Pathology
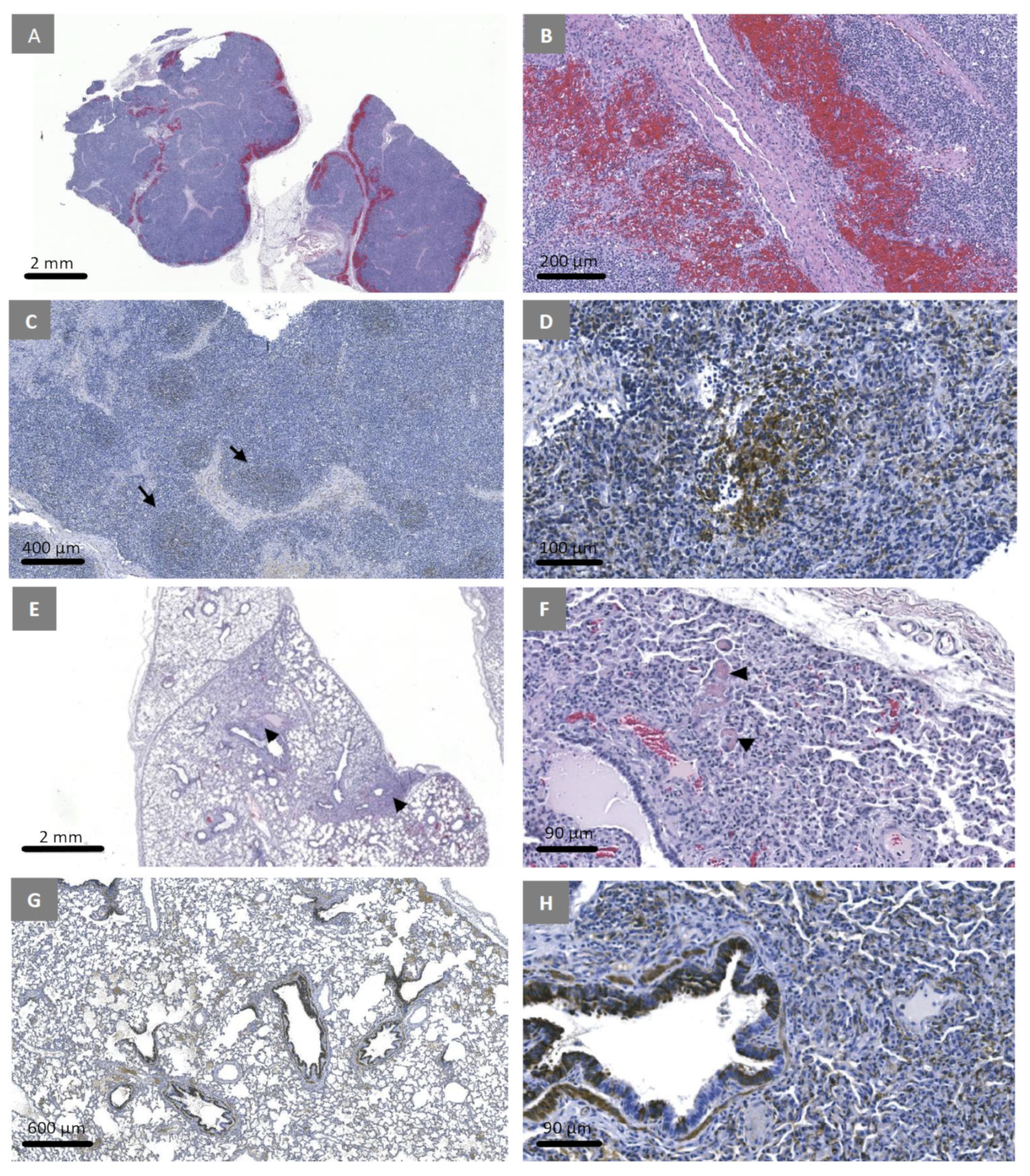
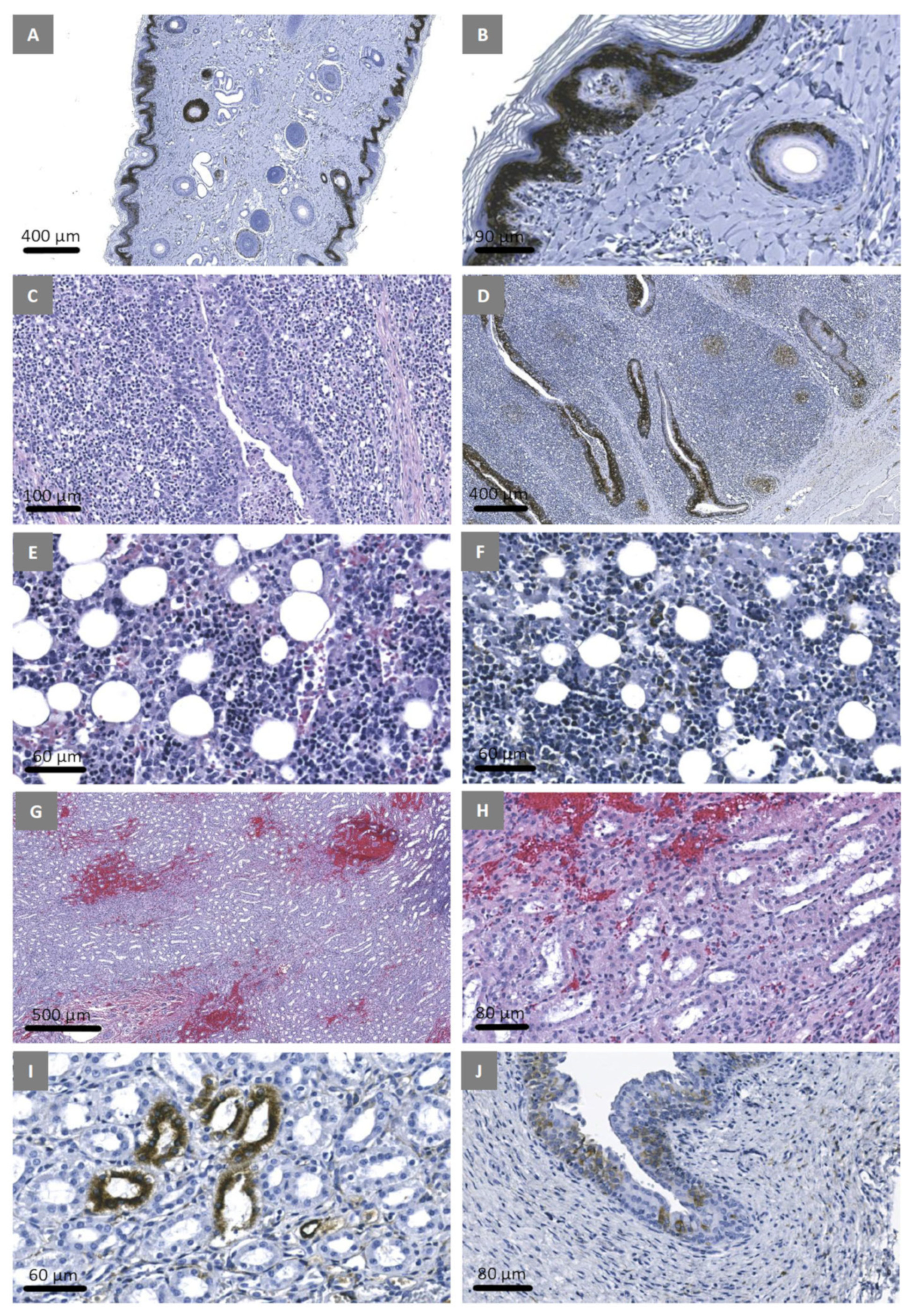
3.7. Experimental Inoculation—Histopathology and Immunohistochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical Swine Fever-An Updated Review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef]
- List of CSF Free Members According to Resolution No. 18 (90th General Session, May 2023). Available online: https://www.woah.org/app/uploads/2023/05/a-r18-2023-csf-1.pdf (accessed on 20 August 2023).
- Rios, L.; Núñez, J.I.; Díaz de Arce, H.; Ganges, L.; Pérez, L.J. Revisiting the genetic diversity of classical swine fever virus: A proposal for new genotyping and subgenotyping schemes of classification. Transbound. Emerg. Dis. 2018, 65, 963–971. [Google Scholar] [CrossRef]
- Ganges, L.; Crooke, H.R.; Bohórquez, J.A.; Postel, A.; Sakoda, Y.; Becher, P.; Ruggli, N. Classical swine fever virus: The past, present and future. Virus Res. 2020, 289, 198151. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V.; Floegel-Niesmann, G.; Greiser-Wilke, I. Clinical signs and epidemiology of classical swine fever: A review of new knowledge. Vet. J. 2003, 165, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Mittelholzer, C.; Moser, C.; Tratschin, J.D.; Hofmann, M.A. Analysis of classical swine fever virus replication kinetics allows differentiation of highly virulent from avirulent strains. Vet. Microbiol. 2000, 74, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Belák, K.; Koenen, F.; Vanderhallen, H.; Mittelholzer, C.; Feliziani, F.; De Mia, G.M.; Belák, S. Comparative studies on the pathogenicity and tissue distribution of three virulence variants of classical swine fever virus, two field isolates and one vaccine strain, with special regard to immunohistochemical investigations. Acta Vet. Scand. 2008, 50, 34. [Google Scholar] [CrossRef] [PubMed]
- Liess, B. Persistent infections of hog cholera: A review. Prev. Vet. Med. 1984, 2, 109–113. [Google Scholar] [CrossRef]
- Stewart, W.C.; Carbrey, E.A.; Kresse, J.I. Transplacental hog cholera infection in immune sows. Am. J. Vet. Res. 1972, 33, 791–798. [Google Scholar] [PubMed]
- Muñoz-González, S.; Ruggli, N.; Rosell, R.; Pérez, L.J.; Frías-Leuporeau, M.T.; Fraile, L.; Montoya, M.; Cordoba, L.; Domingo, M.; Ehrensperger, F.; et al. Postnatal persistent infection with classical Swine Fever virus and its immunological implications. PLoS ONE 2015, 10, e0125692. [Google Scholar] [CrossRef]
- Cabezón, O.; Colom-Cadena, A.; Muñoz-González, S.; Pérez-Simó, M.; Bohórquez, J.A.; Rosell, R.; Marco, I.; Domingo, M.; Lavín, S.; Ganges, L. Post-natal persistent infection with classical swine fever virus in wild boar: A strategy for viral maintenance? Transbound. Emerg. Dis. 2017, 64, 651–655. [Google Scholar] [CrossRef]
- Coronado, L.; Bohórquez, J.A.; Muñoz-González, S.; Perez, L.J.; Rosell, R.; Fonseca, O.; Delgado, L.; Perera, C.L.; Frías, M.T.; Ganges, L. Investigation of chronic and persistent classical swine fever infections under field conditions and their impact on vaccine efficacy. BMC Vet. Res. 2019, 15, 247. [Google Scholar] [CrossRef]
- Paton, D.J.; McGoldrick, A.; Greiser-Wilke, I.; Parchariyanon, S.; Song, J.Y.; Liou, P.P.; Stadejek, T.; Lowings, J.P.; Bjorklund, H.; Belak, S. Genetic typing of classical swine fever virus. Vet. Microbiol. 2000, 73, 137–157. [Google Scholar] [CrossRef]
- Björklund, H.; Lowings, P.; Stadejek, T.; Vilcek, S.; Greiser-Wilke, I.; Paton, D.; Belak, S. Phylogenetic comparison and molecular epidemiology of classical swine fever virus. Virus Genes 1999, 19, 189–195. [Google Scholar] [CrossRef]
- Cha, S.H.; Choi, E.J.; Park, J.H.; Yoon, S.R.; Kwon, J.H.; Yoon, K.J.; Song, J.Y. Phylogenetic characterization of classical swine fever viruses isolated in Korea between 1988 and 2003. Virus Res. 2007, 126, 256–261. [Google Scholar] [CrossRef]
- Simon, G.; Le Dimna, M.; Le Potier, M.F.; Pol, F. Molecular tracing of classical swine fever viruses isolated from wild boars and pigs in France from 2002 to 2011. Vet. Microbiol. 2013, 166, 631–638. [Google Scholar] [CrossRef]
- Leifer, I.; Hoffmann, B.; Höper, D.; Bruun Rasmussen, T.; Blome, S.; Strebelow, G.; Höreth-Böntgen, D.; Staubach, C.; Beer, M. Molecular epidemiology of current classical swine fever virus isolates of wild boar in germany. J. Gen. Virol. 2010, 91, 2687–2697. [Google Scholar] [CrossRef]
- Blome, S.; Grotha, I.; Moennig, V.; Greiser-Wilke, I. Classical swine fever virus in South-Eastern Europe—Retrospective analysis of the disease situation and molecular epidemiology. Vet. Microbiol. 2010, 146, 276–284. [Google Scholar] [CrossRef]
- Tomar, N.; Sharma, V.; John, J.K.; Sethi, M.; Ray, P.K.; Arya, R.S.; Das, T.; Saikumar, G. Complete Genome Sequence of a Field Isolate of Classical Swine Fever Virus Belonging to Subgenotype 2.2 from India. Genome Announc. 2018, 6, e00288-18. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Rajak, K.K.; Kumar, A.; Yadav, S.K. Classical swine fever in India: Current status and future perspective. Trop. Anim. Heal. Prod. 2018, 50, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Rajak, K.K.; Kumar, R.; Raut, S.D.; Saxena, A.; Muthuchelvan, D.; Singh, R.K.; Pandey, A.B. Changing pattern of classical swine fever virus genogroup from classical 1.1 to emerging 2.2 in India. VirusDisease 2017, 28, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bahoussi, A.N.; Wang, P.H.; Wu, C.; Xing, L. Complete genome sequences of classical swine fever virus: Phylogenetic and evolutionary analyses. Front. Microbiol. 2022, 13, 1021734. [Google Scholar] [CrossRef]
- Zhou, B. Classical Swine Fever in China-An Update Minireview. Front. Vet. Sci. 2019, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Pereda, A.J.; Greiser-Wilke, I.; Schmitt, B.; Rincon, M.A.; Mogollon, J.D.; Sabogal, Z.Y.; Lora, A.M.; Sanguinetti, H.; Piccone, M.E. Phylogenetic analysis of classical swine fever virus (CSFV) field isolates from outbreaks in South and Central America. Virus Res. 2005, 110, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Coronado, L.; Liniger, M.; Muñoz-González, S.; Postel, A.; Perez, L.J.; Perez-Simo, M.; Perera, C.L.; Frias-Lepoureau, M.T.; Rosell, R.; Grundhoff, A.; et al. Novel poly-uridine insertion in the 3’ UTR and E2 amino acid substitutions in a low virulent classical swine fever virus. Vet. Microbiol. 2017, 201, 103–112. [Google Scholar] [CrossRef]
- Júnior, A.A.F.; Laguardia-Nascimento, M.; Barbosa, A.A.S.; da Silva Gonçalves, V.L.; Freitas, T.R.P.; Júnior, A.V.R.; Camargos, M.F. Phylodynamics of classical swine fever virus in Brazil. Braz. J. Microbiol. 2022, 53, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Araínga, M.; Hisanaga, T.; Hills, K.; Handel, K.; Rivera, H.; Pasick, J. Phylogenetic analysis of classical swine fever virus isolates from Peru. Transbound. Emerg. Dis. 2010, 57, 262–270. [Google Scholar] [CrossRef]
- Postel, A.; Schmeiser, S.; Perera, C.L.; Rodríguez, L.J.; Frias-Lepoureau, M.T.; Becher, P. Classical swine fever virus isolates from Cuba form a new subgenotype 1.4. Vet. Microbiol. 2013, 161, 334–338. [Google Scholar] [CrossRef]
- Sabogal, Z.Y.; Mogollón, J.D.; Rincón, M.A.; Clavijo, A. Phylogenetic analysis of recent isolates of classical swine fever virus from Colombia. Virus Res. 2006, 115, 99–103. [Google Scholar] [CrossRef]
- Garrido Haro, A.D.; Barrera Valle, M.; Acosta, A.; JFlores, F. Phylodynamics of classical swine fever virus with emphasis on Ecuadorian strains. Transbound. Emerg. Dis. 2018, 65, 782–790. [Google Scholar] [CrossRef]
- Pineda, P.; Deluque, A.; Peña, M.; Diaz, O.L.; Allepuz, A.; Casal, J. Descriptive epidemiology of classical swine fever outbreaks in the period 2013–2018 in Colombia. PLoS ONE 2020, 15, e0234490. [Google Scholar] [CrossRef]
- Hoffmann, B.; Beer, M.; Schelp, C.; Schirrmeier, H.; Depner, K. Validation of a real-time RT-PCR assay for sensitive and specific detection of classical swine fever. Virol. Methods 2005, 130, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Peterk87/Nf-Ionampliseq [Internet]. CFIA NCFAD—Genomics Unit. 2021. Available online: https://github.com/CFIA-NCFAD/nf-ionampliseq (accessed on 10 May 2023).
- Tommaso, P.D.; Chatzou, M.; Floden, E.W.; Barja, P.P.; Palumbo, E.; Notredame, C. Nextflow enables reproducible computational workflows. Nat. Biotechnol. 2017, 35, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Ondov, B.D.; Starrett, G.J.; Sappington, A.; Kostic, A.; Koren, S.; Buck, C.B.; Phillippy, A.M. Mash Screen: High-throughput sequence containment estimation for genome discovery. Genome Biol. 2019, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Iontorrent/TS [Internet]. Ion Torrent. 2023. Available online: https://github.com/iontorrent/TS (accessed on 10 May 2023).
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics. 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Martin, D.P.; Varsani, A.; Roumagnac, P.; Botha, G.; Maslamoney, S.; Schwab, T.; Kelz, Z.; Kumar, V.; Murrell, B. RDP5: A computer program for analyzing recombination in, and removing signals of recombination from, nucleotide sequence datasets. Virus Evol. 2020, 7, veaa087. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Sagulenko, P.; Puller, V.; Neher, R.A. TreeTime: Maximum-likelihood phylodynamic analysis. Virus Evol. 2018, 4, vex042. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T. GGTREE:anRpackage for visualization and annotation ofphylogenetic trees with their covariates and otherassociated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- Paton, D.J.; Sands, J.J.; Lowings, J.P.; Smith, J.E.; Ibata, G.; Edwards, S. A proposed division of the pestivirus genus using monoclonal antibodies, supported by cross-neutralisation assays and genetic sequencing. Vet. Res. 1995, 26, 92–109. [Google Scholar] [PubMed]
- Edwards, S.; Sands, J.J. Antigenic comparisons of hog cholera virus isolates from Europe, America and Asia using monoclonal antibodies. DTW Dtsch. Tieraerztl. Wochenschr. 1990, 97, 79–81. [Google Scholar]
- Lin, M.; Lin, F.; Mallory, M.; Clavijo, A. Deletions of structural glycoprotein E2 of classical swine fever virus strain alfort/187 resolve a linear epitope of monoclonal antibody WH303 and the minimal N-terminal domain essential for binding immunoglobulin G antibodies of a pig hyperimmune serum. J. Virol. 2000, 74, 11619–11625. [Google Scholar] [CrossRef]
- Instituto Colombiano Agropecuario. In Censo Pecu. Nac. 2017. Available online: https://www.ica.gov.co/areas/pecuaria/servicios/epidemiologia-veterinaria/censos-2016/censo-2018.aspx (accessed on 14 January 2020).
- Instituto Colombiano Agropecuario (ICA). Programa de Erradicación Peste Porcina Clásica. 2018. Available online: https://www.ica.gov.co/getdoc/ea9c6aa0-a5fc-472f-869b-975b27d7ac35/Peste-Porcina-Clasica-(1).aspx (accessed on 14 January 2020).
- CSF Free Zones in Colombia. Available online: https://www.woah.org/app/uploads/2023/05/csf-colombia-eng.png (accessed on 5 July 2023).
- Pineda, P.; Santa, C.; Deluque, A.; Peña, M.; Casal, J. Evaluation of the sensitivity of the classical swine fever surveillance system in two free zones in Colombia. Transbound. Emerg. Dis. 2022, 69, 1294–1306. [Google Scholar] [CrossRef]
- Choe, S.; Le, V.P.; Shin, J.; Kim, J.H.; Kim, K.S.; Song, S.; Cha, R.M.; Park, G.N.; Nguyen, T.L.; Hyun, B.H.; et al. Pathogenicity and Genetic Characterization of Vietnamese Classical Swine Fever Virus: 2014–2018. Pathogens 2020, 9, 169. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Nguyen, B.T.P.; Do, D.T.; Nguyen, T.Q.; Nguyen, D.T.M.; Nguyen, M.N. Genetic diversity and molecular characterization of classical swine fever virus envelope protein genes E2 and Erns circulating in Vietnam from 2017 to 2019. Infect. Genet. Evol. 2021, 96, 105140. [Google Scholar] [CrossRef]
- Pauly, T.; König, M.; Thiel, H.J.; Saalmüller, A. Infection with classical swine fever virus: Effects on phenotype and immune responsiveness of porcine T lymphocytes. J. Gen. Virol. 1998, 79, 31–40. [Google Scholar] [CrossRef]
- Bautista, M.J.; Ruiz-Villamor, E.; Salguero, F.J.; Sanchez-Cordon, P.J.; Carrasco, L.; Gomez-Villamandos, J.C. Early platelet aggregation as a cause of thrombocytopenia in classical swine fever. Vet. Pathol. 2002, 39, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; Knoetig, S.M.; Tschudin, R.; McCullough, K.C. Pathogenesis of granulocytopenia and bone marrow atrophy during classical swine fever involves apoptosis and necrosis of unin-fected cells. Virology 2000, 272, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Susa, M.; König, M.; Saalmüller, A.; Reddehase, M.J.; Thiel, H.J. Pathogenesis of classical swine fever: B-lymphocyte deficiency caused by hog cholera virus. J. Virol. 1992, 66, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, G. Classical swine fever and related infections. In Pathology and Pathogenesis of the Disease; Martinus Nijhoff: Boston, MA, USA, 1988; pp. 27–54. [Google Scholar]
- Weiss, E.; Teredesai, A.; Hoffmann, R.; Hoffmann-Fezer, G. Volume distribution and ultrastructure of platelets in acute hog cholera. Thromb. Diath. Haemorrh. 1973, 30, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Izzati, U.Z.; Hoa, N.T.; Lan, N.T.; Diep, N.V.; Fuke, N.; Hirai, T.; Yamaguchi, R. Pathology of the outbreak of subgenotype 2.5 classical swine fever virus in northern Vietnam. Vet. Med. Sci. 2021, 7, 164–174. [Google Scholar] [CrossRef]
- Gong, W.; Li, J.; Wang, Z.; Sun, J.; Mi, S.; Lu, Z.; Cao, J.; Dou, Z.; Sun, Y.; Wang, P.; et al. Virulence evaluation of classical swine fever virus subgenotype 2.1 and 2.2 isolates circulating in China. Vet. Microbiol. 2019, 232, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Nishi, T.; Kameyama, K.I.; Meyer, D.; Suckstorff, O.; Fukai, K.; Becher, P. Reemergence of Classical Swine Fever, Japan. Emerg. Infect. Dis. 2019, 25, 1228–1231. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, M.; Wu, X.; Ma, W.; Zhao, X. Phylogenetic analysis of classical swine fever virus isolates from China. Arch. Virol. 2021, 166, 2255–2261. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Nguyen, P.B.T.; Nguyen, T.Q.; Do, D.T.; Nguyen, M.D.T.; Nguyen, M.N. Genotypic diversity of CSFV field strains: A silent risk reduces vaccination efficacy of CSFV vaccines in Vietnam. Virology 2022, 571, 39–45. [Google Scholar] [CrossRef]



| Sample | Sample # | Type | Department | Municipality | Type of Operation | Date of Collection | RRT-PCR | GenBank # |
|---|---|---|---|---|---|---|---|---|
| 1 | 2013-409S | Serum | La-Guajira | Urumita | Backyard | 1 June 2013 | 0.00 | |
| 2 | 2013-409T | Tonsil | La-Guajira | Urumita | Backyard | 1 June 2013 | 33.36 | |
| 4 | 2013-1070 | Serum | Cesar | El-Paso | Backyard | 3 September 2013 | 35.65 | |
| 3 | 2013-922 | Lymph Node | Cesar | Chiriguana | Backyard | 29 September 2013 | 0.00 | |
| 5 | 2013-1185 | Serum | Cesar | Chiriguana | Backyard | 2 November 2013 | 18.75 | OL963693 |
| 6 | 2014-58 | Serum | Cesar | El Paso | Backyard | 24 January 2014 | 26.15 | OL963694 |
| 7 | 2014-257 | Spleen | Magdalena | Santa-Ana | Backyard | 7 March 2014 | 25.05 | |
| 8 | 2014-519 | Tonsil | Cesar | Chiriguana | Backyard | 11 April 2014 | 24.69 | |
| 9 | 2014-598 | Kidney | Boliver | Talaigua-Nuevo | Backyard | 12 May 2014 | 26.85 | |
| 10 | 2014-947 | Serum | Norte-Santander | Ocana | Backyard | 9 August 2014 | 0.00 | |
| 11 | 2014-1343 | Serum | Magdalena | San-Zenzon | Backyard | 11 October 2014 | 26.49 | |
| 12 | 2015-113 | Serum | Magdalena | El-Banco | Backyard | 1 February 2015 | 0.00 | |
| 13 | 2015-246 | Tonsil | La Guajira | Riohacha | Backyard | 1 March 2015 | 36.90 | |
| 14 | 2015-389 | Serum | Sucre | San-Benito-Abad | Backyard | 5 March 2015 | 28.12 | |
| 15 | 2015-302 | Serum | Cesar | La-Jagua-De-Ibirico | Backyard | 10 March 2015 | 0.00 | |
| 16 | 2015-338 | Tonsil | Magdalena | Santa-Marta | Backyard | 21 March 2015 | 34.09 | |
| 17 | 2015-915 | Serum | Bolivar | Barranco -De-Loba | Backyard | 10 July 2015 | 0.00 | |
| 18 | 2016-11 | Tonsil | Bolivar | Morales | Backyard | 2 January 2016 | 22.59 | OL963695 |
| 19 | 2016-666 | Serum | Cordoba | Lorica | Backyard | 8 June 2016 | 25.26 | |
| 20 * | 2016-686 | Serum | Bolivar | Pinnillos | Backyard | 4 July 2016 | 22.36 | OL963696 |
| 21 | 2016-860 | Serum | Magdalena | Santa Ana | Backyard | 24 July 2016 | 23.52 | OL963697 |
| 22 | 2017-356 | Lymph Node | Atlantico | Polonuevo | Commercial | 28 April 2017 | 23.74 | OL963699 |
| 23 | 2017-848 | Lymph Node | Sucre | Galeras | Backyard | 15 September 2017 | 22.52 | OL963698 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robert, E.; Goonewardene, K.; Lamboo, L.; Perez, O.; Goolia, M.; Lewis, C.; Erdelyan, C.N.G.; Lung, O.; Handel, K.; Moffat, E.; et al. Molecular and Pathological Characterization of Classical Swine Fever Virus Genotype 2 Strains Responsible for the 2013–2018 Outbreak in Colombia. Viruses 2023, 15, 2308. https://doi.org/10.3390/v15122308
Robert E, Goonewardene K, Lamboo L, Perez O, Goolia M, Lewis C, Erdelyan CNG, Lung O, Handel K, Moffat E, et al. Molecular and Pathological Characterization of Classical Swine Fever Virus Genotype 2 Strains Responsible for the 2013–2018 Outbreak in Colombia. Viruses. 2023; 15(12):2308. https://doi.org/10.3390/v15122308
Chicago/Turabian StyleRobert, Erin, Kalhari Goonewardene, Lindsey Lamboo, Orlando Perez, Melissa Goolia, Charles Lewis, Cassidy N. G. Erdelyan, Oliver Lung, Katherine Handel, Estella Moffat, and et al. 2023. "Molecular and Pathological Characterization of Classical Swine Fever Virus Genotype 2 Strains Responsible for the 2013–2018 Outbreak in Colombia" Viruses 15, no. 12: 2308. https://doi.org/10.3390/v15122308






