Sida Golden Mosaic Virus, an Emerging Pathogen of Snap Bean (Phaseolus vulgaris L.) in the Southeastern United States
Abstract
:1. Introduction
2. Materials and Methods
2.1. SiGMV Idenfication and Inoculum Source
2.1.1. SiGMV Identification in Georgia
2.1.2. SiGMV Identification in Florida
2.1.3. SiGMV Inoculum Source for Greenhouse Experiments
2.2. Plants for Host Range Evaluation
2.3. Insects
2.4. SiGMV Symptoms and Accumulation in Inoculated Host Plants
2.5. Virus Accumulation in Whiteflies Feeding on SiGMV-Infected Plants
2.6. Back Transmission of SiGMV from Different Host Plants to Snap Bean
2.7. Whitefly Survival, Development, and Fecundity on SiGMV-Infected and/or Non-Infected Host Plants
2.8. Statistical Analyses
2.9. Nucleotide Similarity and Phylogenetic Analysis
2.9.1. SiGMV DNA A Sequencing for Phylogenetic Analyses
2.9.2. Phylogenetic Analysis
3. Results
3.1. SiGMV Symptoms and Accumulation in Inoculated Host Plants
3.2. SiGMV Detection and Accumulation in Whiteflies
3.3. Back Transmission of SiGMV from Different Host Plants to Snap Bean Plants
3.4. Whitefly Survival, Development, and Fecundity on Infected and/or Non-Infected Host Plant Species
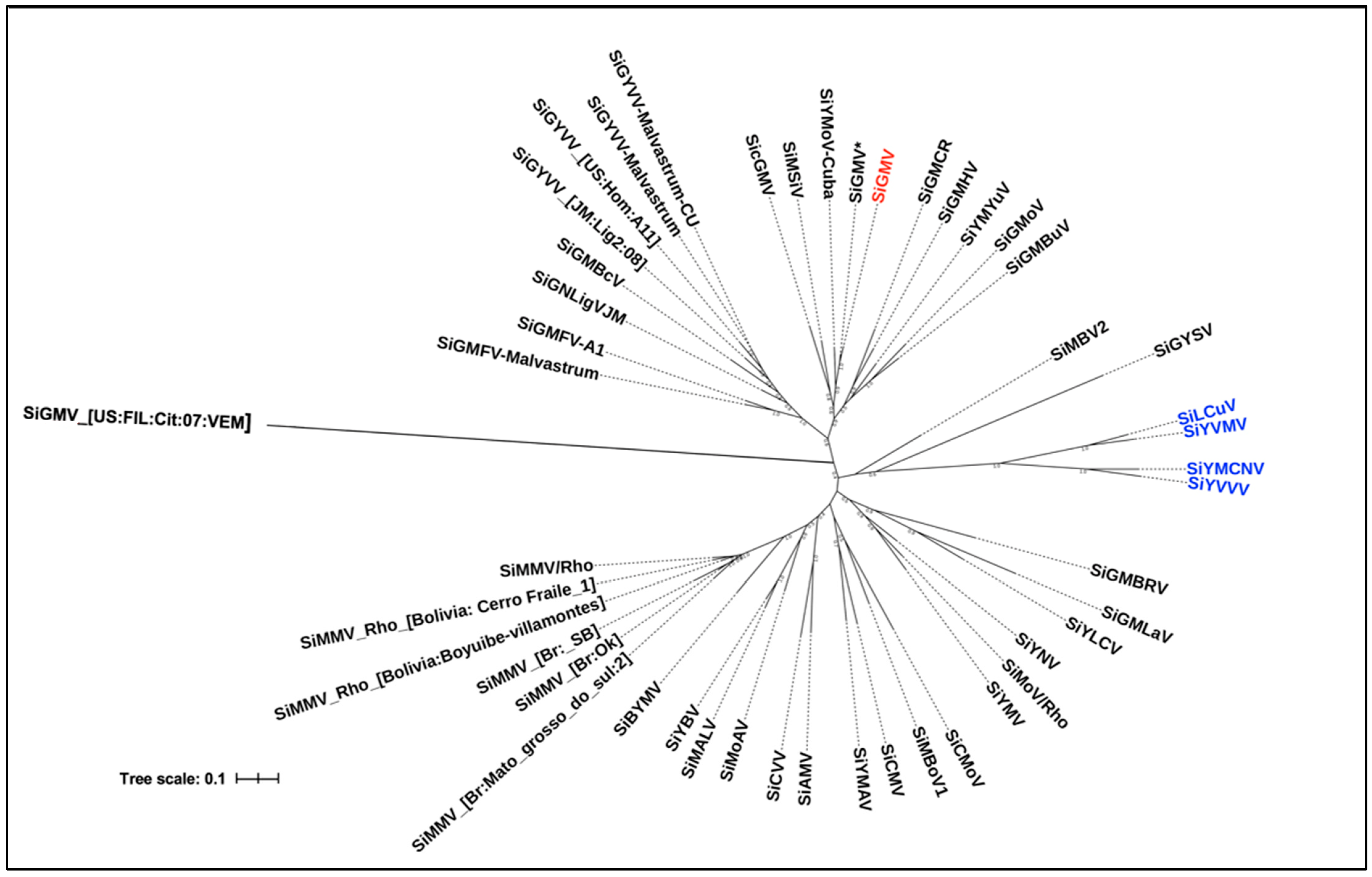
3.5. Nucleotide Similarity and Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elena, S.F.; Fraile, A.; García-Arenal, F. Evolution and Emergence of Plant Viruses. Adv. Virus Res. 2014, 88, 161–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.A.C. Plant Virus Emergence and Evolution: Origins, New Encounter Scenarios, Factors Driving Emergence, Effects of Changing World Conditions, and Prospects for Control. Virus Res. 2009, 141, 113–130. [Google Scholar] [CrossRef]
- Whitfield, A.E.; Falk, B.W.; Rotenberg, D. Insect Vector-Mediated Transmission of Plant Viruses. Virology 2015, 479–480, 278–289. [Google Scholar] [CrossRef] [Green Version]
- McLeish, M.J.; Fraile, A.; García-Arenal, F. Evolution of Plant–Virus Interactions: Host Range and Virus Emergence. Curr. Opin. Virol. 2019, 34, 50–55. [Google Scholar] [CrossRef]
- Adkins, S.; Webster, C.G.; Kousik, C.S.; Webb, S.E.; Roberts, P.D.; Stansly, P.A.; Turechek, W.W. Ecology and Management of Whitefly-Transmitted Viruses of Vegetable Crops in Florida. Virus Res. 2011, 159, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Navas-Castillo, J.; Fiallo-Olivé, E.; Sánchez-Campos, S. Emerging Virus Diseases Transmitted by Whiteflies. Annu Rev. Phytopathol. 2011, 49, 219–248. [Google Scholar] [CrossRef]
- Brown, J.K. Current Status of Bemisia tabaci as a Plant Pest and a Virus Vector in Agro-Ecosystems Worldwide. Plant Prot. Bull. 1994, 42, 3–32. [Google Scholar]
- Brown, J.K. The Molecular Epidemiology of Begomoviruses. In Trends in Plant Virolgy; Khan, J.A., Dykstra, J., Eds.; Haworth Press: Binghamton, NY, USA, 2001; pp. 279–316. [Google Scholar]
- Rojas, M.R.; Hagen, C.; Lucas, W.J.; Gilbertson, R.L. Exploiting Chinks in the Plant’s Armor: Evolution and Emergence of Geminiviruses. Annu. Rev. Phytopathol. 2005, 43, 361–394. [Google Scholar] [CrossRef]
- Briddon, R.W.; Patil, B.L.; Bagewadi, B.; Nawaz-ul-Rehman, M.S.; Fauquet, C.M. Distinct Evolutionary Histories of the DNA-A and DNA-B Components of Bipartite Begomoviruses. BMC Evol. Biol. 2010, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Rojas, M.R.; Macedo, M.A.; Maliano, M.R.; Soto-Aguilar, M.; Souza, J.O.; Briddon, R.W.; Kenyon, L.; Rivera Bustamante, R.F.; Zerbini, F.M.; Adkins, S.; et al. World Management of Geminiviruses. Annu. Rev. Phytopathol. 2018, 56, 637–677. [Google Scholar] [CrossRef]
- Seal, S.E.; VandenBosch, F.; Jeger, M.J. Factors Influencing Begomovirus Evolution and Their Increasing Global Significance: Implications for Sustainable Control. CRC Crit. Rev. Plant Sci. 2006, 25, 23–46. [Google Scholar] [CrossRef]
- Brown, J.K. Whitefly-Transmitted Geminiviruses and Associated Disorders in the Americas and the Caribbean Basin. Plant Dis. 1992, 76, 220. [Google Scholar] [CrossRef]
- Varma, A.; Malathi, V.G. Emerging Geminivirus Problems: A Serious Threat to Crop Production. Ann. Appl. Biol. 2003, 142, 145–164. [Google Scholar] [CrossRef]
- Pan, H.; Chu, D.; Yan, W.; Su, Q.; Liu, B.; Wang, S.; Wu, Q.; Xie, W.; Jiao, X.; Li, R.; et al. Rapid Spread of tomato yellow leaf curl virus in China Is Aided Differentially by Two Invasive Whiteflies. PLoS ONE 2012, 7, e34817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boykin, L.M.; Shatters, R.G., Jr.; Rosell, R.C.; McKenzie, C.L.; Bagnall, R.A.; de Barro, P.; Frohlich, D.R. Global Relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) Revealed Using Bayesian Analysis of Mitochondrial COI DNA Sequences. Mol. Phylogenet Evol. 2007, 44, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Mugerwa, H.; Seal, S.; Wang, H.-L.; Patel, M.V.; Kabaalu, R.; Omongo, C.A.; Alicai, T.; Tairo, F.; Ndunguru, J.; Sseruwagi, P.; et al. African Ancestry of New World, Bemisia tabaci-Whitefly Species. Sci. Rep. 2018, 8, 2734. [Google Scholar] [CrossRef] [Green Version]
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; de Barro, P. Refined Global Analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) Mitochondrial Cytochrome Oxidase 1 to Identify Species Level Genetic Boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- Czosnek, H.; Hariton-Shalev, A.; Sobol, I.; Gorovits, R.; Ghanim, M. The Incredible Journey of Begomoviruses in Their Whitefly Vector. Viruses 2017, 9, 273. [Google Scholar] [CrossRef] [Green Version]
- Gadhave, K.R.; Gautam, S.; Dutta, B.; Coolong, T.; Adkins, S.; Srinivasan, R. Low Frequency of Horizontal and Vertical Transmission of cucurbit leaf crumple virus in Whitefly Bemisia tabaci Gennadius. Phytopathology 2020, 110, 1235–1241. [Google Scholar] [CrossRef]
- Guo, Q.; Shu, Y.-N.; Liu, C.; Chi, Y.; Liu, Y.-Q.; Wang, X.-W. Transovarial Transmission of tomato yellow leaf curl virus by Seven Species of the Bemisia tabaci Complex Indigenous to China: Not All Whiteflies Are the Same. Virology 2019, 531, 240–247. [Google Scholar] [CrossRef]
- Stansly, P.A.; Naranjo, S.E.; Accotto, G.P.; Sardo, L. Transovarial Transmission of Begomoviruses in Bemisia tabaci. In Bemisia: Bionomics and Management of a Global Pest; Stansly, P.A., Naranjo, S.E., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 339–348. [Google Scholar]
- Wei, J.; He, Y.-Z.; Guo, Q.; Guo, T.; Liu, Y.-Q.; Zhou, X.-P.; Liu, S.-S.; Wang, X.-W. Vector Development and Vitellogenin Determine the Transovarial Transmission of Begomoviruses. Proc. Nat. Acad. Sci. USA 2017, 114, 201701720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, F.J. History and Current Distribution of Begomoviruses in Latin America. Adv. Virus Res. 2006, 67, 127–162. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Crossley, M.S.; Dutta, B.; Coolong, T.; Simmons, A.M.; da Silva, A.; Snyder, W.E.; Srinivasan, R. Low Genetic Variability in Bemisia tabaci MEAM1 Populations within Farmscapes of Georgia, USA. Insects 2020, 11, 834. [Google Scholar] [CrossRef] [PubMed]
- Hamon, A.; Salguero, V. Bemisia tabaci, Sweetpotato Whitefly, in Florida (Homoptera: Aleyrodidae: Aleyrodinae); Florida Department of Agriculture and Consumer Services, Division of Plant Industry: Gainesville, FL, USA, 1987. [Google Scholar]
- Hoelmer, K.A.; Osborne, L.S.; Yokomi, R.K. Foliage Disorders in Florida Associated with Feeding by Sweetpotato Whitefly, Bemisia tabaci. Fla Entomol. 1991, 74, 162. [Google Scholar] [CrossRef]
- Schuster, D.J.; Price, J.F.; King, J.B.; Everett, P.H. Integrated Management of the Sweetpotato Whitefly on Commercial Tomato; University of Florida, Institute of Food and Agricultural Science, Bradenton Gulf Coast Research and Education Center Research Report; BRA: Gainesville, FL, USA, 1989. [Google Scholar]
- Agarwal, G.; Kavalappara, S.R.; Gautam, S.; Silva, A.D.; Simmons, A.; Srinivasan, R.; Dutta, B. Field Screen and Genotyping of Phaseolus vulgaris against Two Begomoviruses in Georgia, USA. Insects 2021, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S. The Role of Bemisia tabaci in the Transmission of Vegetable Viruses in the Farmscape of Georgia. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2019. [Google Scholar]
- Durham, T.C.; Baker, C.; Jones, L.; Snyder, L.U. First Report of sida golden mosaic virus Infecting Snap Bean ( Phaseolus vulgaris ) in Florida. Plant Dis. 2010, 94, 487. [Google Scholar] [CrossRef] [PubMed]
- Karen, S. Georgia Farm Gate Value Report 2019; Center for Agribusiness & Economic Development, University of Georgia: Athens, GA, USA, 2019. [Google Scholar]
- Gautam, S.; Gadhave, K.R.; Buck, J.W.; Dutta, B.; Coolong, T.; Adkins, S.; Srinivasan, R. Virus-Virus Interactions in a Plant Host and in a Hemipteran Vector: Implications for Vector Fitness and Virus Epidemics. Virus Res. 2020, 286, 198069. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.R. Use of Degenerate Primers in the Polymerase Chain Reaction to Detect Whitefly-Transmitted Geminiviruses. Plant Dis. 1993, 77, 340. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, S. Detection of Subgroup III Geminivirus Isolates in Leaf Extracts by Degenerate Primers and Polymerase Chain Reaction. Phytopathology 1996, 86, 1288. [Google Scholar] [CrossRef]
- Muñiz, M.; Nombela, G. Bemisia tabaci: A New Clip-Cage for Biological Studies; European Whiteflies Studies Network: Norwich, UK, 2001; Volume 5. [Google Scholar]
- Srinivasan, R.; Riley, D.; Diffie, S.; Sparks, A.; Adkins, S. Whitefly Population Dynamics and Evaluation of Whitefly-Transmitted tomato yellow leaf curl virus (TYLCV)-Resistant Tomato Genotypes as Whitefly and TYLCV Reservoirs. J. Econ. Entomol. 2012, 105, 1447–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legarrea, S.; Barman, A.; Marchant, W.; Diffie, S.; Srinivasan, R. Temporal Effects of a Begomovirus Infection and Host Plant Resistance on the Preference and Development of an Insect Vector, Bemisia tabaci, and Implications for Epidemics. PLoS ONE 2015, 10, e0142114. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage Publications: Thousand Oaks CA, USA, 2011. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V. Least-Squares Means: The R Package Lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Bodenhofer, U.; Bonatesta, E.; Horejš-Kainrath, C.; Hochreiter, S. Msa: An R Package for Multiple Sequence Alignment. Bioinformatics 2015, 31, 3997–3999. [Google Scholar] [CrossRef] [PubMed]
- Schliep, K.P. Phangorn: Phylogenetic Analysis in R. Bioinformatics 2011, 27, 592–593. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree Of Life (ITOL) v4: Recent Updates and New Developments. Nucl. Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, W.; Shi, X.; Liu, B.; Pan, H.; Wei, W.; Zeng, Y.; Sun, X.; Xie, W.; Wang, S.; Wu, Q.; et al. Transmission of tomato yellow leaf curl virus by Bemisia tabaci as Affected by Whitefly Sex and Biotype. Sci. Rep. 2015, 5, 10744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapidot, M.; Friedmann, M.; Pilowsky, M.; Ben-Joseph, R.; Cohen, S. Effect of Host Plant Resistance to tomato yellow leaf curl virus (TYLCV) on Virus Acquisition and Transmission by Its Whitefly Vector. Phytopathology 2001, 91, 1209–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legarrea, S.; Barman, A.; Diffie, S.; Srinivasan, R. Virus Accumulation and Whitefly Performance Modulate the Role of Alternate Host Species as Inoculum Sources of tomato yellow leaf curl virus. Plant Dis. 2020, 104, 2958–2966. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Liu, S.-S. Tomato yellow leaf curl virus Infection of Tomato Does Not Affect the Performance of the Q and ZHJ2 Biotypes of the Viral Vector Bemisia tabaci. Insect Sci. 2011, 18, 40–49. [Google Scholar] [CrossRef]
- Shi, X.; Pan, H.; Xie, W.; Wu, Q.; Wang, S.; Liu, Y.; Fang, Y.; Chen, G.; Gao, X.; Zhang, Y. Plant Virus Differentially Alters the Plant’s Defense Response to Its Closely Related Vectors. PLoS ONE 2013, 8, e83520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, S.R. Sida, in Jepson Flora Project; University of California: Berkeley, CA, USA, 2012; Available online: https://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=44401 (accessed on 25 January 2023)Jepson eFlora.
- Webster, T.M.; MACDONALD, G.E. A Survey of Weeds in Various Crops in Georgia 1. Weed Technol. 2001, 15, 771–790. [Google Scholar] [CrossRef]
- Sanz, A.I.; Fraile, A.; García-Arenal, F.; Zhou, X.; Robinson, D.J.; Khalid, S.; Butt, T.; Harrison, B.D. Multiple Infection, Recombination and Genome Relationships among Begomovirus Isolates Found in Cotton and Other Plants in Pakistan. Microbiology 2000, 81, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- García-Andrés, S.; Monci, F.; Navas-Castillo, J.; Moriones, E. Begomovirus Genetic Diversity in the Native Plant Reservoir Solanum nigrum: Evidence for the Presence of a New Virus Species of Recombinant Nature. Virology 2006, 350, 433–442. [Google Scholar] [CrossRef] [Green Version]
- labi, O.J.; Ogbe, F.O.; Bandyopadhyay, R.; Lava Kumar, P.; Dixon, A.G.O.; d’A. Hughes, J.; Naidu, R.A. Alternate Hosts of African cassava mosaic virus and East African cassava mosaic Cameroon virus in Nigeria. Arch. Virol. 2008, 153, 1743–1747. [Google Scholar] [CrossRef]
- Power, A.G. Insect Transmission of Plant Viruses: A Constraint on Virus Variability. Curr. Opin. Plant Biol. 2000, 3, 336–340. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Martínez-Zubiaur, Y.; Moriones, E.; Navas-Castillo, J. Complete Nucleotide Sequence of sida golden mosaic Florida virus and Phylogenetic Relationships with Other Begomoviruses Infecting Malvaceous Weeds in the Caribbean. Arch. Virol. 2010, 155, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.; Zerbini, F.M.; Navas-Castillo, J.; Moriones, E.; Ramos-Sobrinho, R.; Silva, J.C.F.; Fiallo-Olivé, E.; Briddon, R.W.; Hernández-Zepeda, C.; Idris, A.; et al. Revision of Begomovirus Taxonomy Based on Pairwise Sequence Comparisons. Arch. Virol. 2015, 160, 1593–1619. [Google Scholar] [CrossRef]
- Hily, J.M.; García, A.; Moreno, A.; Plaza, M.; Wilkinson, M.D.; Fereres, A.; Fraile, A.; García-Arenal, F. The Relationship between Host Lifespan and Pathogen Reservoir Potential: An Analysis in the System Arabidopsis thaliana-cucumber mosaic virus. PLoS Pathog. 2014, 10, e1004492. [Google Scholar] [CrossRef]
- DaPalma, T.; Doonan, B.P.; Trager, N.M.; Kasman, L.M. A Systematic Approach to Virus–Virus Interactions. Virus Res. 2010, 149, 1–9. [Google Scholar] [CrossRef]
- Mascia, T.; Gallitelli, D. Synergies and Antagonisms in Virus Interactions. Plant Sci. 2016, 252, 176–192. [Google Scholar] [CrossRef]
- Roossinck, M.J.; García-Arenal, F. Ecosystem Simplification, Biodiversity Loss and Plant Virus Emergence. Curr. Opin. Virol. 2015, 10, 56–62. [Google Scholar] [CrossRef]
- Syller, J. Facilitative and Antagonistic Interactions between Plant Viruses in Mixed Infections. Mol. Plant Pathol. 2012, 13, 204–216. [Google Scholar] [CrossRef]
- Gil-Salas, F.M.; Peters, J.; Boonham, N.; Cuadrado, I.M.; Janssen, D. Co-Infection with cucumber vein yellowing virus and cucurbit yellow stunting disorder virus Leading to Synergism in Cucumber. Plant Pathol. 2012, 61, 468–478. [Google Scholar] [CrossRef]
- Rentería-Canett, I.; Xoconostle-Cázares, B.; Ruiz-Medrano, R.; Rivera-Bustamante, R.F. Geminivirus Mixed Infection on Pepper Plants: Synergistic Interaction between PHYVV and PepGMV. Virol. J. 2011, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, R.M.A. Effect of Mixed Viral Infections (potato virus Y-potato leafroll virus) on Biology and Preference of Vectors Myzus persicae and Macrosiphum euphorbiae (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 646–655. [Google Scholar] [CrossRef]

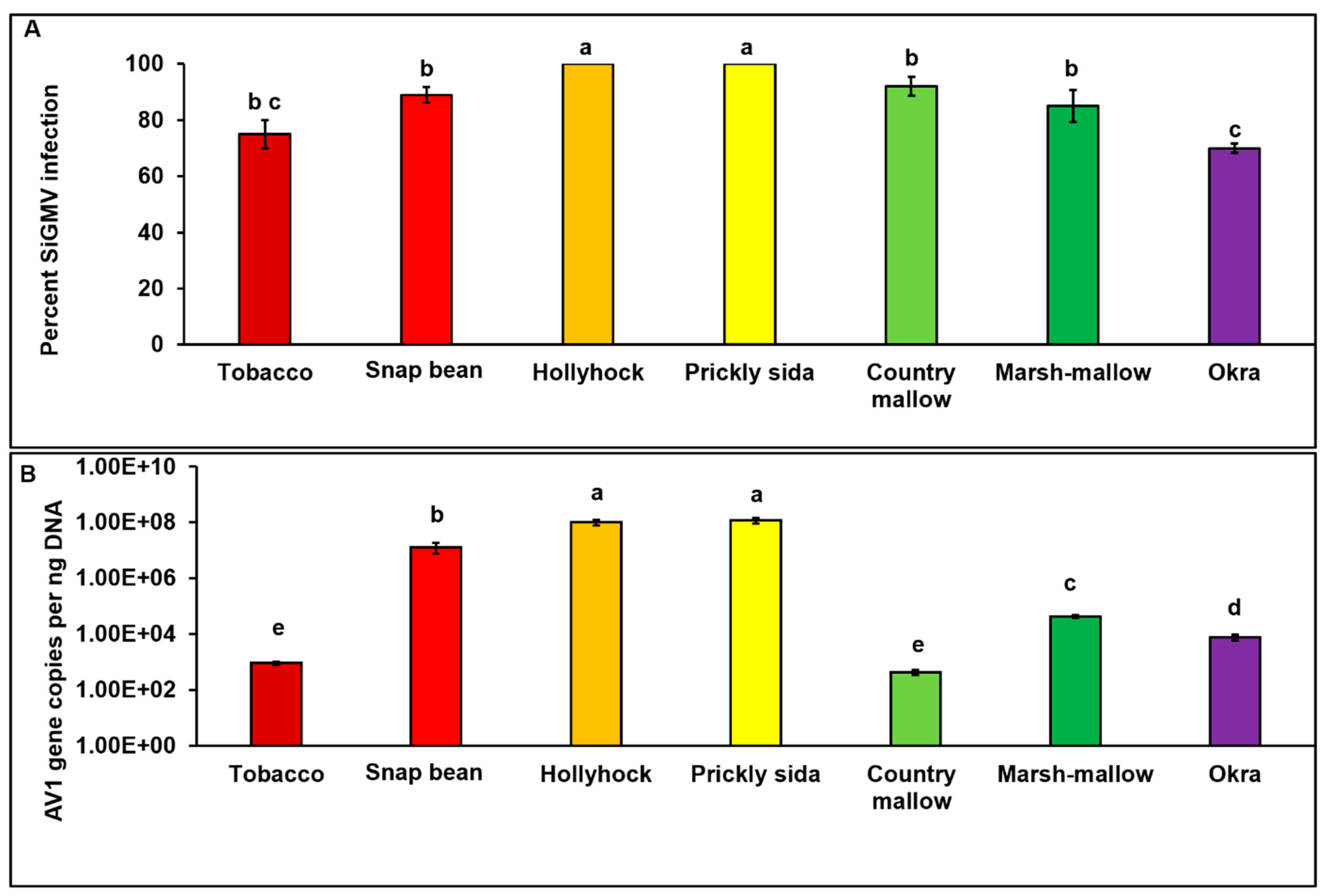




| Plant Species | Treatment | N a | Egg-to-Adult Survival b | Egg-to-Adult Developmental Time c | N d | Fecundity e | |||
|---|---|---|---|---|---|---|---|---|---|
| Tobacco | Non-infected | 45 | 46.22± 11.75 c | χ26, 308 = 31.56; p < 0.001 | 24 (22–35) b | χ26, 195 = 63.03; p < 0.001 | 30 | 77.5 ± 15.37 c | F6, 203 = 19.39; p < 0.001 |
| Snap bean | Non-infected | 45 | 62.33 ± 2.22 b | 22 (18–26) c | 30 | 153.3 ± 10.81 a | |||
| Hollyhock | Non-infected | 45 | 86.67 ± 3.84 a | 22 (19–36) c | 30 | 108.3 ± 12.10 b c | |||
| Prickly sida | Non-infected | 45 | 58.12 ± 9.23 b c | 26 (20–34) a b | 30 | 72.67 ± 9.67 c | |||
| Country mallow | Non-infected | 45 | 50 ± 10.18 b c | 26 (24–31) a b | 30 | 77.45 ± 7.58 c | |||
| Marsh mallow | Non-infected | 45 | 54.65 ± 9.21 b c | 20 (21–37) d | 30 | 112.04 ± 10.38 b | |||
| Okra | Non-infected | 45 | 56.23 ± 13.22 b c | 23 (21–33) c b | 30 | 162.34 ± 11.75 a |
| Plant Species | Treatment | N a | Egg-to-Adult Survival b | Egg-to-Adult Developmental Time c | N d | Fecundity e | |||
|---|---|---|---|---|---|---|---|---|---|
| Tobacco | Non-infected | 45 | 46.22 ± 11.75 | χ21.88 = 2.28; p = 0.13 | 24 (22–35) | U1.46 = 32, p = 0.17 | 30 | 77.5 ± 15.37 | F1.58= −1.44; p = 0.07 |
| Infected | 45 | 57.77 ± 5.87 | 25 (19–34) | 30 | 87.5 ± 22.1 | ||||
| Snap bean | Non-infected | 45 | 62.33 ± 2.22 | χ21.88 = 0.63; p = 0.43 | 22 (18–26) | U1.58 = 73, p = 0.41 | 30 | 153.3 ± 10.8 | F1.58 = 0.77; p = 0.22 |
| Infected | 45 | 64.44 ± 8.01 | 22 (20–31) | 30 | 158.3 ± 9.1 | ||||
| Hollyhock | Non-infected | 45 | 86.67 ± 3.84 | χ21.88 = 2.26; p = 0.42 | 22 (19–36) | U1.72 = 97, p = 0.74 | 30 | 108.3 ± 12.1 | F1.58 = −0.15; p = 0.43 |
| Infected | 45 | 80.00 ± 3.84 | 21 (20–29) | 30 | 111.4 ± 6.57 | ||||
| Prickly sida | Non-infected | 45 | 58.12 ± 9.23 | χ21.88 = 2.25; p= 0.13 | 26 (20–34) | U1.56 = 42, p < 0.001 | 30 | 72.67 ± 9.67 | F1.58 = −4.3; p < 0.001 |
| Infected | 45 | 68.4 ± 10.11 | 20 (18–29) | 30 | 112.23 ± 8.87 | ||||
| Country mallow | Non-infected | 45 | 50 ± 10.18 | χ21.88 = 1.61; p = 0.20 | 26 (24–31) | U1.68 = 13, p = 0.013 | 30 | 77.45 ± 7.58 | F1.58 = −3.72; p < 0.001 |
| Infected | 45 | 60.55 ± 8.01 | 22 (21–28) | 30 | 101.60 ± 10.87 | ||||
| Marsh mallow | Non-infected | 45 | 54.65 ± 9.21 | χ21.88 = 0.84; p = 0.36 | 20 (18–37) | U1.50 = 82, p = 0.136 | 30 | 112.04 ± 10.38 | F 1.58= −1.09; p = 0.13 |
| Infected | 45 | 60.25 ± 7.89 | 21 (17–31) | 30 | 126.60 ± 9.87 | ||||
| Okra | Non-infected | 45 | 56.23 ± 13.22 | χ21.48 = 6.26; p = 0.09 | 23 (21–33) | U1.88 = 39, p < 0.001 | 30 | 162.34 ± 11.75 | F1.58 = 5.81; p < 0.001 |
| Infected | 45 | 51.11 ± 14.57 | 28 (22–32) | 30 | 89.00 ± 22.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gautam, S.; Buck, J.W.; Dutta, B.; Coolong, T.; Sanchez, T.; Smith, H.A.; Adkins, S.; Srinivasan, R. Sida Golden Mosaic Virus, an Emerging Pathogen of Snap Bean (Phaseolus vulgaris L.) in the Southeastern United States. Viruses 2023, 15, 357. https://doi.org/10.3390/v15020357
Gautam S, Buck JW, Dutta B, Coolong T, Sanchez T, Smith HA, Adkins S, Srinivasan R. Sida Golden Mosaic Virus, an Emerging Pathogen of Snap Bean (Phaseolus vulgaris L.) in the Southeastern United States. Viruses. 2023; 15(2):357. https://doi.org/10.3390/v15020357
Chicago/Turabian StyleGautam, Saurabh, James W. Buck, Bhabesh Dutta, Timothy Coolong, Tatiana Sanchez, Hugh A. Smith, Scott Adkins, and Rajagopalbabu Srinivasan. 2023. "Sida Golden Mosaic Virus, an Emerging Pathogen of Snap Bean (Phaseolus vulgaris L.) in the Southeastern United States" Viruses 15, no. 2: 357. https://doi.org/10.3390/v15020357





