Development of an IgY-Based Treatment to Control Bovine Coronavirus Diarrhea in Dairy Calves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Egg Powder Enriched with IgY Abs to BCoV
2.2. BCoV IgY Specific Antibody ELISA
2.3. Fluorescent Focus Reduction virus Neutralization Test
2.4. Virus and Inoculum Production
2.5. Experimental Design to Test the Efficacy of Egg Yolk Powder to Prevent BCoV Diarrhea in Calves

2.6. Coronavirus Antigen Detection
2.7. Quantitative Real-Time BCoV PCR
2.8. Cell Culture Immunofluorescence Assay (CCIF)
2.9. Bovine Isotype-Specific Antibody ELISA
2.10. Statistical Analysis
3. Results
3.1. Development of a Product Based on IgY Abs to BCoV
3.2. Quality Control of Egg Yolk Powder DS Batches Enriched in IgY to BCoV
3.3. BCoV IgY-Specific Antibody ELISA Validation
3.3.1. Positive Control: Admission Range and Linearity
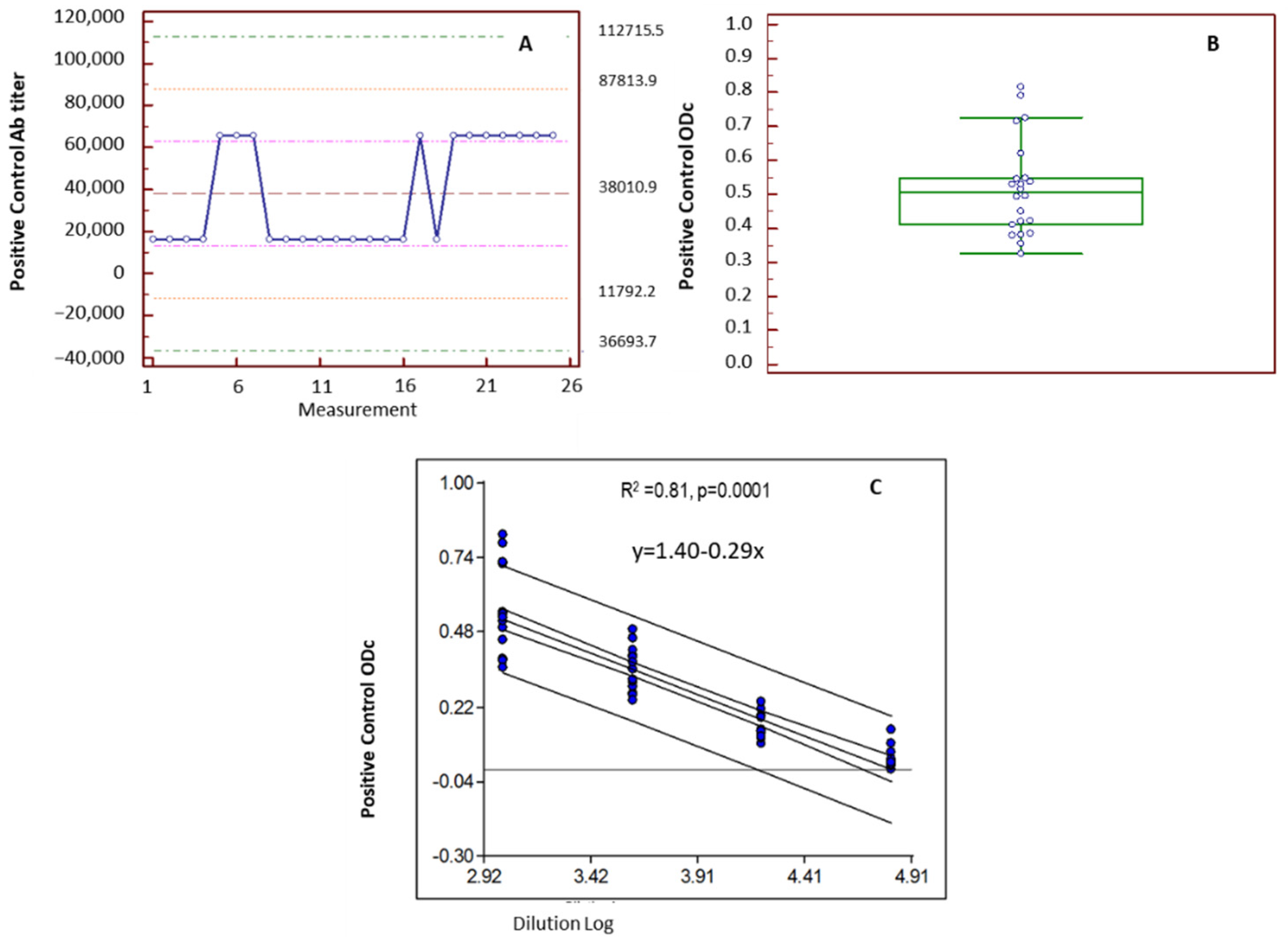
3.3.2. ROC Curve: Cut-Off Value and Associated Sensitivity and Specificity

3.3.3. Assay Intermediate Precision
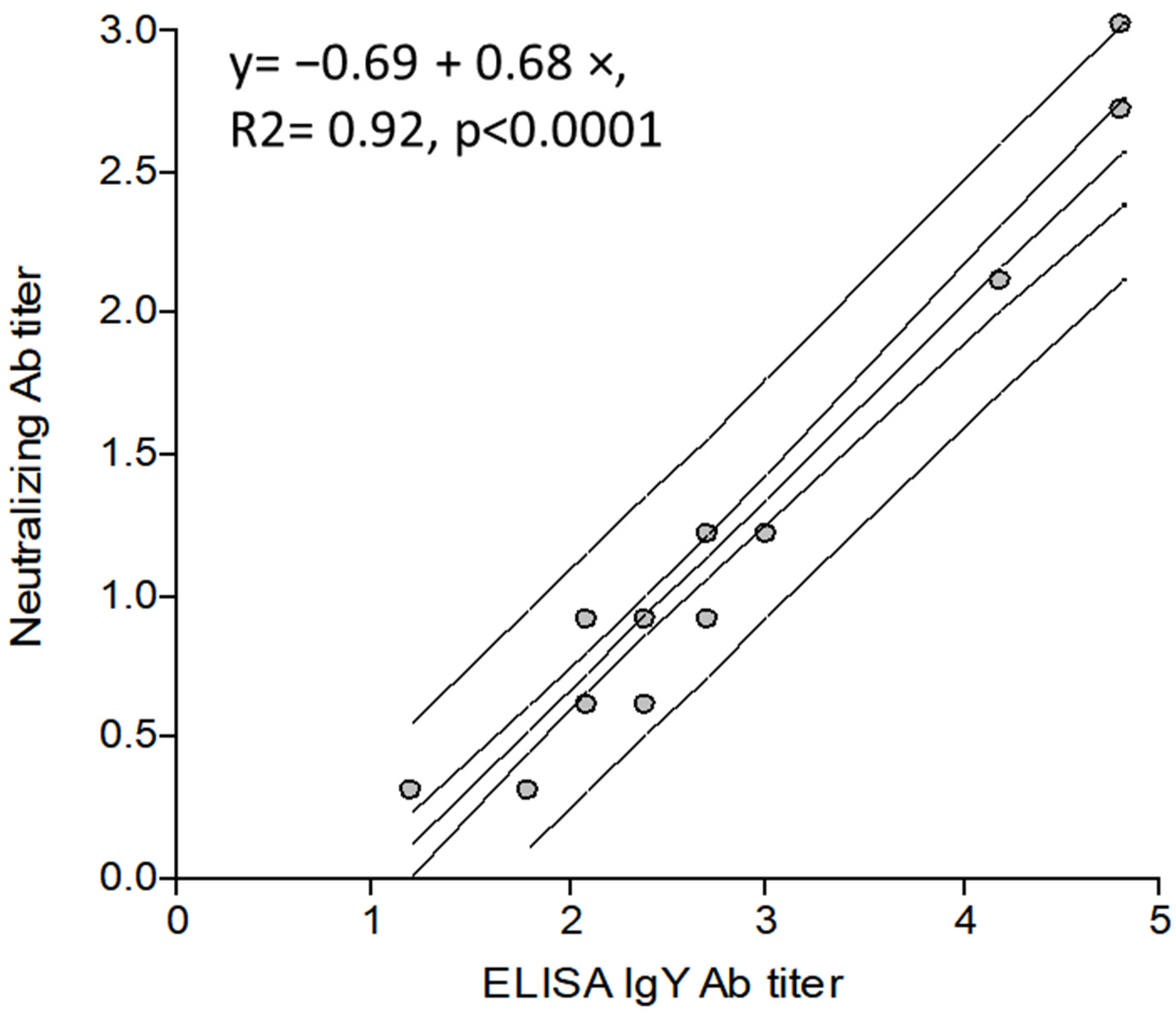
3.3.4. Correlation between the IgY ELISA Ab Titers and Neutralizing Ab Titers
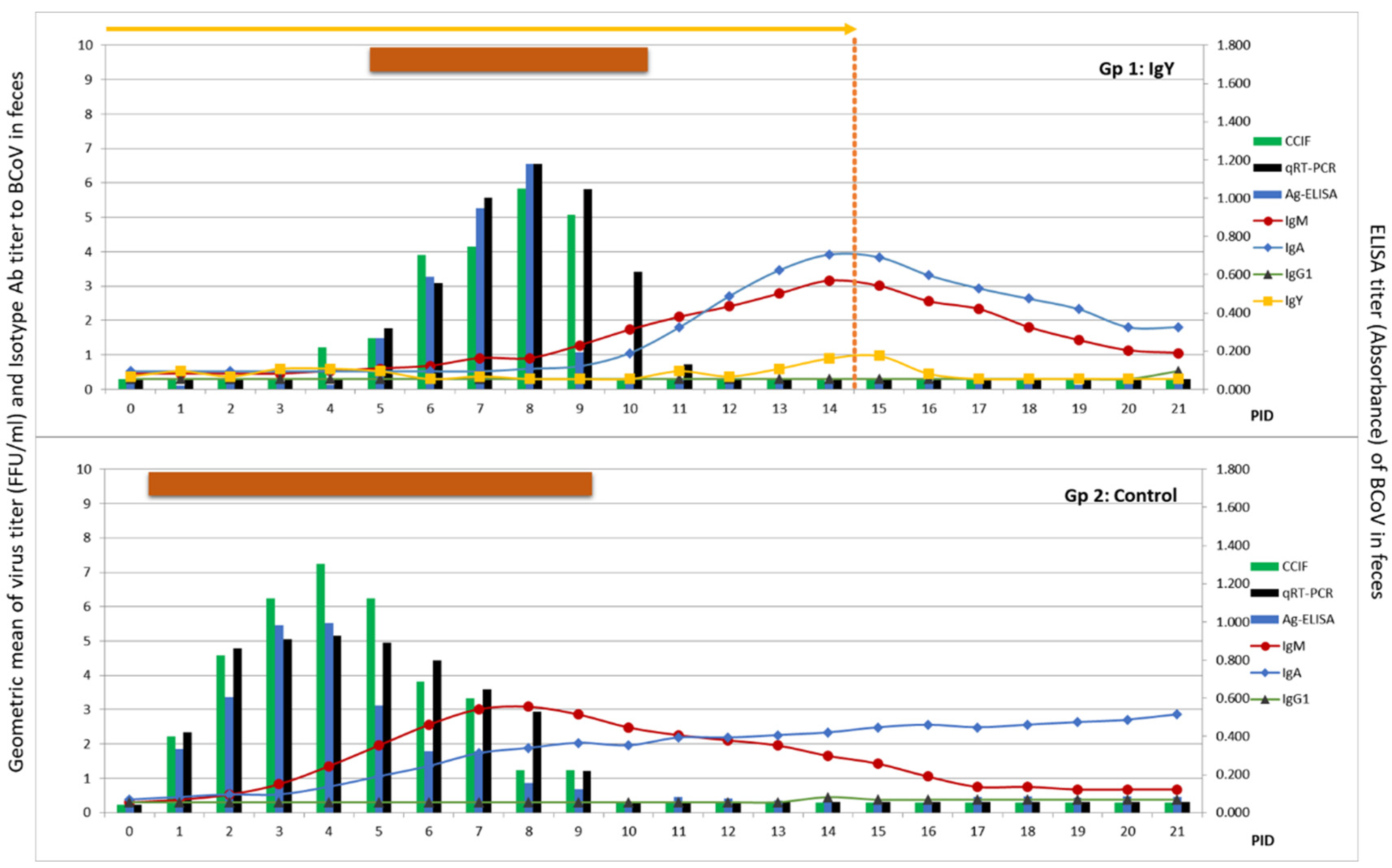
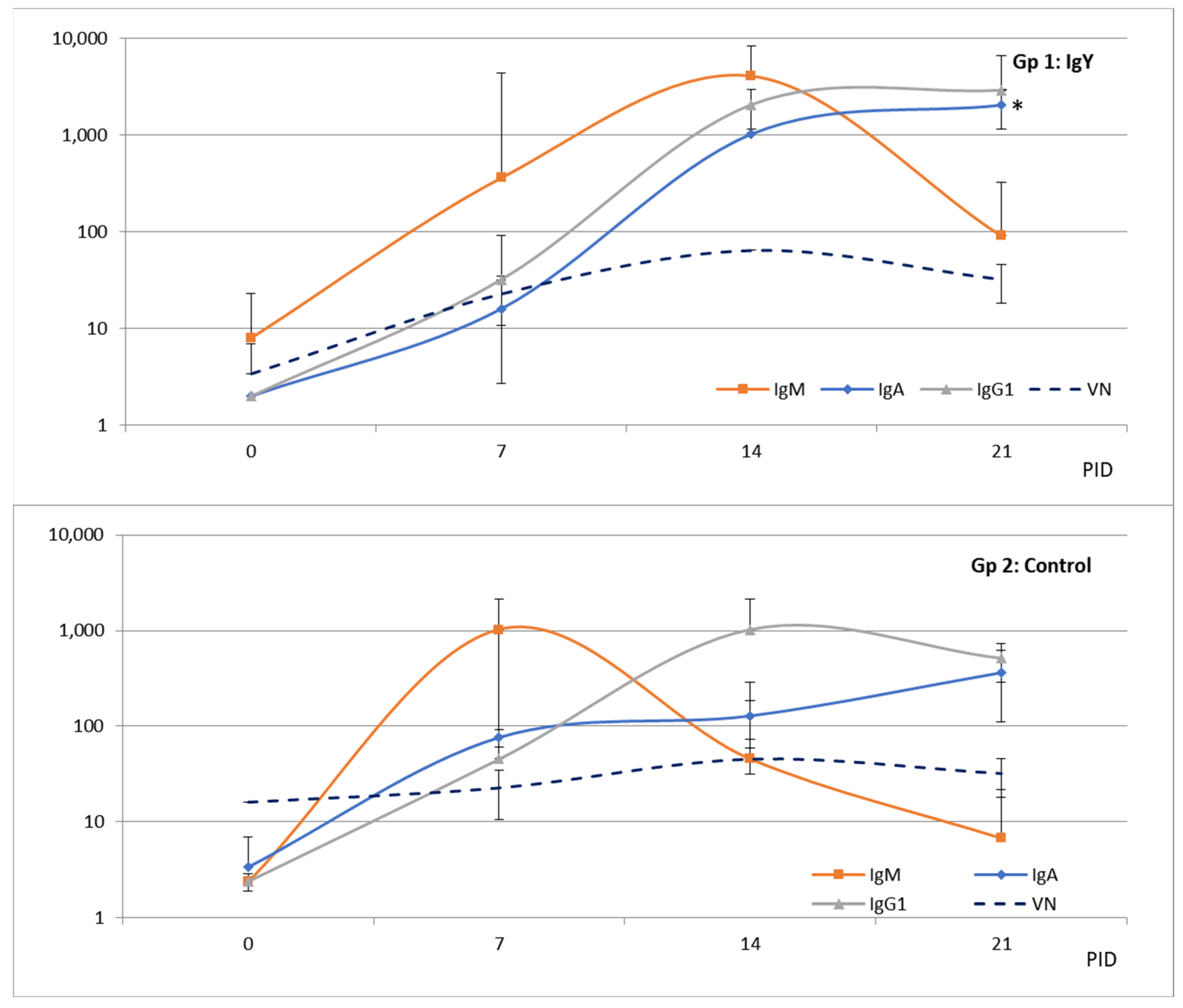
3.4. Reproduction of BCoV Infection and Diarrhea in Colostrum-Deprived Calves
3.5. Egg Powder Containing BCoV IgY Abs Reduced Diarrhea Duration and Severity in a Colostrum-Deprived Calf Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA. Dairy 2007, Heifer Calf Health and Management Practices on U.S. Dairy Operations, 2007; USDA:APHIS:VS.; CEAH: Fort Collins, CO, USA, 2010.
- Boileau, M.J.; Kapil, S. Bovine Coronavirus Associated Syndromes. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 123–146. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20117547 (accessed on 6 February 2023). [CrossRef]
- Vlasova, A.N.; Saif, L.J. Biological aspects of the interspecies transmission of selected coronaviruses. In Viral Infections and Global Change; Singh, S.K., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2014; pp. 393–418. [Google Scholar] [CrossRef] [Green Version]
- Blanchard, P.C. Diagnostics of Dairy and Beef Cattle Diarrhea. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 443–464. [Google Scholar] [CrossRef]
- Castells, M.; Giannitti, F.; Caffarena, R.D.; Casaux, M.L.; Schild, C.; Castells, D.; Riet-Correa, F.; Victoria, M.; Parreño, V.; Colina, R. Bovine coronavirus in Uruguay: Genetic diversity, risk factors and transboundary introductions from neighboring countries. Arch. Virol. 2019, 164, 2715–2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.R. Field Disease Diagnostic Investigation of Neonatal Calf Diarrhea. Vet. Clin. N. Am.—Food Anim. Pract. 2012, 28, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, E.; Aduriz, M.; Bok, M.; Vega, C.G.; Saif, L.; Aguirre, D.; Cimino, R.O.; Miño, S.; Parreño, V. First report of group A rotavirus and bovine coronavirus associated with neonatal calf diarrhea in the northwest of Argentina. Trop. Anim. Health Prod. 2020, 52, 2761–2768. [Google Scholar] [CrossRef]
- Bok, M.; Alassia, M.; Frank, F.; Vega, C.G.; Wigdorovitz, A.; Parreño, V. Passive immunity to control Bovine coronavirus diarrhea in a dairy herd in Argentina. Rev. Argent. Microbiol. 2017, 50, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Crouch, C.F.; Acres, S.D. Prevalence of rotavirus and coronavirus antigens in the feces of normal cows. Can. J. Comp. Med. 1984, 48, 340–342. [Google Scholar]
- Marsolais, G.; Assaf, R.; Montpetit, C.; Marois, P. Diagnosis of viral agents associated with neonatal calf diarrhea. Can. J. Comp. Med. 1978, 42, 168–171. [Google Scholar]
- Langpap, T.J.; Bergeland, M.E.; Reed, D.E. Coronaviral enteritis of young calves: Virologic and pathologic findings in naturally occurring infections. Am. J. Vet. Res. 1979, 40, 1476–1478. [Google Scholar]
- Bok, M.; Mino, S.; Rodriguez, D.; Badaracco, A.; Nunes, I.; Souza, S.P.; Bilbao, G.; Louge Uriarte, E.; Galarza, R.; Vega, C.G.; et al. Molecular and antigenic characterization of bovine Coronavirus circulating in Argentinean cattle during 1994–2010. Vet. Microbiol. 2015, 181, 221–229. [Google Scholar] [CrossRef]
- Saif, L.J. Bovine respiratory coronavirus. Vet. Clin. N. Am.—Food Anim. Pract. 2010, 26, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Vlasova, A.N.; Saif, L.J. Bovine Coronavirus and the Associated Diseases. Front. Vet. Sci. 2021, 8, 643220. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.A.; Naylor, J.A.; House, J.K. Diarrhea. In Large Animal Internal Medicine; BP, S., Ed.; Mosby/Elsevier: St. Louis, MO, USA, 2009; pp. 340–363. [Google Scholar]
- Meganck, V.; Goddeeris, B.M.; Stuyven, E.; Piepers, S.; Cox, E.; Opsomer, G. Development of a method for isolating bovine colostrum mononuclear leukocytes for phenotyping and functional studies. Vet. J. 2014, 200, 294–298. [Google Scholar] [CrossRef]
- Saif, L.J.; Redman, D.R.; Brock, K.V.; Kohler, E.M.; Heckert, R.A. Winter Dysentery in Adult Dairy Cattle: Detection of Coronavirus in the Faeces. Vet. Rec. 1988, 123, 300–301. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=284835020117547 (accessed on 6 February 2023). [CrossRef]
- Cho, K.O.; Hoet, A.E.; Loerch, S.C.; Wittum, T.E.; Saif, L.J. Evaluation of Concurrent Shedding of Bovine Coronavirus via the Respiratory Tract and Enteric Route in Feedlot Cattle. Am. J. Vet. Res. 2001, 62, 1436–1441. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11560274284835020117547 (accessed on 6 February 2023). [CrossRef] [PubMed]
- Decaro, N.; Elia, G.; Campolo, M.; Desario, C.; Mari, V.; Radogna, A.; Colaianni, M.L.; Cirone, F.; Tempesta, M.; Buonavoglia, C. Detection of Bovine Coronavirus Using a TaqMan-Based Real-Time RT-PCR Assay. J. Virol. Methods 2008, 151, 167–171. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18579223284835020117547 (accessed on 6 February 2023). [CrossRef]
- Cortese, V.S. Neonatal immunology. Vet. Clin. N. Am. Food Anim. Pract. 2009, 25, 221–227. [Google Scholar] [CrossRef]
- Besser, T.E.; McGuire, T.C.; Gay, C.C.; Pritchett, L.C. Transfer of Functional Immunoglobulin G (IgG) Antibody into the Gastrointestinal Tract Accounts for IgG Clearance in Calves. J. Virol 1988, 62, 2234–2237. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3373569284835020117547 (accessed on 6 February 2023). [CrossRef] [Green Version]
- Barrington, G.M.; McFadden, T.B.; Huyler, M.T.; Besser, T.E. Regulation of colostrogenesis in cattle. Livest. Prod. Sci. 2001, 70, 95–104. [Google Scholar] [CrossRef]
- Parreño, V.; Marcoppido, G.; Vega, C.G.; Garaicoechea, L.; Rodriguez, D.; Saif, L.; Fernández, F. Milk supplemented with immune colostrum: Protection against rotavirus diarrhea and modulatory effect on the systemic and mucosal antibody responses in calves experimentally challenged with bovine rotavirus. Vet. Immunol. Immunopathol. 2010, 136, 12–27. [Google Scholar] [CrossRef]
- Kuroki, M.; Ohta, M.; Ikemori, Y.; Peralta, R.C.; Yokoyama, H.; Kodama, Y. Passive Protection against Bovine Rotavirus in Calves by Specific Immunoglobulins from Chicken Egg Yolk. Arch. Virol. 1994, 138, 143–148. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7980004 (accessed on 6 February 2023). [CrossRef] [PubMed]
- Vega, C.; Bok, M.; Chacana, P.; Saif, L.; Fernandez, F.; Parreno, V. Egg Yolk IgY: Protection against Rotavirus Induced Diarrhea and Modulatory Effect on the Systemic and Mucosal Antibody Responses in Newborn Calves. Vet. Immunol. Immunopathol. 2011, 142, 156–169. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21652087 (accessed on 6 February 2023). [CrossRef] [PubMed] [Green Version]
- Vega, C.; Bok, M.; Saif, L.; Fernandez, F.; Parreño, V. Egg yolk IgY antibodies: A therapeutic intervention against group A rotavirus in calves. Res. Vet. Sci. 2015, 103, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agurto-Arteaga, A.; Poma-Acevedo, A.; Rios-Matos, D.; Choque-Guevara, R.; Montesinos-Millán, R.; Montalván, Á.; Isasi-Rivas, G.; Cauna-Orocollo, Y.; Cauti-Mendoza, M.d.G.; Pérez-Martínez, N.; et al. Preclinical Assessment of IgY Antibodies against Recombinant SARS-CoV-2 RBD Protein for Prophylaxis and Post-Infection Treatment of COVID-19. Front. Immunol. 2022, 13, 1948. [Google Scholar] [CrossRef] [PubMed]
- Frumkin, L.R.; Lucas, M.; Scribner, C.L.; Ortega-Heinly, N.; Rogers, J.; Yin, G.; Hallam, T.J.; Yam, A.; Bedard, K.; Begley, R.; et al. Egg-Derived Anti-SARS-CoV-2 Immunoglobulin Y (IgY) with Broad Variant Activity as Intranasal Prophylaxis Against COVID-19. Front. Immunol. 2022, 13, 899617. [Google Scholar] [CrossRef] [PubMed]
- Klemperer, F. Ueber natürliche Immunität und ihre Verwerthung für die Immunisirungstherapie. Arch. Exp. Pathol. Pharmakol. 1893, 31, 356–382. [Google Scholar] [CrossRef]
- Schade, R.; Calzado, E.G.; Sarmiento, R.; Chacana, P.A.; Porankiewicz-Asplund, J.; Terzolo, H.R. Chicken egg yolk antibodies (IgY-technology): A review of progress in production and use in research and human and veterinary medicine. Altern. Lab. Anim. 2005, 33, 129–154. [Google Scholar] [CrossRef]
- Carlander, D. Avian IgY Antibody; Uppsala University: Uppsala, Sweden, 2002; ISBN 9155452272. [Google Scholar]
- Schade, C.; Hendriksen, M.; Erhard, H.; Hugl, G.; Koch, A.; Larsson, W.; Pollmann, M.; van Regenmortel, E.; Rijke, H.; Spielmann, H.; et al. The Production of Avian (Egg Yolk) Antibodies: IgY. ATLA 1996, 24, 925–934. [Google Scholar] [CrossRef]
- Fu, C.-Y.; Huang, H.; Wang, X.-M.; Liu, Y.-G.; Wang, Z.-G.; Cui, S.-J.; Gao, H.-L.; Li, Z.; Li, J.-P.; Kong, X.-G. Preparation and evaluation of anti-SARS coronavirus IgY from yolks of immunized SPF chickens. J. Virol. Methods 2006, 133, 112–115. [Google Scholar] [CrossRef]
- Lee, D.H.; Jeon, Y.-S.; Park, C.-K.; Kim, S.; Lee, D.S.; Lee, C. Immunoprophylactic effect of chicken egg yolk antibody (IgY) against a recombinant S1 domain of the porcine epidemic diarrhea virus spike protein in piglets. Arch. Virol. 2015, 160, 2197–2207. [Google Scholar] [CrossRef]
- Sitnik, O.; Jawor, P.; Kopec, W.; Skiba, T.; Stefaniak, T. Production and characterization of egg yolk antibodies against bovine alimentary tract pathogens. Pol. J. Vet. Sci. 2013, 16, 283–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikemori, Y.; Ohta, M.; Umeda, K.; Icatlo, F.C.; Kuroki, M.; Yokoyama, H.; Kodama, Y. Passive protection of neonatal calves against bovine coronavirus-induced diarrhea by administration of egg yolk or colostrum antibody powder. Vet. Microbiol. 1997, 58, 105–111. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health (OIE). Chapter 1.1.6. Principles and methods of validation of diagnostic assays for infectious diseases (NB: Version adopted in May 2013). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organisation for Animal Health (OIE): Paris, France, 2013. [Google Scholar]
- Parreño, V.; Bejar, C.; Vagnozzi, A.; Barrandeguy, M.; Costantini, V.; Craig, M.I.; Yuan, L.; Hodgins, D.; Saif, L.; Fernandez, F. Modulation by Colostrum-Acquired Maternal Antibodies of Systemic and Mucosal Antibody Responses to Rotavirus in Calves Experimentally Challenged with Bovine Rotavirus. Vet. Immunol. Immunopathol. 2004, 100, 7–24. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15182992 (accessed on 6 February 2023). [CrossRef] [PubMed]
- Smith, D.R.; Tsunemitsu, H.; Heckert, R.A.; Saif, L.J. Evaluation of Two Antigen-Capture ELISAs Using Polyclonal or Monoclonal Antibodies for the Detection of Bovine Coronavirus. J. Vet. Diagn. Investig. 1996, 8, 99–105. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9026089 (accessed on 6 February 2023). [CrossRef] [Green Version]
- Cho, Y.I.; Kim, W.I.; Liu, S.; Kinyon, J.M.; Yoon, K.J. Development of a panel of multiplex real-time polymerase chain reaction assays for simultaneous detection of major agents causing calf diarrhea in feces. J. Vet. Diagn. Investig. 2010, 22, 509–517. [Google Scholar] [CrossRef] [Green Version]
- World Organisation for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organisation for Animal Health: Paris, France, 2022; Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-manual-online-access/ (accessed on 6 February 2023).
- Carrasco, J.L.; Jover, L. Métodos estadísticos para evaluar la concordancia. Med. Clin. 2004, 122, 28–34. [Google Scholar] [CrossRef]
- Decaro, N.; Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef]
- Cho, Y.I.; Yoon, K.J. An overview of calf diarrhea—Infectious etiology, diagnosis, and intervention. J. Vet. Sci. 2014, 15, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Zedan, H.T.; Yassine, H.M.; Al-Sadeq, D.W.; Liu, N.; Qotba, H.; Nicolai, E.; Pieri, M.; Bernardini, S.; Abu-Raddad, L.J.; Nasrallah, G.K. Evaluation of commercially available fully automated and ELISA-based assays for detecting anti-SARS-CoV-2 neutralizing antibodies. Sci. Rep. 2022, 12, 19020. [Google Scholar] [CrossRef]
- Han, M.G.; Cheon, D.-S.; Zhang, X.; Saif, L.J. Cross-protection against a human enteric coronavirus and a virulent bovine enteric coronavirus in gnotobiotic calves. J. Virol. 2006, 80, 12350–12356. [Google Scholar] [CrossRef] [Green Version]
- Heckert, R.A.; Saif, L.J.; Myers, G.W. Mucosal and Systemic Isotype-Specific Antibody Responses to Bovine Coronavirus Structural Proteins in Naturally Infected Dairy Calves. Am. J. Vet. Res. 1991, 52, 852–857. Available online: http://www.ncbi.nlm.nih.gov/pubmed/1652905 (accessed on 6 February 2023). [PubMed]
- Arthington, J.D.; Jaynes, C.A.; Tyler, H.D.; Kapil, S.; Quigley, J.D. The use of bovine serum protein as an oral support therapy following coronavirus challenge in calves. J. Dairy Sci. 2002, 85, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, K.; Nassiri, M.R.; Tahmoorespur, M.; Haghparast, A.; Zibaee, S. Production and characterization of egg yolk antibody (IgY) against recombinant VP8-S2 antigen. Pol. J. Vet. Sci. 2016, 19, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandao, P.E.; Gregori, F.; Richtzenhain, L.J.; Rosales, C.A.; Villarreal, L.Y.; Jerez, J.A. Molecular Analysis of Brazilian Strains of Bovine Coronavirus (BCoV) Reveals a Deletion within the Hypervariable Region of the S1 Subunit of the Spike Glycoprotein also Found in Human Coronavirus OC43. Arch. Virol. 2006, 151, 1735–1748. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16583154 (accessed on 6 February 2023). [CrossRef] [Green Version]
- Nelson, R.; Katayama, S.; Mine, Y.; Duarte, J.; Matar, C. Immunomodulating effects of egg yolk low lipid peptic digests in a murine model. Food Agric. Immunol. 2007, 18, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Polanowski, A.; Zabłocka, A.; Sosnowska, A.; Janusz, M.; Trziszka, T. Immunomodulatory activity accompanying chicken egg yolk immunoglobulin Y. Poult. Sci. 2012, 91, 3091–3096. [Google Scholar] [CrossRef]
- Pereira, E.P.V.; van Tilburg, M.F.; Florean, E.O.P.T.; Guedes, M.I.F. Egg yolk antibodies (IgY) and their applications in human and veterinary health: A review. Int. Immunopharmacol. 2019, 73, 293–303. [Google Scholar] [CrossRef]
- Leiva, C.L.; Gallardo, M.J.; Casanova, N.; Terzolo, H.; Chacana, P. IgY-technology (egg yolk antibodies) in human medicine: A review of patents and clinical trials. Int. Immunopharmacol. 2020, 81, 106269. [Google Scholar] [CrossRef]

| Product | DS Batch | ELISA | VN (Mebus) | VN (Arg95) | Humidity 1 (%) | Fat 1(%) | Proteins 1 (%) | Coliforms 2 (CFU/g) | Salmonella 2 (P-A/25 g) |
|---|---|---|---|---|---|---|---|---|---|
| IgY to BCoV | 1 | 1024 | 64 | 64 | 2.97 | 39.63 | 48.31 | <10 | A |
| IgY to BCoV | 2 | 1024 | 64 | 64 | 3.85 | 40.61 | 47.06 | <10 | A |
| supplemented Milk | 512 | 32 | 32 | - | - | - | - | - |
| Parameter | Result | Acceptance Criteria | |
|---|---|---|---|
| Accuracy and precision measurements | IgY in avian and bovine samples | ||
| Associated parameters | ODc 405 nm | P% | |
| Threshold (cut-off) | >0.110 | 18.68% | ODc = 0.100 P% 13–20% |
| Diagnosis Specificity (DSp) | 98.2% | >90% | |
| Diagnosis Sensitivity (DSe) | 97.7% | >90% | |
| Intermediate precision Coefficient of Variation (CV) | 27% (Figure 3B) | <30% | |
| Linearity | YES R2 = 0.91 p = 0.0001 | Linear relationship between OD and sample dilution | |
| Reference standard reagent | Sera from chicken immunized with experimental vaccines with known BCoV antigen concentration. | ||
| Operating range of assay | Starting sample dilution 1:32 Linearity was demonstrated between 1024–65536 reciprocal dilutions (Figure 3C) | ||
| “Specificity” | The assay detects IgY antibodies against BCoV but not to Rotavirus, Salmonella or Escherichia coli | No cross reaction against NCD vaccine antigens | |
| IgY ELISA (Cut-Off: 18.68 P%) Sample Dilution 1/256 | |||
|---|---|---|---|
| Reference Population | Negative 0 | Positive 1 | |
| Negative (0) | 108 | 5 | 113 (46.9%) |
| Positive (1) | 3 | 125 | 128 (53.1%) |
| 111 (46.1%) | 130 (53.9%) | 241 | |
| Kappa | 0.933 | ||
| Standard error | 0.023 | ||
| 95% CI | 0.888 to 0.979 | ||
| Sample | BCoV Infectious Titer (FFU/mL) |
|---|---|
| Inoculum | 105 |
| SIC | 106 |
| Colon | 109 |
| LIC | 1010 |
| Feces | 1010 |
| Diarrhea | Shedding of Infectious Virus | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment Group | n | Onset (PID) | Duration (Days) | Severity (AUC) | Hyperthermia (Days) | Onset (PID) | Duration (Days) | Magnitude (AUC) |
| Gp 1: BCoV-IgY | 4 | 6.25 A | 3.8 B | 17.7 B | 1.5 B | 6.3 A | 3.0 B | 23.8 A |
| Gp 2: Control | 4 | 1.5 B | 7.8 A | 25.4 A | 4.75 A | 2.0 B | 6.0 A | 36.4 A |
| p-value | 0.0019 | 0.0003 | 0.0036 | - | 0.0041 | 0.0104 | 0.103 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bok, M.; Vega, C.G.; Castells, M.; Colina, R.; Wigdorovitz, A.; Parreño, V. Development of an IgY-Based Treatment to Control Bovine Coronavirus Diarrhea in Dairy Calves. Viruses 2023, 15, 708. https://doi.org/10.3390/v15030708
Bok M, Vega CG, Castells M, Colina R, Wigdorovitz A, Parreño V. Development of an IgY-Based Treatment to Control Bovine Coronavirus Diarrhea in Dairy Calves. Viruses. 2023; 15(3):708. https://doi.org/10.3390/v15030708
Chicago/Turabian StyleBok, Marina, Celina G. Vega, Matias Castells, Rodney Colina, Andrés Wigdorovitz, and Viviana Parreño. 2023. "Development of an IgY-Based Treatment to Control Bovine Coronavirus Diarrhea in Dairy Calves" Viruses 15, no. 3: 708. https://doi.org/10.3390/v15030708
APA StyleBok, M., Vega, C. G., Castells, M., Colina, R., Wigdorovitz, A., & Parreño, V. (2023). Development of an IgY-Based Treatment to Control Bovine Coronavirus Diarrhea in Dairy Calves. Viruses, 15(3), 708. https://doi.org/10.3390/v15030708






